Abstract
Active matrix metalloproteinases and degraded collagen are observed in disease states, such as atherosclerosis. To examine whether degraded collagen fragments have distinct effects on vascular smooth muscle cells (SMC), collagenase-digested type I collagen was added to cultured human arterial SMC. After addition of collagen fragments, adherent SMC lose their focal adhesion structures and round up. Analysis of components of the focal adhesion complex demonstrates rapid cleavage of the focal adhesion kinase (pp125FAK), paxillin, and talin. Cleavage is suppressed by inhibitors of the proteolytic enzyme, calpain I. In vitro translated pp125FAK is a substrate for both calpain I– and II–mediated processing. Mapping of the proteolytic cleavage fragments of pp125FAK predicts a dissociation of the focal adhesion targeting (FAT) sequence and second proline-rich domain from the tyrosine kinase domain and integrin-binding sequence. Coimmunoprecipitation studies confirm that the ability of pp125FAK to associate with paxillin, vinculin, and p130cas is significantly reduced in SMC treated with degraded collagen fragments. Further, there is a significant reduction in the association of intact pp125FAK with the cytoskeletal fraction, while pp125FAK cleavage fragments appear in the cytoplasm in SMC treated with degraded collagen fragments. Integrin-blocking studies indicate that integrin-mediated signals are involved in degraded collagen induction of pp125FAK cleavage. Thus, collagen fragments induce distinct integrin signals that lead to initiation of calpain-mediated cleavage of pp125FAK, paxillin, and talin and dissolution of the focal adhesion complex.
Keywords: extracellular matrix, matrix metalloproteinase, calpain, focal adhesion kinase, pp125FAK
Extracellular matrix (ECM)1 molecules regulate cell function by acting as both structural support and signaling mediators (Hay 1989; Hynes 1992). Integration of the intracellular signaling and structural responses initiated by ECM-integrin engagement may be mediated through the focal adhesion complex (Burridge et al. 1988). Focal adhesions are localized to sites of integrin-ECM adhesion (Burridge and Chrzanowska-Wodnicka 1996). They consist of numerous, tightly associated, structural and signaling proteins and the cytoplasmic tail of β integrins, which provide intracellular signal transduction machinery but also serve as a physical link between the ECM and the cytoskeleton (Sastry and Horwitz 1993).
Focal adhesion kinase (pp125FAK) is a cytoplasmic tyrosine kinase that localizes to focal adhesions and may play an important role in the integration of signals from integrins and growth factors (Hanks and Polte 1996; Zachary and Rozengurt 1992). The pp125FAK protein can be subdivided into three functional domains: an NH2-terminal integrin-binding domain necessary for association with the cytoplasmic domain of β1 integrin subunits (Schaller et al. 1995), an internal kinase domain, and a COOH-terminal domain consisting of two proline-rich motifs and a focal adhesion targeting sequence required for association with other focal adhesion proteins, including p130cas, paxillin, and talin (Chen et al. 1995; Hildebrand et al. 1995; Polte and Hanks 1995). pp125FAK also contains a number of sites of tyrosine phosphorylation involved in binding to the SH2 domains of signaling proteins such as P-I3 kinase, pp60src and Grb2 (Schaller et al. 1994; Schlaepfer et al. 1994; Bachelot et al. 1996). Through these protein-binding domains, pp125FAK plays a central role in the recruitment of signaling molecules to the focal adhesion complex and in the transduction of downstream signals derived from integrin-ECM interactions (reviewed in Guan 1997). Previous studies have implicated tyrosine phosphorylation of pp125FAK as the primary mechanism whereby integrin-ECM interactions regulate pp125FAK activity and recruitment of signaling molecules (Schaller and Parsons 1995). However, a recent study carried out on platelets proposed a novel mechanism of pp125FAK regulation, in which the calcium-activated proteolytic enzyme calpain I induces cleavage of pp125FAK, leading to downregulation of pp125FAK autokinase activity and altered intracellular localization (Cooray et al. 1996).
ECM-integrin engagement may affect numerous biological processes in vivo, including cell participation in the development of lesions of atherosclerosis (Thyberg et al. 1990). Smooth muscle cells within the medial layer of normal arteries are surrounded by a dense network of interstitial type I and III collagen fibers (Barnes 1985). However, disruption of collagen fibers during atherogenesis is thought to occur as a result of increased activation of matrix metalloproteinases derived from either infiltrating macrophages or activated SMC (Bendeck et al. 1994; Galis et al. 1994). SMC are arrested at the G1 stage of the cell cycle in vitro on polymerized type I collagen fibrils, whereas monomer collagen supports SMC proliferation (Koyama et al. 1996).
From these studies, we hypothesized that the ECM surrounding SMC in the normal media may be nonpermissive for SMC proliferation. Degradation of the ECM during different stages of lesion development may release SMC from this nonpermissive state and promote SMC migration and proliferation. Previous studies have indicated that denaturation or proteolysis of the type I collagen triple helix may reveal cryptic RGD integrin-binding motifs (Davis 1992; Montgomery et al. 1994). Recent studies also demonstrate that processing of the ECM protein laminin 5 by matrix metalloproteinases generates fragments that have different cell signaling properties from intact laminin 5 (Gianelli et al. 1997). These studies raise the possibility that fragments of degraded collagen generated within the atherosclerotic lesion may modulate SMC behavior by possessing specific integrin signaling properties, which are distinct from those of intact native collagen found in normal arteries.
In this study, we describe the rapid induction of calpain-mediated cleavage of pp125FAK, paxillin, and talin in response to treatment of SMC with collagenase-degraded collagen fragments. The cleavage of pp125FAK, paxillin, and talin in adherent SMC occurs in parallel with functional and molecular focal adhesion disassembly and cell rounding. We propose that calpain-mediated cleavage of pp125FAK represents a dynamic mechanism for regulating pp125FAK activity and together with paxillin and talin cleavage promotes focal adhesion disassembly in response to matrix degradation.
Materials and Methods
Cell Culture and Collagen Matrix Preparation
Human newborn arterial SMC were isolated from the thoracic aorta as described previously (Bornfeldt et al. 1994). SMC between passages 5 and 9 were cultured on the surface or in the presence of the following collagen type I preparation: polymerized collagen gels (1.0 mg/ml final concentration) prepared by neutralization of the collagen solution (Vitrogen 100, Collagen Corp.) with 1/6 volume of 7× DME concentrate, dilution to a final 1× DME solution, and incubation at 37°C for 24 h. Monomer collagen-coated dishes were prepared by incubating with 0.1 mg/ml of collagen solution in 0.1 M acetic acid for 24 h. Monomer collagen-coated dishes were washed twice with DME before cell seeding. Degraded type I collagen was prepared by incubating polymerized collagen gels (prepared as above) with 2.5 mg/ml collagenase type 1 (Worthington Biochemical Corp.) for 30 min at 37°C. After digestion, collagenase activity was inhibited by the addition of an equal volume of 1× DME containing 2% plasma-derived serum (Raines and Ross 1985). Fibronectin was purified from human plasma as previously described (Faull et al. 1994). Fibronectin-coated dishes (0.625 μg/cm2) were prepared by overnight incubation at room temperature.
Inhibitors and Antibodies
Calpain and caspase inhibition studies were performed with two inhibitors: calpain inhibitor 1 (ALLN; Calbiochem-Novabiochem Corp.) and the caspase inhibitor benzyloxycarbonyl Val-Ala-Asp fluoromethyl ketone (ZVAD-fmk; Alexis Biochemicals). SMC were preincubated with ALLN or ZVAD (100 μm) for 3 h or overnight before treatment with degraded collagen in the presence of ALLN or ZVAD. To block integrin function, SMC were preincubated at 37°C for 30 min with nonblocking (P1H6) and blocking (P1H5) Fab antibody fragments against α2, blocking anti-α3 Fab antibody (P1B5), blocking αvβ3 (LM609; Chemicon) at (5 μg/200,000 cells) and αv blocking (cyclic penRGD) and control (RGE) peptides (100 μM) (GIBCO BRL). Monoclonal antibody supernatants for α2 and α3 were provided by W.G. Carter (Fred Hutchinson Cancer Research Center, Seattle, WA). Matrix metalloproteinase inhibition studies were performed with recombinant human tissue inhibitors of metalloproteinase (TIMP1 and TIMP2; 3 μg/ml; Oncogene Research Products). Antibodies for Western blot detection, immunoprecipitation, and immunostaining included: 2–18N pp125FAK and 903–1058C pp125FAK (Santa Cruz Biotechnology), 354–534N pp125FAK (Transduction Laboratories), α-actinin (Sigma), p130cas, paxillin (Transduction Laboratories), talin-8d4 (Sigma), and vinculin (Calbiochem-Novabiochem Corp.). Anti–mouse and –rabbit peroxidase-conjugated secondary antibodies were purchased from Vector Laboratories Inc.
Immunocytochemistry
SMC were plated on monomeric collagen- or fibronectin-coated plastic chamber slides for 24 h and either left untreated or treated with degraded collagen fragments for 1–5 min. Cells were fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100 in PBS and washed serially in PBS, 0.15 M glycine/PBS + 0.02% NaN3, and PBS. Cells were blocked in 5% normal goat serum, 0.05% Tween in PBS for 30 min and incubated with primary antibodies to paxillin and vinculin for 1 h at room temperature followed by several washes in PBS and subsequent incubation with FITC-labeled secondary antibodies (Vector Laboratories Inc.). Cells were also incubated with FITC-labeled phalloidin (Sigma). Immunostaining of cells was analyzed by confocal microscopy.
Cell Lysis, Immunoblotting, and Immunoprecipitations
Cells were washed twice with PBS and lysed in lysis buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 5 mM EDTA, 2.5 mM EGTA, 1 mM DTT, 1 mM NaF, 0.1 mM Na3VO4, 10 mM β-glycerophosphate, 0.5 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) for 30 min on ice. Cell lysates were clarified by centrifugation at 27,000 g for 20 min, and protein concentration was determined using the BCA protein assay (Pierce). Lysates were separated on 10% or 7.5% SDS-page; proteins were transferred to Immobilon membrane (Millipore) and immunoblotted with specific antibodies. All immunoblots were visualized by enhanced chemiluminescence (ECL, Amersham Corp.). For pp125FAK coimmunoprecipitation studies, cell lysates were precleared with protein A-agarose (Santa Cruz Biotech.), incubated with 2 μg of the 2-18N pp125FAK antibody, and immunoblotted with specific antibodies against p130cas, paxillin, and vinculin.
Triton X-100–soluble (cytoplasmic) and –insoluble (cytoskeletal) fractions were prepared as previously described (Jackson et al. 1994), with the exception of modification of Triton X-100 and radioimmunoprecipitation assay (RIPA) lysis buffers. In brief, SMC were lysed in Triton X-100 lysis buffer (20 mM Tris-HCl, pH 7.4, 1% Triton X-100, 5 mM EGTA, 0.4 mM leupeptin, 0.2 mM Na3VO4, and 0.1 mM PMSF) for 1 h at 4°C. Triton X-100 insoluble and soluble extracts were separated by centrifugation at 15,000 g for 5 min. The cytoskeletal pellet was washed twice with Triton-free lysis buffer, and proteins were extracted using RIPA buffer (10 mM Tris-HCl [pH 7.2], 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 1 mM PMSF, and 1 mM Na3VO4).
In Vitro Cleavage of pp125FAK
In vitro transcription and translation of pp125FAK were performed with the TNT®-coupled reticulocyte lysate system (Promega) and [35S]methionine (1,000 Ci/mmol, Amersham Corp.), according to the manufacturer's instructions. The expression plasmid for chicken pp125FAK was given by J.T. Parsons (University of Virginia, Charlottesville, VA). Of the reaction, 1/25 was used as a substrate and incubated with a range of 0.25–2 activity units of purified calpain I and calpain II (Calbiochem-Novabiochem Corp.) for 30 min at 30°C in reaction buffer (50 mM Tris-HCl, pH 7.4, 10 mM CaCl2, 5 mM β-mercaptoethanol, and 30 mM NaCl) in the presence and absence of the human recombinant endogenous calpain inhibitor, calpstatin (30 μM; Calbiochem-Novabiochem Corp.). Reactions were terminated by the addition of 4× SDS sample buffer. Common molecular mass standards (Bio-Rad Laboratories) were used to determine Rf values and proteolytic fragment size for both in vitro and cellular pp125FAK cleavage analysis.
Collagen Radiolabeling and Degradation Assay
Vitrogen (Collagen Corp.) concentration was adjusted to 1 mg/ml and neutralized after dialysis against 10 mM borate, 0.2 M CaCl2, pH 8. The vitrogen solution was then radiolabeled by acetylation with [3H]acetic anhydride (NEN Life Science) as described previously (Mookhtiar et al. 1986). 3H-labeled collagen degradation was assayed by modification of a previously described procedure (Aimes and Quigley 1995). In brief, an aliquot of radiolabeled vitrogen was used to generate polymerized fibrillar collagen gels, as described above. SMC were cultured on the labeled polymerized collagen, and at subsequent time points after cell seeding culture supernatants were analyzed for degraded 3H-labeled collagen fragments by liquid scintillation spectroscopy.
Zymography
Cell lysates were prepared in lysis buffer as described above and serum-free conditioned media was collected from SMC cultures. Samples were prepared in nondenaturing loading buffer and separated on 10% SDS–polyacrylamide gel impregnated with 1 mg/ml gelatin. After electrophoresis, gels were washed twice in 2.5% Triton X-100 for 30 min, briefly rinsed with water, and incubated for 24 h at 37°C in collagenase buffer (50 mM Tris-HCl buffer, pH 7.5, 200 mM NaCl, and 10 mM CaCl2). Gels were subsequently fixed and stained in Coomassie blue fixative solution (50% methanol and 10% acetic acid containing 0.25% Coomassie blue R250).
Results
Degraded Collagen Fragments Induce Cell Rounding and Promote Cleavage of pp125FAK
Human arterial SMC cultured on polymerized type I collagen fibrils are arrested in the G1 phase of the cell cycle and do not respond to growth factor stimulation, whereas SMC on monomeric type I collagen proliferate in response to growth factors (Koyama et al. 1996). We hypothesized that matrix alteration or degradation may be necessary to release cells from a nonpermissive state, such as culture on polymerized collagen. To test whether degraded type I collagen has unique properties, we added degraded collagen to SMC that had been plated on monomeric collagen or fibronectin for 24 h. Within 5 min after addition of degraded collagen, the SMC round up but remain loosely attached (Fig. 1B and Fig. E). This cell rounding is reversible; after incubation with degraded collagen for 24 h, SMC attach and spread if the degraded collagen is removed and the cells are plated on fresh monomeric collagen or fibronectin-coated dishes (results not shown).
Figure 1.
SMC spreading and substrate attachment are impaired after treatment with degraded collagen. Human arterial SMC were cultured on monomeric collagen (A–C, G–J) or fibronectin (D–F) for 24 h before addition of degraded collagen fragments. SMC shape and substrate attachment 5 and 30 min after addition of degraded collagen were evaluated by phase-contrast microscopy (A–F). 5 min after treatment, SMC cultured on monomeric collagen were fixed and immunostained with antibodies to paxillin (G and H) and vinculin (I and J) and examined by confocal microscopy. Bars: 20 μm.
To evaluate effects on focal adhesions, we immunostained SMC cultures with antibodies against paxillin (Fig. 1G and Fig. H) and vinculin (Fig. 1I and Fig. J). On monomeric collagen, SMC exhibit abundant vinculin- and paxillin-containing focal adhesion structures (Fig. 1G and Fig. I), while these structures are almost totally absent when SMC are treated for only 5 min with degraded collagen (Fig. 1H and Fig. J). The loss of focal adhesions is also reversible, and focal adhesions recover within 3 h after removal of degraded collagen and replating of SMC on fresh monomeric collagen (data not shown).
Degraded Collagen Promotes Calpain-mediated Cleavage of pp125FAK in Smooth Muscle Cells
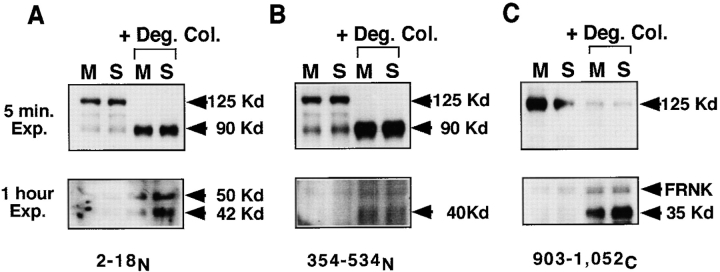
Because degraded type I collagen reversibly alters cell spreading and focal adhesion formation, we asked whether it alters a major component of focal adhesions, focal adhesion kinase (pp125FAK). Incubation with degraded collagen (1 mg/ml concentration) rapidly induces pp125FAK processing, resulting in a significant reduction in the levels of native pp125FAK, as monitored by Western analysis with a polyclonal antibody to the NH2 terminus of pp125FAK (Fig. 2 A). This reduction in native pp125FAK is accompanied by an increase in levels of 90-, 50-, and 42-kD bands. By immunoblotting the same lysates with an antibody to pp125FAK directed against residues 354–534 (see Fig. 4 B), we detect 90- and 40-kD fragments (Fig. 2 B). Using a polyclonal antibody against the COOH terminus of pp125FAK, we identify a cleaved 35-kD fragment (Fig. 2 C). This cleavage of pp125FAK is reversible, and native pp125FAK is restored to maximal levels within 3 h when the degraded collagen is removed and the SMC are replated on fresh monomeric collagen (data not shown). The cleavage of pp125FAK is also specific for treatment with degraded collagen, and is not observed upon addition of BSA (Fig. 3 A) or laminin treated with collagenase (results not shown).
Figure 2.
Cleavage of pp125FAK in response to treatment with degraded type I collagen. SMC were cultured on monomeric collagen (M) or in suspension (S) for 30 min in the presence or absence of degraded type I collagen fragments (+ Deg. Col.). Cell lysates were prepared, separated on SDS-PAGE (10% separating gel), transferred to Immobilon membranes, and immunoblotted with three antibodies against pp125FAK: (A) a polyclonal antibody to residues 2–18 at the NH2 terminus (2–18N); (B) a monoclonal antibody to residues 354–534 (354–534N); and (C) a polyclonal antibody to residues 903–1052 at the COOH terminus (903–1052C). Arrowheads indicate approximate molecular masses of proteolytic cleavage fragments of pp125FAK determined from molecular mass standards used to calculate Rf values.
Figure 4.
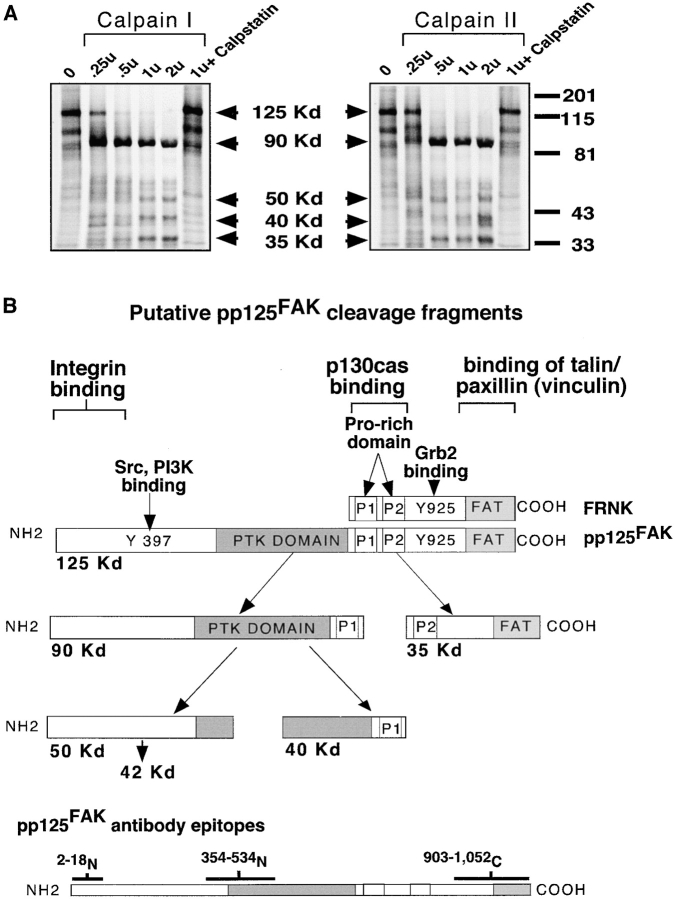
Calpain I and II cleave pp125FAK in vitro into multiple fragments that separate domains. (A) In vitro translated pp125FAK labeled with [35S]methionine was used as a substrate and incubated with increasing concentrations of purified calpain I and calpain II enzyme (0.25–2 units), and cleavage fragments were evaluated by SDS-page and autoradiography. Recombinant calpain inhibitor calpstatin was added to reactions with 1 unit calpain I and calpain II. The mobility of molecular mass standards (201 kD, myosin; 115 kD, β-galactosidase; 81 kD, bovine serum albumin; 43 kD, carbonic anhydrase; 33 kD, soybean trypsin inhibitor [Bio-Rad Laboratories]) used to determine Rf values and proteolytic fragment size (arrows) are indicated on the right. (B) The protein-binding domains of pp125FAK and FRNK are indicated, and putative cleavage fragments resulting from calpain cleavage are shown. Epitopes of pp125FAK-specific antibodies used to evaluate the cleavage fragments are also indicated.
Figure 3.
pp125FAK cleavage is blocked by calpain inhibition. (A) SMC in suspension were incubated, with collagenase degraded collagen (+Deg. Col.) or degraded BSA (+ Deg. BSA) for 30 min. Cell lysates were separated by SDS-PAGE transferred to an Immobilon membrane and immunoblotted with 2–18N-pp125FAK antibody. (B) SMC cultured on monomeric collagen (M) or in suspension (S) were exposed to degraded collagen (+ Deg. Col.) for 30 min in the presence or absence of inhibitors of calpains (ALLN, 100 μM) and caspases (ZVAD, 100 μM). Total cell lysates were prepared, separated by SDS-PAGE, transferred to a membrane, and immunoblotted with 2–18N-pp125FAK antibody.
pp125FAK cleavage mediated by caspases has been demonstrated in Jurkat T cells (Wen et al. 1997) and endothelial cells (Levkau et al. 1998). Cleavage of pp125FAK has also been observed in platelets, where it is mediated by the calcium-activated protease calpain I, which results in proteolytic fragments of 90, 45, and 40 kD (Cooray et al. 1996), similar in size to those observed when SMC are treated with degraded collagen. To determine whether calpain and/or caspases are responsible for pp125FAK processing, we treated SMC with degraded collagen in the presence of the calpain inhibitor ALLN (100 μM) or the broad caspase inhibitor ZVAD (100 μM). As shown in Fig. 3, inhibition of calpain partially reduces processing of native pp125FAK, whereas the caspase inhibitor (ZVAD) has no influence on pp125FAK processing.
To identify which calpain(s) is capable of cleaving pp125FAK, we incubated in vitro translated pp125FAK with purified calpain I or calpain II. As shown in Fig. 4 A, both calpain I and II induce dose-dependent processing of pp125FAK to 90-, 50-, 40-, and 35-kD fragments. The proteolytic activity of either calpain I or II can be blocked by incubation with the specific endogenous calpain inhibitor calpstatin (30 μM). The size of the proteolytic pp125FAK fragments obtained in vitro correlates with the proteolytic cleavage of pp125FAK, observed when SMC are treated with degraded collagen fragments (Fig. 2). The putative proteolytic fragments are illustrated in Fig. 4 B with the epitopes of pp125FAK antibodies used to characterize the cleavage.
α2β1 Integrins Interact with Degraded Collagen to Induce pp125FAK Cleavage
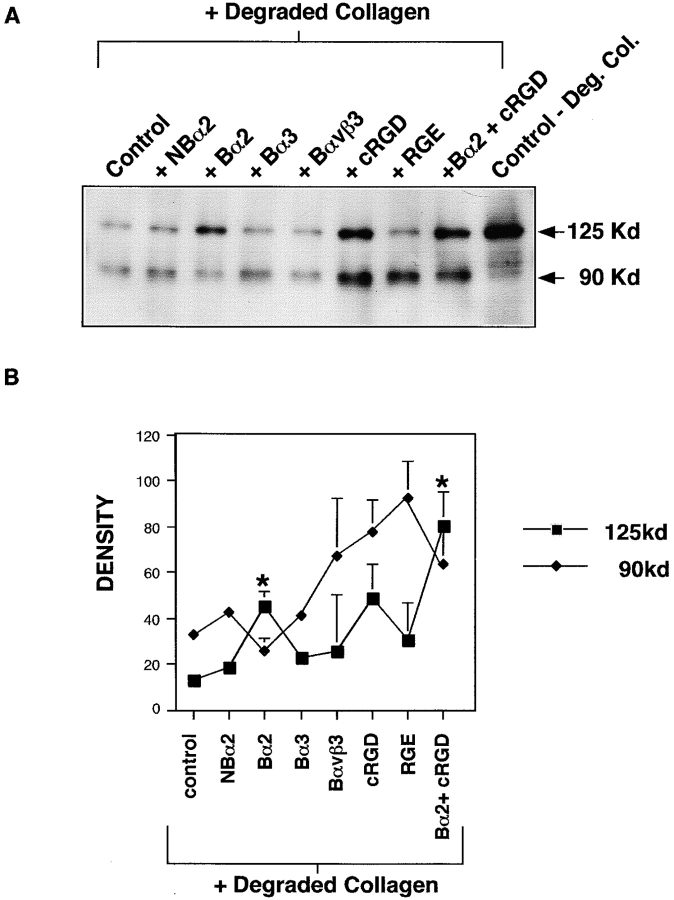
We asked whether the ability of degraded collagen to promote pp125FAK cleavage was mediated through ligation with cell surface integrin receptors. SMC cultured on fibronectin were preincubated with Fab fragments of blocking (P1H5) or nonblocking (P1H6) antibodies against the α2 integrin subunit, blocking α3 integrin subunit antibody (P1D6), blocking αvβ3 antibody (LM609), or αv-blocking (cyclic RGD) or control (RGE) peptides, followed by 30 min incubation in the presence of degraded collagen fragments (0.25 mg/ml). Preincubation with nonblocking anti-α2 Fab fragments, a blocking anti-α3 Fab, blocking anti-αvβ3, or RGE peptide did not prevent pp125FAK cleavage. However, when degraded collagen was added to SMC preincubated with either blocking anti-α2 Fab, cyclic RGD peptide, or α2 Fab and cyclic RGD peptide in combination, the induction of pp125FAK cleavage was suppressed (Fig. 5 A). Changes in pp125FAK cleavage with integrin blockade were quantified by densitometric scanning of both native 125 kD pp125FAK and the 90-kD cleavage fragment. Densitometry values from three separate experiments were combined and are shown in Fig. 5 B. Asterisks indicate significant difference from control + degraded collagen (P < 0.05 by Student's t test). Although suppression of pp125FAK cleavage by preincubation with αv-blocking cyclic RGD peptide is a consistent observation, statistical analysis of three quantified experiments reveal that this effect is not quite significant. The blocking anti-αvβ3 is ineffective because the SMC do not express detectable αvβ3 (Skinner et al. 1994). These results suggest that α2- and to some extent, αv-containing integrins may contribute to the induction of pp125FAK cleavage stimulated by degraded collagen fragments.
Figure 5.
Interaction of degraded collagen with α2-containing integrins promotes pp125FAK cleavage. (A) SMC adherent to fibronectin were preincubated for 30 min with Fab fragments (5 μg/200,000 cells) of a nonblocking α2 antibody (P1H6; NBα2) and a blocking anti-α2 integrin antibody (P1H5, Bα2); blocking anti-α3 antibody (P1B5, Bα3); blocking αvβ3 (LM609, Bαvβ3); and αv-blocking (cyclic RGD, CRGD) and control (RGE) peptides (100 μM) for 30 min. Cells were subsequently incubated with degraded collagen for 30 min. Control samples consist of cells on fibronectin incubated with degraded collagen, without prior antibody preincubation (control + Degraded Collagen), or cells on fibronectin not treated with either antibody preincubation or degraded collagen (control − Deg. Col.). Total cell lysates were prepared, separated by SDS-PAGE, transferred to a membrane, and immunoblotted with 2–18N-pp125FAK antibody. (B) The consequence of integrin blocking on degraded collagen induction of pp125FAK cleavage was quantified by performing densitometry on Western blots representing three separate experiments. Data for intact 125-kD pp125FAK and the 90-kD fragment are shown. *P < 0.05 by Student's t test.
Degraded Collagen Promotes Calpain-mediated Cleavage of Paxillin and Talin but Not α-Actinin
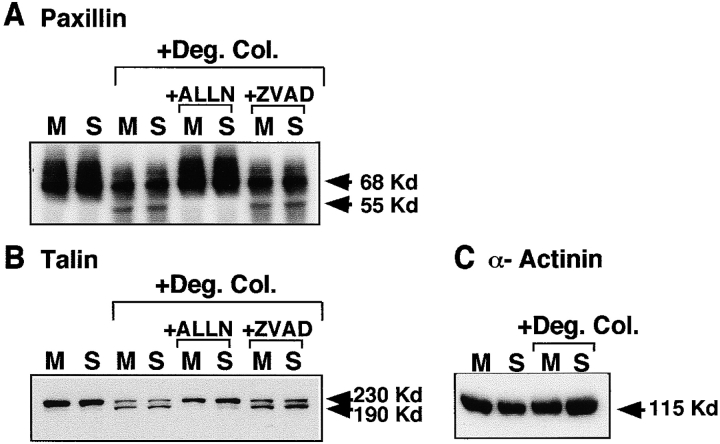
Three other focal adhesion proteins, paxillin, talin, and α-actinin, have been shown to be substrates for calpain (Yamaguchi et al. 1994; Selliah et al. 1996; Schoenwaelder et al. 1997). We asked whether they are also cleaved in SMC exposed to degraded collagen. Native paxillin levels (68 kD) are slightly reduced in SMC cultured with degraded collagen, and a 55-kD band appears (Fig. 6 A). Inhibition of calpain (ALLN, 100 μM) prevents processing to the 55-kD paxillin fragment, whereas caspase inhibition with ZVAD (100 μM) has no effect. Similarly native talin (230 kD) also appears to be proteolytically cleaved giving rise to a 190-kD fragment in SMC treated with degraded collagen (Fig. 6 B). The generation of a 190-kD cleavage fragment of talin is identical in size to a previously identified calpain-mediated cleavage product of talin (Schoenwaelder et al. 1997). Inhibition of calpain but not caspase activity suppresses talin cleavage (Fig. 6 B). In contrast to paxillin, talin and pp125FAK, α-actinin is not processed in response to degraded collagen (Fig. 6 C), nor is actin, another known substrate of calpain (data not shown).
Figure 6.
Degraded collagen induces calpain-mediated cleavage of paxillin and talin but not α-actinin. SMC cultured on monomeric collagen (M) or in suspension (S) were exposed to degraded collagen (+ Deg. Col.) for 30 min in the presence (A and B) or absence (A–C) of inhibitors of calpains (ALLN, 100 μM) and caspases (ZVAD, 100 μM). Total cell lysates were prepared, separated by SDS-PAGE, transferred to Immobilon membrane, and immunoblotted with (A) anti-paxillin antibody, (B) anti-talin antibody, or (C) anti–α-actinin antibody.
Degraded Collagen-induced Cleavage of pp125FAK Decreases Its Interaction with Components of the Focal Adhesion Complex and Alters Its Intracellular Localization
The COOH-terminal cleavage fragment of pp125FAK (35 kD) contains the focal adhesion targeting (FAT) sequence and second proline-rich domain (p2) of pp125FAK, sites involved in pp125FAK interaction with other focal adhesion proteins, such as p130cas, paxillin, and vinculin (Fig. 4 B). We asked whether the ability of pp125FAK to associate with other focal adhesion signaling molecules is altered in SMC after treatment with degraded collagen. Coimmunoprecipitation studies using the 2–18NH2-terminal pp125FAK antibody demonstrate a significant reduction in the association of paxillin, vinculin, and p130cas with pp125FAK in SMC cultured with degraded collagen compared with cells on monomeric collagen (Fig. 7). Control coimmunoprecipitation assays using a normal rabbit IgG were performed in parallel with pp125FAK immunoprecipitations, and rabbit IgG did not associate with paxillin, vinculin, or p130cas in either untreated or degraded collagen-treated SMC samples (results not shown).
Figure 7.
Decreased association of cleaved pp125FAK with components of the focal adhesion complex. pp125FAK was immunoprecipitated from SMC on monomeric collagen incubated for 30 min in the absence (−) or presence (+) of degraded collagen fragments. pp125FAK-associated paxillin, vinculin, and p130cas were detected after SDS-PAGE, transferred to a membrane, and immunoblotted (left). Direct Western blots of the same total cell lysates used for immunoprecipitation are shown on the right.
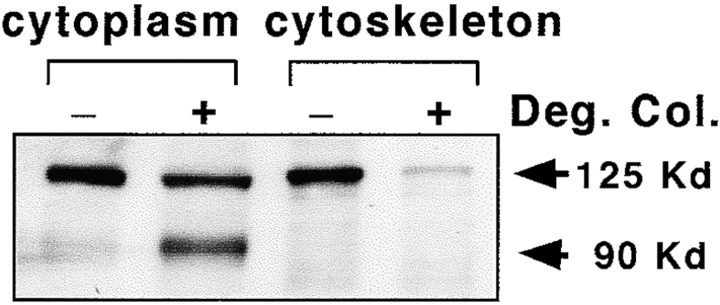
The subcellular localization of pp125FAK between cytoplasmic and cytoskeletal fractions is also altered in SMC treated with degraded collagen (Fig. 8). Significantly less native pp125FAK is in the cytoskeletal fraction, and the NH2-terminal proteolytic fragments of pp125FAK are predominantly found in the cytoplasmic fraction. Levels of native pp125FAK in the cytoplasmic fraction are only slightly reduced in response to treatment with degraded collagen, suggesting that cytoskeleton-associated pp125FAK may be more sensitive to calpain-mediated processing.
Figure 8.
Intracellular translocation of pp125FAK cleavage fragments to the cytoplasm. SMC cultured on monomeric collagen in the presence (+) or absence (−) of degraded collagen (Deg. Col.) fragments were lysed with Triton X-100 lysis buffer and fractionated into Triton X-100–soluble (cytoplasmic) and –insoluble (cytoskeletal) extracts. Extracts were then separated by SDS-PAGE, transferred to a membrane, and immunoblotted with 2-18N-pp125FAK antibodies.
Pretreatment with Calpain Inhibitor Suppresses Degraded Collagen-induced Cell Rounding and Disassembly of Actin Cytoskeleton
To directly test the role of calpain activity in focal adhesion disassembly and cell rounding induced by degraded collagen, SMC cultured on fibronectin were preincubated with or without calpain inhibitor 1 (ALLN, 100 μM) for 18 h before addition of degraded collagen (0.25 mg/ml). Immunostaining with antibodies against paxillin (Fig. 9, A–C) and FITC-labeled phalloidin (Fig. 9, D–F) demonstrate that, in the absence of calpain inhibition, SMC round up and retain limited numbers of focal adhesions one minute after the addition of degraded collagen (Fig. 9 A). In contrast, SMC preincubated with the calpain inhibitor round up to a lesser extent after incubation with degraded collagen, and many of these cells contain more focal adhesions than cells not treated with calpain inhibitor (Fig. 9B and Fig. C). SMC incubated with degraded collagen in the absence of calpain inhibition do not maintain organized stress fibers (Fig. 9 D), whereas cells preincubated with the calpain inhibitor retain organized actin stress fibers and a spread morphology after treatment with degraded collagen (Fig. 9E and Fig. F).
Figure 9.
Preincubation with calpain inhibitor suppresses loss of substrate attachment and cell rounding induced by degraded collagen. Human arterial SMC were cultured on fibronectin in the absence (A and D) or presence (B, C, E, and F) of 100 μM calpain inhibitor (ALLN) for 18 h. Degraded collagen was then added to these cultures. 1 and 5 min after the addition of degraded collagen, SMC were fixed and immunostained with antibodies to paxillin (A–C) or FITC-labeled phalloidin (D–F) and examined by confocal microscopy. Bars: 20 μM.
Prolonged Culture of SMC on Polymerized Collagen Results in Increased Matrix Metalloproteinase (MMP) Activity, Collagen Degradation, and Calpain-mediated Cleavage of pp125FAK
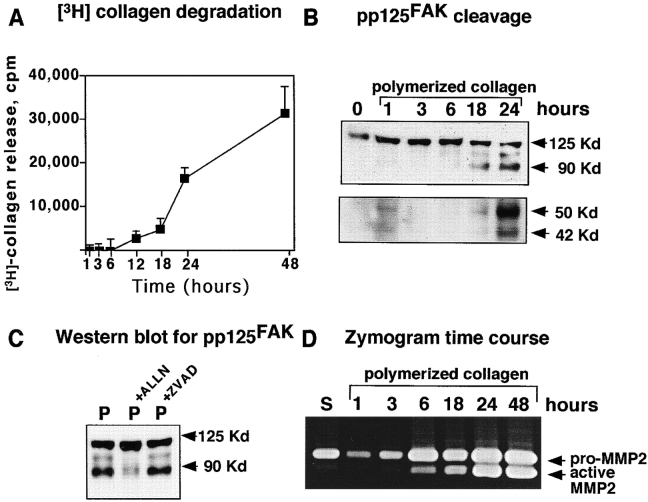
Culture of cells, including SMC, for extended periods on polymerized collagen or three-dimensional matrices, is associated with gradual dissolution of the matrix mediated by induction of metalloproteinase activity (Riikonen et al. 1995; Sudbeck et al. 1997). Therefore, we asked whether extended culture of SMC on polymerized collagen is associated with collagen breakdown and alterations in pp125FAK. Using 3H-labeled polymerized collagen, we monitored the release of collagen fragments at sequential time points after addition of SMC. Collagen degradation is observed as early as 6 h after plating on polymerized collagen, and increases at subsequent time points (Fig. 10 A). Evaluation of pp125FAK demonstrates cleavage fragments as early as 18 h after plating on polymerized collagen (Fig. 10 B), which increases with time. The cleavage of pp125FAK on polymerized collagen can be significantly suppressed by incubation with the calpain inhibitor ALLN (100 μM) (Fig. 10 C). Calpain-mediated cleavage of paxillin and talin can also be observed in response to long-term culture of SMC on polymerized collagen (results not shown).
Figure 10.
Increased endogenous SMC MMP activity, generation of degraded collagen fragments, and pp125FAK cleavage in response to culture on polymerized collagen. (A) Time course of release of 3H-labeled collagen fragments into culture media of SMC plated on 3H-labeled polymerized collagen. (B) Time course of pp125FAK cleavage in response to culture on polymerized collagen. (C) Protein extracts from SMC plated on polymerized collagen for 24 h in the presence of the calpain inhibitor (ALLN, 100 μM) or the caspase inhibitor (ZVAD, 100 μM) were separated by SDS-PAGE, transferred to a membrane, and immunoblotted with antibodies to 2–18N-pp125FAK. (D) Gelatin zymography of serum-free conditioned media from SMC cultured on polymerized collagen at sequential time points after plating on polymerized collagen.
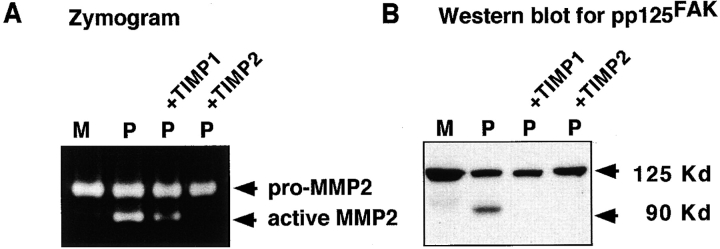
We hypothesized that the pp125FAK cleavage observed when SMC are cultured on polymerized collagen may be initiated as a result of generation of degraded collagen fragments from an induction of endogenous SMC MMP activity. Gelatin zymography demonstrates that pro and active forms of MMP2 are increased in SMC cultured on polymerized collagen as early as 6 h after plating (Fig. 10 D). Western blotting of cell lysates also demonstrates significant increases in intracellular levels of both MMP1 and MMP2 when SMC are cultured on polymerized collagen (data not shown). Addition of recombinant TIMP1 and TIMP2 (3 μg/ml) to SMC cultured on polymerized collagen for 24 h significantly reduces both the induction of MMP2 activity as determined by gelatin zymography (Fig. 11 A) and subsequent degradation of 3H-labeled polymerized collagen (data not shown). Western blotting of total cell lysates demonstrates that inhibition of MMP activity and collagen degradation by TIMP1 and TIMP2 is accompanied by a significant reduction in the extent of pp125FAK cleavage (Fig. 11 B).
Figure 11.
Inhibition of endogenous SMC MMP activity and collagen degradation by TIMP1 and TIMP2 suppresses cleavage of pp125FAK. (A) MMP activity was evaluated in serum-free conditioned media from SMC cultured for 24 h on monomeric (M) or polymerized (P) collagen in the absence or presence of 3 μg/ml TIMP1 (P+TIMP1) or TIMP2 (P+TIMP2). (B) pp125FAK cleavage was monitored in total cell lysates from SMC cultured for 24 h on monomeric (M) or polymerized (P) collagen in the absence or presence of 3 μg/ml TIMP1 (P+TIMP1) or TIMP2 (P+TIMP2). Cell lysates were separated by SDS-PAGE, transferred to a membrane, and immunoblotted with the 2–18N-pp125FAK antibody.
Discussion
Matrix Proteolysis Exposes Cryptic Sites with Distinct Activities
The possibility that structural changes in the ECM may be necessary for cell migration during tissue remodeling and tumor invasion is supported by two examples of matrix degradation products promoting distinct migratory responses. MMP2 digestion of laminin 5 confers unique signaling properties that promote cell migration, which are not possessed by native laminin 5 (Gianelli et al. 1997). MMP2 cleavage of the laminin 5 γ2 chain exposes a cryptic promigratory site on laminin 5. This altered form of laminin 5 is found in tumors and in tissues undergoing remodeling, but not in quiescent tissues. It has also been shown that thrombin cleavage of osteopontin enhances its haptotactic activity (Senger and Perruzzi 1996), and the NH2-terminal cleavage fragment contains a cryptic adhesive sequence recognized by α9β1 integrin (Smith et al. 1996).
The studies presented in this report demonstrate unique signaling properties of degraded collagen primarily mediated through the integrin α2β1 that rapidly promotes focal adhesion disassembly and cell rounding. Previous work has proposed that denaturation or metalloproteinase degradation of collagen reveals cryptic RGD integrin-binding motifs (Davis 1992; Montgomery et al. 1994). MMP degradation of collagen differentially affects α2β1 binding to the collagen fragments (Messent et al. 1998) and reveals a cryptic β3 integrin binding motif, which induces specific integrin-signaling events in SMC, resulting in increased tenascin-C expression (Jones et al. 1997). In our studies, the molecular changes mediated by the interaction with degraded collagen fragments are not mimicked by RGD peptides, nor mediated by αvβ3. The effects of degraded collagen are independent of the substrate (and integrins) involved in cell adhesion.
Rapid and Selective Cleavage of Focal Adhesion Components by Calpain after Stimulation by Degraded Collagen Fragments
Studies have postulated that limited proteolysis of components of the focal adhesion complex by proteolytic enzymes is a potential mediator for focal adhesion disassembly (Beckerle et al. 1987; Huttenlocher et al. 1997). Particularly interesting candidate enzymes are the calpains, a family of intracellular, calcium-activated cysteine proteases, consisting of two ubiquitously expressed isozymes, calpain I and II (reviewed in Molanari and Carafoli 1997). Calpains are localized to sites of focal adhesions in several cell types (Beckerle et al. 1987), and have also been demonstrated to cleave a number of focal adhesion proteins in platelets or in vitro, including pp125FAK, pp60 src, paxillin, talin, actin, and α-actinin (Yoshida et al. 1984; Beckerle et al. 1987; Oda et al. 1993; Yamaguchi et al. 1994; Cooray et al. 1996; Selliah et al. 1996). However, a direct link between calpain-mediated proteolytic activity and disassembly of focal adhesion complexes in adherent cell populations has not been established.
In this study, we demonstrate that proteolytic cleavage of pp125FAK, paxillin, and talin is rapidly induced in adherent vascular SMC treated with degraded collagen fragments, and can be observed in longer term SMC cultures on polymerized collagen after MMP induction and the endogenous generation of degraded collagen fragments. Calpain inhibitor studies and in vitro cleavage assays indicate that pp125FAK, paxillin, and talin cleavage is mediated by calpain. Cleavage of other focal adhesion proteins, including known calpain substrates, such as α-actinin and actin is not observed, indicating that calpain proteolysis induced by degraded collagen is fairly selective for the focal adhesion components pp125FAK, paxillin, and talin. Our kinetic studies demonstrate that calpain proteolysis of pp125FAK, paxillin, and talin is observed within 5 min after addition of degraded collagen and occurs in parallel with focal adhesion disassembly and loss of cell attachment.
The mechanisms that regulate calpain proteolytic activity in vivo are still poorly understood. Studies have proposed that translocation of calpain to the plasma membrane and focal adhesion sites promotes activity in response to elevated calcium (Molanari and Carafoli 1997). Integrin ligation with ECM ligands increases intracellular calcium levels (Jaconi et al. 1991), and rapid initiation of calpain-mediated cleavage of substrates is observed in response to integrin αIIbβ3 ligation on platelets (Fox et al. 1993; Inomata et al. 1996; Schoenwaelder et al. 1997). The rapid kinetics of calpain-mediated cleavage of pp125FAK, paxillin, and talin that we observe in SMC in response to exposure to degraded collagen fragments is consistent with the time course of integrin-mediated calpain activation observed previously (Cooray et al. 1996; Inomata et al. 1996). Our integrin-blocking studies further suggest that initiation of pp125FAK cleavage is at least partially dependent on the association of the collagen fragments with α2- and possibly also αv-containing integrins. This raises the possibility that degraded collagen fragments possess specific integrin-signaling properties, including initiation of calpain activation. However, the precise downstream signaling mechanisms responsible for initiating calpain cleavage of pp125FAK, paxillin, and talin remain to be elucidated.
pp125FAK, a Critical Regulator of Focal Adhesion Turnover, Is Functionally Modulated by Calpain Cleavage
Numerous studies have established pp125FAK phosphorylation as a key step in regulating focal adhesion turnover (Burridge et al. 1992; Schaller et al. 1992). Autophosphorylation of pp125FAK on tyrosine 397 generates a high affinity binding site for the src family kinases (Schaller et al. 1994; Polte and Hanks 1995). Formation of a pp125FAK/src signaling complex enhances the catalytic activity of both pp125FAK and src and recruitment of other focal adhesion proteins, further promoting assembly of the focal adhesion signaling complex (Schlaepfer et al. 1994; Polte and Hanks 1997). Our studies suggested that calpain-mediated cleavage of pp125FAK (Fig. 4 B) should dissociate the COOH-terminal focal adhesion targeting sequence (FAT) and second proline-rich domain from the NH2-terminal catalytic kinase and integrin binding domains. Further, we demonstrate that calpain cleavage of pp125FAK induced by degraded collagen impairs the ability of pp125FAK to function as an adapter protein and to associate with paxillin, p130cas, and vinculin. pp125FAK cleavage also results in decreased levels of native pp125FAK associated with the cytoskeletal fraction and accumulation of NH2-terminal cleavage fragments in the cytoplasmic fraction. This translocation of NH2-terminal fragments to the cytoplasm is most likely a consequence of cleavage of the COOH-terminal FAT sequence and second proline-rich domain, which are required for localization of pp125FAK to focal adhesion sites (Hildebrand et al. 1993; Harte et al. 1996; Polte and Hanks 1997). Thus, intracellular translocation of pp125FAK, combined with impaired ability to associate with and recruit other focal adhesion proteins, strongly suggests that calpain cleavage represents an important mechanism for regulating pp125FAK activity and focal adhesion disassembly.
Previous studies suggest that pp125FAK activity may also be regulated by expression of an alternatively spliced form called pp125FAK-related non-kinase (FRNK) (Schaller et al. 1993). FRNK is a truncated 41-kD protein identical to the COOH-terminal domain of pp125FAK. It is not catalytically active because it lacks the kinase domain, but localizes to focal adhesions through protein associations mediated by the COOH-terminal proline-rich domains and FAT sequence (Schaller et al. 1993). Previous studies suggest FRNK may regulate pp125FAK activity by acting as a competitive inhibitor (Richardson and Parsons 1996). Our results demonstrate that calpain cleavage of pp125FAK generates a 35-kD COOH-terminal fragment consisting of the COOH-terminal FAT sequence and second proline-rich domain and thus is structurally similar to FRNK. Due to very low levels of the 35-kD COOH-terminal fragment, we have been unable to detect whether it localizes to the cytoskeletal fraction or is associated with other focal adhesion proteins, such as paxillin. The domain structure of the 35-kD COOH-terminal fragment indicates that it has the potential to act as a competitive inhibitor of pp125FAK, and further contribute to disassembly of focal adhesions that accompanies calpain cleavage of pp125FAK. However, its effectiveness in such a role would depend on the maintenance of this fragment at high levels within focal adhesion sites.
Tyrosine phosphorylation of pp125FAK is not required for focal adhesion assembly and cell spreading in response to SMC adhesion to a fibronectin substrate (Wilson et al. 1995). This is consistent with our studies, which demonstrate that, with adhesion of SMC to distinct collagen or fibronectin substrates, there is no significant regulation of pp125FAK tyrosine phosphorylation. We, therefore, propose that, under certain conditions, the primary mechanism for regulating pp125FAK activity and focal adhesion assembly in SMC is mediated through calpain-dependent proteolytic processing, rather than tyrosine phosphorylation. The calpain-mediated proteolysis impairs recruitment of other signaling proteins, decreases association with the cytoskeletal fraction, and generates fragments structurally similar to FRNK that may further contribute to focal adhesion disassembly by acting as competitive inhibitors of pp125FAK.
Our studies demonstrate that pretreatment of SMC with a calpain inhibitor before incubation with degraded collagen fragments suppresses the cell rounding and loss of substrate attachment. The influence of calpain inhibition on focal adhesion disassembly is less clear, as many of the weakly attached SMC, after treatment with degraded collagen, are lost during immunostaining, and only the most adherent cells remain attached and are evaluated by confocal microscopy. However, results presented in this study indicate that pretreatment with calpain inhibitors before addition of degraded collagen appears to enhance the number of focal adhesion structures, compared with cells not pretreated with the calpain inhibitor. More significantly, calpain inhibition maintains substrate anchorage, a spread morphology, and organized actin stress fibers in all cells incubated with degraded collagen fragments. These results further implicate calpain cleavage of pp125FAK, paxillin, and talin in promoting focal adhesion disassembly and cell rounding induced by degraded collagen. Our data are also consistent with previous studies demonstrating that proteolytic cleavage of pp125FAK occurs in parallel with cell rounding and loss of substrate attachment (Fincham et al. 1995; Crouch et al. 1996).
Our studies have focused primarily on the influence calpain cleavage has on pp125FAK function and focal adhesion integrity. However, calpain cleavage of paxillin and talin is also likely to have significant consequences on focal adhesion complexes and may contribute to disassembly of focal adhesions observed when SMC are treated with degraded collagen fragments.
In Vivo Implications of Calpain Dissolution of SMC Focal Adhesions in Response to Increased MMP Activity and Collagen Degradation
Focal adhesions are dynamic structures that are assembled and disassembled at different stages in the cell cycle, indicating their possible involvement in regulation of cell proliferation (David-Pfeuty and Singer 1980; Herman and Pledger 1985). It has also been proposed that focal adhesion assembly and disassembly control cell migration by regulating attachment to the substratum at the leading edge with detachment from the posterior end of the cell (Huttenlocher et al. 1995). Cells derived from pp125FAK−/− embryos exhibit a reduced capacity for cell migration and have more focal adhesions than pp125FAK+ cells (Ilic et al. 1995). Thus, pp125FAK may play a critical role in the regulation of focal adhesion turnover and cell migration.
During the development of atherosclerotic and restenotic lesions, the ECM surrounding SMC is modified by increased MMP activity derived from both vascular SMC and inflammatory monocytes (Bendeck et al. 1994; Galis et al. 1994). Modulation of the vascular ECM may contribute to transition of SMC to a “synthetic” phenotype, which results in enhanced SMC proliferation, migration, and lesion progression (Thyberg et al. 1990; Cheng et al. 1998). Our demonstration that degraded collagen fragments promote calpain-mediated disassembly of SMC focal adhesions suggests the possibility that, in localized sites of MMP expression, SMC anchorage to the surrounding ECM may be disrupted, thereby promoting their migration and invasion into the arterial intima. Loss of focal adhesion contacts with the surrounding ECM may contribute to enhanced SMC proliferation by releasing SMC from a nonpermissive matrix environment, such as polymerized collagen fibrils (Pauly et al. 1994; Koyama et al. 1996).
In conclusion, our results characterize an alternative to phosphorylation of pp125FAK for regulating pp125FAK activity and focal adhesion assembly in a viable adherent cell population, one dependent on calpain-mediated cleavage of pp125FAK, paxillin, and talin. We further demonstrate that degraded collagen fragments initiate this cleavage through integrin signals distinct from those of native collagen.
Acknowledgments
We would like to thank Bonnie Ashleman and the W.M. Keck Center for Advanced Studies in Neural Signaling for technical assistance with confocal microscopy. We would also like to thank Drs. W.G. Carter and J.T. Parsons for kindly providing reagents.
This study was supported by a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health, HL18645.
Footnotes
1.used in this paper: ALLN, calpain inhibitor 1; ECL, enhanced chemiluminescence; ECM, extracellular matrix; FAK, focal adhesion kinase; FAT, focal adhesion targeting (sequence); FRNK, pp125FAK-related non-kinase; MMP, matrix metalloproteinase; pp125FAK, focal adhesion kinase; RIPA, radioimmunoprecipitation assay; SMC, smooth muscle cells; TIMP, tissue inhibitors of metalloproteinase; ZVAD-fmk, benzyloxycarbonyl Val-Ala-Asp fluoromethyl ketone (caspase inhibitor)
Dr. Levkau's present address is Institute for Arteriosclerosis Research, Münster, Germany.
References
- Aimes R.T., Quigley J.P. Matrix metalloproteinase 2 is an interstitial collagenase. J. Biol. Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Bachelot C., Rameh L., Parsons T., Cantley L.C. Association of phosphatidylinositol 3-kinase, via the SH2 domains of p85 with focal adhesion kinase in polyoma middle t-transformed fibroblasts. Biochim. Biophys. Acta. 1996;1311:45–52. doi: 10.1016/0167-4889(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Barnes M.J. Collagens in atherosclerosis. Collagen Rel. Res. 1985;5:65–97. doi: 10.1016/s0174-173x(85)80048-0. [DOI] [PubMed] [Google Scholar]
- Beckerle M.C., Burridge K., DeMartino G.N., Croall D.E. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987;51:569–577. doi: 10.1016/0092-8674(87)90126-7. [DOI] [PubMed] [Google Scholar]
- Bendeck M.P., Zempo N., Clowes A.W., Galardy R.E., Reidy M.A. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ. Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Bornfeldt K.E., Raines E.W., Nakano T., Graves L.M., Krebs E.G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J. Clin. Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M. Focal adhesions, contractility, and cell signaling. Annu. Rev. Cell Dev. Biol. 1996;2:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burridge K., Faith T., Nuckolls G., Turner C. Focal adhesionstransmembrane junctions between the extracellular matrix and the cytoskeleton. Ann. Rev. Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C.E., Romer L.H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrixa role in cytoskeleton assembly. J. Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-C., Appeddu P.A., Parsons J.T., Hildebrand J.D., Schaller M.D., Guan J.-L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Cheng L., Mantile G., Pauly R., Nater C., Felici A., Monticone R., Bilato C., Gluzband Y.A., Crow M.T., Stetler-Stevenson W., Capogrossi M.C. Adenovirus-mediated gene transfer of the human tissue inhibitor of metalloproteinase-2 blocks vascular smooth muscle invasiveness in vitro and modulates neointimal development in vivo. Circulation. 1998;98:2195–2201. doi: 10.1161/01.cir.98.20.2195. [DOI] [PubMed] [Google Scholar]
- Cooray P., Yuan Y., Schoenwaelder S.M., Mitchell C.A., Salem H.H., Jackson S.P. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 1996;318:41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch D.H., Fincham V.J., Frame M.C. Targeted proteolysis of the focal adhesion kinase pp125FAK during c-Myc-induced apoptosis is suppressed by integrin signalling. Oncogene. 1996;12:2689–2696. [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S.J. Altered distributions of the cytoskeletal proteins vinculin and α-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc. Natl. Acad. Sci. USA. 1980;77:6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. Affinity of integrins for damaged extracellular matrixαVβ3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- Faull R.J., Du X., Ginsberg M.H. Receptors on platelets. Methods Enzymol. 1994;245:183–194. doi: 10.1016/0076-6879(94)45011-0. [DOI] [PubMed] [Google Scholar]
- Fincham V.J., Wyke J.A., Frame M.C. v-src-induced degradation of focal adhesion kinase during morphological transformation of chicken embryo fibroblasts. Oncogene. 1995;10:2247–2252. [PubMed] [Google Scholar]
- Fox J.E.B., Taylor R.G., Taffarel M., Boyels J.K., Goll D.E. Evidence that activation of platelet calpain is induced as a consequence of binding of adhesive ligand to the integrin, glycoprotein IIb-IIIa. J. Cell Biol. 1993;120:1501–1507. doi: 10.1083/jcb.120.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z.S., Sukhova G.K., Lark M.W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianelli G., Falk-Marzillier J., Schiraldi O., Stetler-Stevenson W.G., Quaranta V. Induction of cell migration by matrix metalloproteinase-2 cleavage of laminin 5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Guan J.-L. Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell. Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- Hanks S.K., Polte T.R. Signaling through focal adhesion kinase. Bioessays. 1996;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- Harte M.T., Hildebrand J.D., Burnham M.R., Bouton A.H., Parsons J.T. p130Cas, a substrate associated with v-src and v-crk, localizes to focal adhesions and binds to focal adhesion kinase. J. Biol. Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- Hay E.D. Extracellular matrix, cell skeletons, and embryonic development. Am. J. Med. Genet. 1989;1:14–29. doi: 10.1002/ajmg.1320340107. [DOI] [PubMed] [Google Scholar]
- Herman B., Pledger W.J. Platelet-derived growth factor induced alterations in vinculin and actin distribution in BALB/c 3T3 cells. J. Cell Biol. 1985;100:1031–1040. doi: 10.1083/jcb.100.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J.T., Schaller M.D., Parsons J.T. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J.D., Schaller M.D., Parsons J.T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein, binds to the carboxy terminal domain of focal adhesion kinase. Mol. Biol. Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A., Sandborg R.R., Horwitz A.F. Adhesion in cell migration. Curr. Opin. Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A., Palecek S.P., Lu Q., Zhang W., Mellgren R.L., Lauffenburger D.A., Ginsberg M.H., Horwitz A.F. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Integrinsversatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Inomata M., Hayashi M., Ohno-Iwashita Y., Tsubuki S., Saido T.C., Kawashima S. Involvement of calpain in integrin-mediated signal transduction. Arch. Biochem. Biophys. 1996;328:129–134. doi: 10.1006/abbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- Jackson S.P., Schoenwaelder S.M., Yuan Y., Rabinowitz I., Salem H.H., Mitchell C.A. Adhesion receptor activation of phosphatidylinositol 3-kinase. von Willebrand factor stimulates the cytoskeletal association and activation of phosphatidylinositol 3-kinase and pp60c-src in human platelets. J. Biol. Chem. 1994;269:27093–27099. [PubMed] [Google Scholar]
- Jaconi M.E.E., Theler J.M., Schlegel W., Appel R.D., Wright S.D., Lew P.D. Multiple elevations of cytosolic free Ca2+ in human neutrophilsinitiation by adherence receptors of the integrin family. J. Cell Biol. 1991;112:1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.L., Crack J., Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the αvβ3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J. Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H., Raines E.W., Bornfeldt K.E., Roberts J.M., Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Levkau B., Herren B., Koyama H., Ross R, Raines E.W. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J. Exp. Med. 1998;187:579–586. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messent A.J., Tuckwell D.S., Knäuper V., Humphries M.J., Murphy G., Gavrilovic J. Effects of collagenase-cleavage of type I collagen on α2β1 integrin-mediated cell adhesion. J. Cell Sci. 1998;111:1127–1135. doi: 10.1242/jcs.111.8.1127. [DOI] [PubMed] [Google Scholar]
- Molanari M., Carafoli E. CalpainA cytosolic proteinase active at the membranes. J. Membrane Biol. 1997;156:1–8. doi: 10.1007/s002329900181. [DOI] [PubMed] [Google Scholar]
- Montgomery A.M.P., Reisfeld R.A., Cheresh D.A. Integrin αvβ3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc. Natl. Acad. Sci. USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookhtiar K.A., Mallya S.K., Van Wart H.E. Properties of radiolabelled type I, II and III collagens related to their use as substrates in collagenase assays. Anal. Biochem. 1986;158:322–333. doi: 10.1016/0003-2697(86)90557-9. [DOI] [PubMed] [Google Scholar]
- Oda A., Druker B.J., Ariyoshi H., Smith M., Salzman E.W. pp60src is an endogenous substrate for calpain in human blood platelets. J. Biol. Chem. 1993;268:12603–12608. [PubMed] [Google Scholar]
- Pauly R.R., Passaniti A., Bilato C., Monticone R., Cheng L., Papadopoulos N., Gluzband Y.A., Smith L., Weinstein C., Lakatta E.G., Crow M.T. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ. Res. 1994;75:41–54. doi: 10.1161/01.res.75.1.41. [DOI] [PubMed] [Google Scholar]
- Polte T.R., Hanks S.K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130cas. Proc. Natl. Acad. Sci. USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte T.R., Hanks S.K. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130(cas)) are elevated in cytoskeleton-associated fractions following adhesion and Src transformation. Requirements for Src kinase activity and FAK proline-rich motifs. J. Biol. Chem. 1997;272:5501–5510. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- Raines E.W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Richardson A., Parsons J.T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- Riikonen T., Westermarck J., Koivisto L., Broberg A., Kahari V.-M., Heino J. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J. Biol. Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- Sastry S.K., Horwitz A.F. Integrin cytoplasmic domainsmediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr. Opin. Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- Schaller M.D., Parsons J.T. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for crk. Mol. Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Borgman C.A., Cobb B.S., Vines R.R., Reynolds A.B., Parsons J.T. pp125FAK, a structurally unique protein tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Borgman C.A., Parsons J.T. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK . Mol. Biol. Cell. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Hildebrand J.D., Shannon J.D., Fox J.X., Vines R.R., Parsons J.T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src . Mol. Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Otey C.A., Hildebrand J.D., Parsons J.T. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J. Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hanks S.K., Hunter T., van der Greer P. Integrin-mediated signal transduction linked to the ras pathway by Grb2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Yuan Y., Cooray P., Salem H.H., Jackson S.P. Calpain cleavage of focal adhesion proteins regulates the cytoskeletal attachment of integrin αIIbβ3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin clots. J. Biol. Chem. 1997;272:1694–1702. doi: 10.1074/jbc.272.3.1694. [DOI] [PubMed] [Google Scholar]
- Selliah N., Brooks W.H., Roszman T.L. Proteolytic cleavage of alpha-actinin by calpain in T cells stimulated with anti-CD3 monoclonal antibody. J. Immunol. 1996;159:3215–3221. [PubMed] [Google Scholar]
- Senger D.R., Perruzzi C.A. Cell migration promoted by a potent GRGDS-containing thrombin-cleavage fragment of osteopontin. Biochim. Biophys. Acta. 1996;1314:13–24. doi: 10.1016/s0167-4889(96)00067-5. [DOI] [PubMed] [Google Scholar]
- Skinner M.P., Raines E.W., Ross R. Dynamic expression of α1β1 and α2β1 integrin receptors by human vascular smooth muscle cellsα2β1 integrin is required for chemotaxis across type I collagen-coated membranes. Am. J. Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- Smith L.L., Cheung H.K., Ling L.E., Chen J., Sheppard D., Pytela R., Giachelli C.M. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by α9β1 integrin. J. Biol. Chem. 1996;271:28485–28491. [PubMed] [Google Scholar]
- Sudbeck B.D., Pilcher B.K., Welgus H.G., Parks W.C. Induction and repression of collagenase-1 by keratinocytes is controlled by distinct components of different extracellular matrix compartments. J. Biol. Chem. 1997;272:22103–22110. doi: 10.1074/jbc.272.35.22103. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hedin U., Sjolund M., Palmberg L., Bottger B.A. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990;10:966–990. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- Wen L.P., Fahrni J.A., Troie S., Guan J.-L., Orth K., Rosen G.D. Cleavage of focal adhesion kinase by caspases during apoptosis. J. Biol. Chem. 1997;272:56–61. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- Wilson L., Carrier M.J., Kellie S. pp125FAK tyrosine kinase activity is not required for the assembly of F-actin stress fibres and focal adhesions in cultured mouse aortic smooth muscle cells. J. Cell Sci. 1995;108:2381–2391. doi: 10.1242/jcs.108.6.2381. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Maki M., Hatanaka M., Sabe H. Unphosphorylated and tyrosine-phosphorylated forms of a focal adhesion protein, paxillin, are substrates for calpain II in vitroimplications for the possible involvement of calpain II in mitosis-specific degradation of paxillin. FEBS. 1994;356:114–116. doi: 10.1016/0014-5793(94)01246-6. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Murachi T., Tsukahara I. Degradation of actin and vimentin by calpain II, a Ca2+-dependent cysteine proteinase, in bovine lens. FEBS. 1984;170:259–262. doi: 10.1016/0014-5793(84)81324-1. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK)a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992;71:891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]