Abstract
Sphingosine-1-phosphate (SPP) is a bioactive lipid that has recently been identified as the ligand for the EDG family of G protein–coupled cell surface receptors. However, the mitogenic and survival effects of exogenous SPP may not correlate with binding to cell-surface receptors (Van Brocklyn, J.R., M.J. Lee, R. Menzeleev, A. Olivera, L. Edsall, O. Cuvillier, D.M. Thomas, P.J.P. Coopman, S. Thangada, T. Hla, and S. Spiegel. 1998. J. Cell Biol. 142:229–240). The recent cloning of sphingosine kinase, a unique lipid kinase responsible for the formation of SPP, has provided a new tool to investigate the role of intracellular SPP. Expression of sphingosine kinase markedly increased SPP levels in NIH 3T3 fibroblasts and HEK293 cells, but no detectable secretion of SPP into the medium was observed. The increased sphingosine kinase activity in NIH 3T3 fibroblasts was sufficient to promote growth in low- serum media, expedite the G1/S transition, and increase DNA synthesis and the proportion of cells in the S phase of the cell cycle with a concomitant increase in cell numbers. Transient or stable overexpression of sphingosine kinase in NIH 3T3 fibroblasts or HEK293 cells protected against apoptosis induced by serum deprivation or ceramide elevation. N,N-Dimethylsphingosine, a competitive inhibitor of sphingosine kinase, blocked the effects of sphingosine kinase overexpression on cell proliferation and suppression of apoptosis. In contrast, pertussis toxin did not abrogate these biological responses. In Jurkat T cells, overexpression of sphingosine kinase also suppressed serum deprivation- and ceramide-induced apoptosis and, to a lesser extent, Fas-induced apoptosis, which correlated with inhibition of DEVDase activity, as well as inhibition of the executionary caspase-3. Taken together with ample evidence showing that growth and survival factors activate sphingosine kinase, our results indicate that SPP functions as a second messenger important for growth and survival of cells. Hence, SPP belongs to a novel class of lipid mediators that can function inside and outside cells.
Keywords: sphingosine kinase, sphingosine-1-phosphate, cell growth, apoptosis
Sphingosine-1-phosphate (SPP),1 a phosphorylated derivative of sphingosine, the structural backbone of all sphingolipids, is a bioactive lipid that regulates diverse biological processes, such as calcium mobilization, cell growth, differentiation, survival, motility, and cytoskeleton organization (Goetzl and An 1998; Spiegel 1999). Recent interest in SPP has been stimulated by the discovery of a family of cell surface G-protein–coupled receptors (GPCR), encoded by the endothelial differentiation genes (EDG), that specifically bind SPP with high affinity and specificity (An et al. 1997; Goetzl and An 1998; Lee et al. 1998; Van Brocklyn et al. 1998, Van Brocklyn et al. 1999; Kon et al. 1999), supporting a role for SPP as an extracellular mediator. Indeed, previously, some of the biological effects of SPP when added exogenously, such as inhibition of platelet activation and motility and cell shape changes (Yamamura et al. 1997), induction of neurite retraction and soma rounding (Postma et al. 1996; Sato et al. 1997), and activation of Gi protein–gated inward rectifying K+ channels in atrial myocytes (van Koppen et al. 1996), have been attributed to interactions with putative cell surface receptors. SPP receptors, EDG-1, -3, and -5, couple to different Gαs and βγ dimers to signal through cAMP, phospholipase C, Ras, mitogen-activated protein kinase, Rho, and several protein tyrosine kinases (An et al. 1997; Goetzl and An 1998; Lee et al. 1998; Okamoto et al. 1998; Van Brocklyn et al. 1998; Zondag et al. 1998; Gonda et al. 1999). Furthermore, SPP is stored in high concentrations in human platelets, from which it is released upon activation by physiological stimuli (Yatomi et al. 1997a), suggesting that SPP can be considered an autocrine factor involved in endothelial injury, inflammation, thrombosis, and angiogenesis.
However, this sphingolipid metabolite also has many of the hallmarks of classical second messengers (reviewed in Spiegel et al. 1996). The level of SPP is very low in cells and is rapidly increased by activation of sphingosine kinase induced by diverse physiological stimuli, including PDGF (Olivera and Spiegel 1993; Bornfeldt et al. 1995; Coroneos et al. 1995; Pyne et al. 1996), NGF (Edsall et al. 1997; Rius et al. 1997), muscarinic acetylcholine agonists (Meyer zu Heringdorf et al. 1998), tumor necrosis factor (TNF)-α (Xia et al. 1998), activation of PKC (Mazurek et al. 1994; Buehrer et al. 1996), and cross-linking of the immunoglobulin receptors Fc∈R1 (Choi et al. 1996) and FcγR1 (Melendez et al. 1998). Similar to other signaling molecules, SPP has a short half-life due to rapid turnover catalyzed by SPP lyase and/or SPP phosphatase (Van Veldhoven and Mannaerts 1991, Van Veldhoven and Mannaerts 1994; Saba et al. 1997; Mandala et al. 1998; Zhou and Saba 1998). Moreover, prevention of SPP formation by competitive inhibitors of sphingosine kinase blunted the mitogenic response to PDGF (Olivera and Spiegel 1993; Rani et al. 1997), the cytoprotective effects of NGF (Edsall et al. 1997), vitamin D3 (Kleuser et al. 1998), PKC, and cAMP activators (Cuvillier et al. 1996; Machwate et al. 1998), as well as calcium mobilization induced by Fc∈R1, FcγR1, and muscarinic acetylcholine receptors (Choi et al. 1996; Melendez et al. 1998; Meyer zu Heringdorf et al. 1998). In addition, microinjected SPP mobilizes calcium from internal sources (Meyer zu Heringdorf et al. 1998), is mitogenic for Swiss 3T3 fibroblasts (Van Brocklyn et al. 1998), and inhibits apoptosis of mouse oocytes induced by the antitumor drug doxorubicin (Perez et al. 1997). Collectively, these observations provide new insights into the biological functions of SPP and emphasize the importance of sphingosine kinase, the enzyme responsible for SPP formation, whether it functions inter- or intracellularly. Until recently, it had been difficult to critically evaluate the second messenger roles of SPP because most of the relevant enzymes involved in its metabolism had not been purified and cloned. Thus, much of the evidence that led to the elucidation of its importance has been indirect, relying on exogenous application and the use of inhibitors, and is further complicated by the existence of cell-surface receptors. We recently purified sphingosine kinase to apparent homogeneity from rat kidney (Olivera et al. 1998) and subsequently cloned and characterized the first mammalian sphingosine kinases (murine SPHK1a and SPHK1b) (Kohama et al. 1998). In this study, we demonstrate that overexpression of sphingosine kinase markedly increased intracellular mass levels of SPP, enhanced proliferation by promoting the G1 to S phase transition, and suppressed serum deprivation- and ceramide-induced apoptosis. Because these effects were mediated in the absence of detectable secretion of SPP and were pertussis toxin independent, our results support an additional role for SPP as an intracellular second messenger important for cell proliferation and survival.
Materials and Methods
Materials
SPP, dihydroSPP, sphingosine, N,N-dimethylsphingosine, and C2-ceramide were from Biomol Research Laboratory Inc. [γ-32P]ATP (3,000 Ci/mmol) was purchased from Amersham Corp. Alkaline phosphatase (bovine intestinal mucosa, type VII-NT) and pertussis toxin were from Sigma Chemical Co. Serum, medium, and G418 were obtained from Biofluids, Inc. Restriction enzymes were from New England Biolabs Inc. Monoclonal antibodies against c-myc were from Zymed, and anti–mouse Texas red dye–conjugated goat antibody was from Jackson ImmunoResearch Laboratories, Inc. The Anti-Fade kit was from Molecular Probes. The bromodeoxyuridine incorporation detection kit and anti–mouse FITC-conjugated IgG were obtained from Boehringer Mannheim Biochemicals. Bisbenzimide hydrochloride (Hoechst 33258) was from Calbiochem Corp. Acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin (Ac-DEVD-AMC) was from Bachem. Anti-Fas IgM (clone CH-11) was from Upstate Biotechnology Inc.
Cell Culture
NIH 3T3 fibroblasts (ATCC CRL-1658) and human embryonic kidney cells (HEK293, ATCC CRL-1573) were grown in high glucose DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine supplemented with 10% calf serum and fetal bovine serum, respectively (Kohama et al. 1998). Jurkat T leukemia cells were cultured in RPMI 1640 containing 10% fetal bovine serum. Before addition of exogenous stimuli, cells were resuspended at a density of 0.75–1 × 106 cells/ml in medium containing 5 μg/ml transferrin and 5 μg/ml insulin in place of serum (Cuvillier et al. 1998).
Cloning and Expression of Sphingosine Kinase
SPHK1a (accession number AF068748) was subcloned into a modified pcDNA3 vector (Invitrogen Corp.) to express proteins with an NH2-terminal c-myc epitope tag (a gift from Dr. Peter Burbelo, Georgetown University, Washington, DC) by PCR using a 5′ primer with a BamH1 restriction site (5′-GAGGGATCCGAACCAGAATGCCCTCGAGGA-3′), and as the 3′ primer, the last 21 nucleotides of the SPHK1a sequence with an EcoRI overhang (5′-GAGGAATTCTTATGGTTCTTCTGGAGGTGG-3′). For transient expression, plasmids were transfected into cells using Lipofectamine Plus (Life Technologies, Inc.) according to the manufacturer's instructions at a 5:1 ratio with pCEFL-GFP, which encodes green fluorescent protein (GFP; a generous gift of Dr. Silvio Gutkind, NIH, Bethesda, MD). Transfection efficiencies were typically 30 and 40% for NIH 3T3 and HEK293 cells, respectively. Jurkat T cells were transfected by electroporation at a cell density of 106 cells/ml with 15 μg of DNA using a Gene Pulser apparatus (Bio-Rad Laboratories) at 400 V and 960 μFa. Stable transfectants containing pcDNA3 plasmids were selected in medium containing 1 mM sodium pyruvate and 0.5 g/liter G418 (NIH 3T3 fibroblasts) or 1 g/liter G418 (HEK293 and Jurkat cells). For all experiments, nonclonal pools of stably transfected cells were used to avoid clonal variability.
Measurement of SPP
Cells were incubated in low serum or serum-free media for at least 24 h. The media was then removed, cells were washed with PBS and scraped in 1 ml 25 mM HCl/methanol, and SPP levels were measured essentially as described (Edsall and Spiegel 1999). In brief, lipids from the cells were then extracted with 5 ml of chloroform/methanol/1 M NaCl (2:1:2, vol/vol) containing 100 μl 3 M NaOH. SPP is water soluble at alkaline pH, and partitions into the aqueous phase. SPP in the aqueous phase was dephosphorylated with alkaline phosphatase (25–50 U) in buffer containing 1.2 M glycine buffer pH 9.0 and 75 mM MgCl2. After 1 h at 37°C, 40 μl concentration. HCl was added, and sphingosine was extracted and quantitated by phosphorylation with sphingosine kinase and [32P]ATP (Olivera and Spiegel 1998). Total phospholipids in cellular lipid extracts were quantified by a colorimetric reaction with malachite green exactly as previously described (Edsall et al. 1997).
Measurement of [32P]SPP Release
HEK293 cells were grown to subconfluency in 100-mm dishes in 10% FBS media, washed and incubated in serum-free media containing 40 μCi/ml [32P]orthophosphate for 24 h to label the phospholipid pools to isotopic equilibrium. In some experiments, sphingosine (5 μM) was added to the media 10 min before termination of the 24-h incubation period. The media was then collected and the cells scraped from the dishes. Cellular and secreted [32P]SPP were extracted in alkaline conditions as described above, followed by acidic extraction with chloroform/methanol/concentration. HCl (100:100:1, vol/vol), to partition [32P]SPP into the organic phase. [32P]SPP was resolved on TLC with 1-butanol/ethanol/acetic acid/water (80:20:10:20, vol/vol), visualized and quantified as described (Olivera et al. 1998).
Assay of Sphingosine Kinase Activity
Cells were incubated in low serum or serum-free media for at least 24 h, and then harvested and lysed by freeze-thawing in buffer containing 20 mM Tris, pH 7.4, 20% glycerol, 1 mM β-mercaptoethanol, 1 mM EDTA, 1 mM sodium orthovanadate, 40 mM β-glycero-phosphate, 15 mM NaF, 10 μg/ml leupeptin, aprotinin and soybean trypsin inhibitor, 1 mM PMSF, and 0.5 mM 4-deoxypyridoxine. Cell lysates were fractionated into cytosol and membrane fractions by centrifugation at 100,000 g for 60 min 4°C. Sphingosine kinase activity was determined in the presence of 50 μM sphingosine, dissolved in 5% Triton X-100 (final concentration 0.25%), and [32P]ATP (10 μCi, 1 mM) containing MgCl2 (10 mM) as previously described (Kohama et al. 1998), and specific activity was expressed as picomoles of SPP formed per minute per milligram protein.
Immunostaining
Cells grown on glass coverslips coated with collagen I were incubated overnight in DMEM supplemented with 2 μg/ml insulin, 2 μg/ml transferrin, and 20 μg/ml BSA. Cells were washed with PBS and fixed in 3.7% formalin and 0.1% Triton X-100 for 20 min. After washing with PBS, cells were permeabilized for 10 min with 0.5% Triton X-100 in PBS, washed again, and incubated with anti–myc antibody for 20 min at room temperature. After washing, cells were incubated with anti–mouse antibody conjugated with fluorescein or Texas red for 20 min. After washing three times with PBS, coverslips were mounted on slides using an Anti-Fade kit and cells were photographed using an inverted fluorescence microscope (Eclipse TE200; Nikon Inc.) connected to a digital camera (DKC5000; Sony Corp.).
Incorporation of Bromodeoxyuridine
24 h after transfection, NIH 3T3 cells were serum starved in DMEM supplemented with 2 μg/ml insulin, 2 μg/ml transferrin, and 20 μg/ml BSA, and then stimulated with various agents. After 16 h, cells were incubated for 3 h with bromodeoxyuridine (BrdU, 10 μM), and then fixed in 4% paraformaldehyde containing 5% sucrose, pH 7.0, for 20 min at room temperature. After washing with PBS, cells were incubated in permeabilization buffer (0.5% Triton/PBS, pH 7.4, containing 10 mg/ml BSA) for 20 min at room temperature, and then incubated for 1 h at room temperature with monoclonal anti–BrdU antibody in the presence of DNAse (1,000 U/ml) (Van Brocklyn et al. 1998). After washing with PBS, cells were stained with Texas red–conjugated anti–mouse antibody in 5% BSA/PBS for 1 h, washed with PBS, and then photographed using an inverted fluorescence microscope connected to a digital camera. Cells expressing GFP and cells with positive BrdU staining were counted. At least three different fields were scored with a minimum of 100 cells scored per field.
Measurement of DNA Synthesis
Stably transfected NIH 3T3 fibroblasts were plated in 24-well clusters at a density of 5 × 103 cells/well in DMEM containing 10% calf serum. After 24 h, cells were washed with DMEM containing 0.5% calf serum and incubated in same media. The media was replaced every 2–3 d. At the indicated times, cultures were pulsed with 1 μCi of [3H]thymidine for 6 h and radioactivity incorporated into trichloroacetic acid–insoluble material measured as previously described (Olivera and Spiegel 1993). Values are the means of triplicate determinations and standard deviations were routinely <10% of the mean.
Cell Cycle Analysis
Stably transfected NIH 3T3 fibroblasts were trypsinized and counted. Aliquots containing 2 × 106 cells were centrifuged, washed twice with PBS, and resuspended in 40 mM citrate buffer, pH 7.6, containing 250 mM sucrose and 5% DMSO. After propidium iodide staining of cellular DNA, cell cycle analysis was performed with a FACStarplus® flow cytofluorometer (Becton Dickinson & Co.) (Goodemote et al. 1995).
Analysis of Cell Growth
Stably transfected NIH 3T3 fibroblasts (1,000 cells) were plated in 24-well plates in DMEM containing 10% calf serum. After 24 h, cells were washed twice with DMEM and then grown in DMEM containing 0.5 or 10% calf serum. At the indicated times, cells were washed with PBS, fixed with 70% ethanol for 10 min, and stained with crystal violet. Incorporated dye was dissolved in 100 μl of 0.1 M sodium citrate in 50% ethanol, pH 4.2, and the absorbance was measured at 540 nm (Wang et al. 1999a). In some experiments, cells were trypsinized and counted in a hemocytometer.
Determination of Apoptotic Cells
Apoptosis was assessed by staining cells with 8 μg/ml Hoechst in 30% glycerol/PBS for 10 min at room temperature as previously described (Cuvillier et al. 1998). Cells expressing GFP were examined with an inverted fluorescence microscope. Apoptotic cells were distinguished by condensed, fragmented nuclear regions. The percentage of intact and apoptotic nuclei in cells expressing GFP fluorescence was determined (Van Brocklyn et al. 1999). A minimum of 500 cells were scored in a double-blinded manner to minimize subjective interpretations. In some experiments, viable cells were determined by trypan blue exclusion.
Fluorogenic DEVD Cleavage Enzyme Assays
Enzyme reactions were performed in 96-well plates with 20 μg of cytosolic proteins and a final concentration of 20 μM Ac-DEVD-AMC substrate as previously described (Cuvillier et al. 1998). Fluorescent AMC product formation was measured over a 30-min period at excitation and emission wavelengths of 360 and 460 nm using a Cytofluor II fluorometer plate reader (PerSeptive Biosystems).
Western Blotting
Cytosolic fractions (10–25 μg) were boiled in Laemmli sample buffer, separated on 10 or 15% SDS-PAGE, and blotted to nitrocellulose. The membranes were blocked with nonfat dry milk in 0.1% Tween-20–PBS for 2 h, and incubated overnight with anti–c-myc monoclonal antibody (1 μg/ml) or 2 h with rabbit antiserum specific for the p17 subunit of caspase-3 (kindly provided by Dr. Donald Nicholson, Merck), in the same buffer containing 1% nonfat dry milk. Immunocomplexes were detected by enhanced chemiluminescence as described previously (Cuvillier et al. 1998).
Results
Characterization of Cells Expressing Sphingosine Kinase
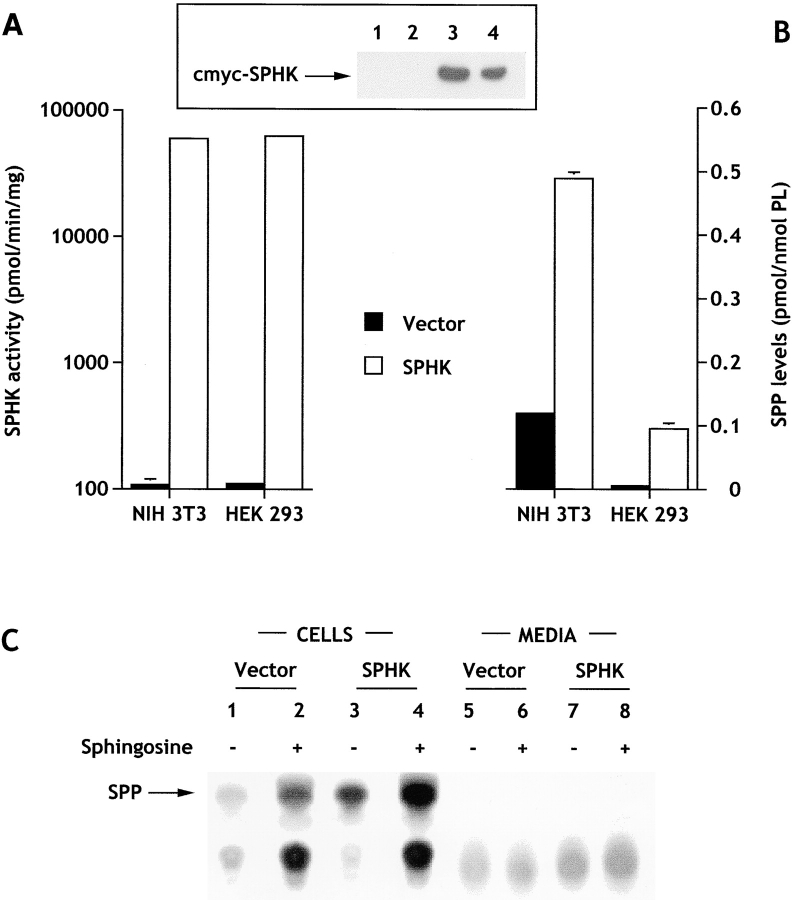
Similar to our previous results with transiently transfected cells (Kohama et al. 1998), sphingosine kinase activity in cell lysates from NIH 3T3 and HEK293 cells stably expressing c-myc–tagged SPHK1a was dramatically increased (500-fold; Fig. 1 A). Western blot analysis of cytosolic fractions using anti–c-myc antibody revealed a specific protein band with an apparent molecular weight consistent with the predicted size of c-myc–sphingosine kinase that was absent in vector-transfected cells (Fig. 1 A, insert). SPP levels were also elevated in cells expressing sphingosine kinase by four- to eightfold (Fig. 1 B), a level that did not correlate with the large fold increase in sphingosine kinase activity measured in vitro. One possible explanation for this discrepancy is that availability of cellular sphingosine might limit the production of SPP. In agreement with our previous results (Zhang et al. 1991), when cells were acutely treated with exogenous sphingosine, which is readily taken up (Olivera et al. 1994, Olivera et al. 1997), levels of SPP were further increased three- to sixfold, suggesting that although availability of sphingosine may be important for regulating SPP levels, it is probably not the only critical factor influencing levels of SPP in cells overexpressing sphingosine kinase.
Figure 1.
Sphingosine kinase expression results in an increase in intracellular but not extracellular levels of sphingosine-1-phosphate. (A) Cytosolic sphingosine kinase activity was measured in cells stably transfected with c-myc–pcDNA3 (filled bars) or c-myc–pcDNA3-SPHK1a (open bars). The sphingosine kinase activity in vector-transfected cells was 108 ± 12 and 111 ± 7 pmol/min per mg for NIH 3T3 and HEK293 cells, respectively. (Inset) Western blot showing expression of sphingosine kinase. (1 and 2) Vector-transfected HEK293 and NIH 3T3 cells, respectively; (3 and 4) SPHK1a-transfected HEK293 and NIH 3T3 cells, respectively. (B) SPP levels in cells stably expressing sphingosine kinase. Transfected cells were washed and incubated for 24 h in serum-free media (HEK293 cells) or serum-free media containing fatty acid–free BSA (20 μg/ml), transferrin (2 μg/ml), and insulin (2 μg/ml) (NIH 3T3 fibroblasts). SPP was extracted and measured as described in Materials and Methods. Levels of SPP in vector-transfected cells were 0.119 ± 0.003 and 0.012 ± 0.002 pmol/nmol phospholipid for NIH 3T3 and HEK293 cells, respectively. (C) Cellular and secreted [32P]SPP. HEK293 cells labeled to isotopic equilibrium with [32]Pi for 24 h. [32P]SPP was then extracted from the cells and the media as described in Materials and Methods. Duplicate cultures were treated with 5 μM sphingosine 10 min before extraction of lipids (lanes 2, 4, 6, and 8). In the autoradiograms of the TLC analyses, the arrow indicates the location of SPP visualized with molybdenum blue spray.
Since activated platelets can release SPP (Yatomi et al. 1995), and the EDG family of G protein–coupled SPP receptors have recently been identified (An et al. 1997; Goetzl and An 1998; Lee et al. 1998; Okamoto et al. 1998; Van Brocklyn et al. 1998, Van Brocklyn et al. 1999; Zondag et al. 1998; Gonda et al. 1999), it was important to determine whether sphingosine kinase–transfected cells, which have notable increases in SPP levels, secrete SPP into the medium. No significant release of SPP into the extracellular media could be detected by our mass measurements, even after addition of sphingosine to both NIH and HEK293 sphingosine kinase–transfected cells. To increase the sensitivity of detection of secreted SPP, we labeled HEK 293 cells to isotopic equilibrium with [32P]Pi and analyzed the labeled SPP in cells as well as in the medium (Fig. 1 C). Despite the large increases in [32P]SPP detected in cells overexpressing sphingosine kinase, there was no detectable labeled SPP released into the medium. Similar results were also found with NIH3T3 fibroblasts. However, in agreement with previous studies (Yatomi et al. 1997a), we could readily detect secretion by human platelets after thrombin treatment. Both methods to measure SPP levels gave identical increases in intracellular SPP in transfected and sphingosine-treated cells. Based on the sensitivity of these methods (1–2 pmol SPP/sample), it is estimated that the concentration of SPP in the extracellular media must be <0.4 nM, a concentration well below the K d for binding of SPP to its receptors (8–25 nM) (Van Brocklyn et al. 1998, Van Brocklyn et al. 1999).
Localization of Sphingosine Kinase and Stimulation by Growth Factors
Both membrane-associated and cytosolic sphingosine kinase activities have been described in mammalian tissues and cell lines (Stoffel et al. 1973; Buehrer and Bell 1992; Olivera and Spiegel 1993; Ghosh et al. 1994; Olivera et al. 1994). However, the amino acid sequence of murine SPHK1a suggests that it should be a cytosolic protein (Kohama et al. 1998). In agreement with our previous study on transiently transfected cells (Kohama et al. 1998), most of the sphingosine kinase activity in cells stably expressing c-myc–tagged sphingosine kinase was cytosolic and the relative amounts of sphingosine kinase activity in cytoplasmic versus membrane fractions were similar in vector- and sphingosine kinase–transfected cells (Table ), suggesting that the small c-myc tag does not affect localization of sphingosine kinase. Immunohistochemistry with antibodies against c-myc revealed that sphingosine kinase has a diffuse distribution in the cytosol and somewhat denser expression in perinuclear sites in both NIH 3T3 and HEK 293 cells (Fig. 2).
Table 1.
PDGF Stimulates Sphingosine Kinase Activity in Sphingosine Kinase–transfected Cells
| Cytosolic sphingosine kinase activity | Cytosolic sphingosine kinase activity | |||
|---|---|---|---|---|
| Treatment | Vector | SPHK | ||
| percent of total activity | pmol/min/mg | |||
| None | 69 | 68 | 68 | 5,900 |
| PDGF (1 ng/ml) | ND | ND | 86 | 8,992 |
| PDGF (20 ng/ml) | 71 | 78 | 124 | 9,092 |
NIH 3T3 cells transfected with c-myc–pcDNA3 (Vector) or c-myc–pcDNA3-SPHK1a (SPHK) were incubated in serum-free media containing fatty acid–free BSA (20 μg/ml), and transferrin (2 μg/ml) overnight, and then stimulated with the indicated concentrations of PDGF for 10 min. Sphingosine kinase activity in cytosol and particulate fractions was measured as described in Materials and Methods and standard deviations were <10%.
Figure 2.
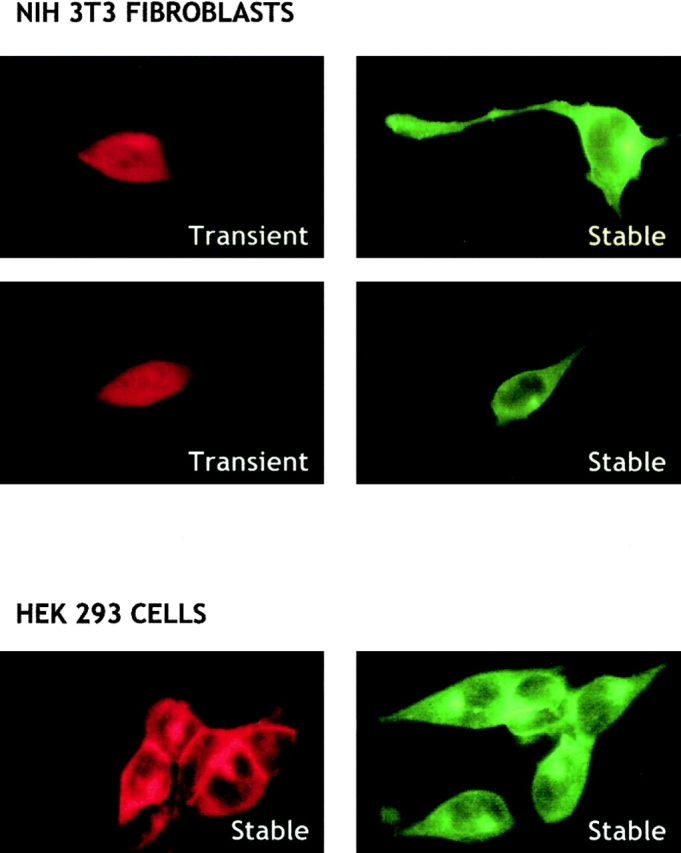
Cellular localization of c-myc–sphingosine kinase. NIH3T3 fibroblasts transiently or stably expressing sphingosine kinase and HEK293 cells stably expressing sphingosine kinase were incubated with a monoclonal c-myc antibody (20 μg/ml) and stained with anti–mouse Texas red–conjugated IgG (1:100 dilution) (left) or anti–mouse FITC-monoclonal IgG (1:10 dilution) (right). Fluorescence micrographs (60×) were taken using an inverted fluorescent microscope. Vector transfectants (c-myc–pcDNA3) did not show any significant fluorescence.
Previously, we and others have shown that PDGF stimulates sphingosine kinase activity in various cell types (Olivera and Spiegel 1993; Bornfeldt et al. 1995; Pyne et al. 1996; Rani et al. 1997). PDGF stimulated cytosolic c-myc–sphingosine kinase activity in transfected NIH 3T3 fibroblasts to a similar extent as its effect on endogenous sphingosine kinase (Table ), indicating that c-myc–sphingosine kinase activity is regulated by the signaling pathways triggered by growth factors in the same manner as the native enzyme. Unlike activation of protein kinase C, activation of sphingosine kinase by PDGF does not induce significant translocation to the membrane fraction. Collectively, these data suggest that cells overexpressing sphingosine kinase are a useful tool to study intracellular actions of SPP. Interestingly, endogenous SPP was present in both the cytoplasm and membrane fractions and overexpression of sphingosine kinase markedly increased both by nearly the same extent (Table ). In contrast to most phospholipids, glycosphingolipids, and other sphingolipid metabolites, such as ceramide and sphingosine, which are found mainly in membranes, 20% of the total cellular SPP is in the cytosol (Table ).
Table 2.
Distribution of SPP in Membrane and Cytosol Fractions
| SPP | SPP | |||
|---|---|---|---|---|
| Fraction | Vector | SPHK | Vector | SPHK |
| pmol/mg protein | pmol/nmol phospholipid | |||
| Cytosol | 0.45 ± 0.014 | 2.17 ± 0.012 | 0.015 ± 0.003 | 0.111 ± 0.044 |
| Membrane | 1.7 ± 0.16 | 9.1 ± 0.37 | 0.009 ± 0.003 | 0.056 ± 0.007 |
SPP in cytosol and particulate fractions of HEK 293 cells transfected with c-myc–pcDNA3 (Vector) or c-myc–pcDNA3-SPHK1a (SPHK) was measured as described in Materials and Methods. The same samples were also analyzed for phospholipid content.
Effect of Sphingosine Kinase Overexpression on Cell Proliferation
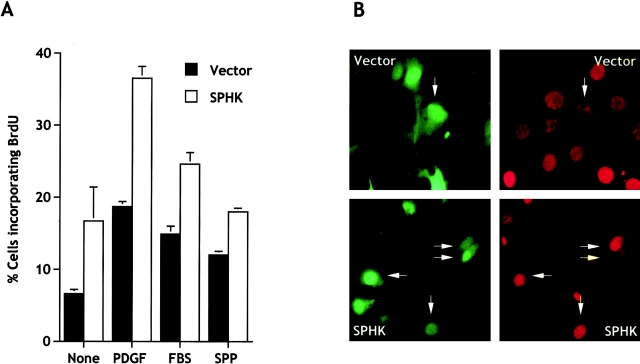
Studies with sphingosine kinase inhibitors suggested that the mitogenic effect of SPP might be due to intracellular actions (Olivera and Spiegel 1993; Van Brocklyn et al. 1998). However, others have suggested that the mitogenic effect is mediated by binding of SPP to the EDG family of G protein–coupled receptors (Goetzl and An 1998). In view of this controversy, it was of interest to examine the proliferation of cells whose intra- rather than extracellular levels of SPP are increased after transfection with sphingosine kinase. Transient expression of c-myc–sphingosine-1-phosphate (SPHK) in NIH 3T3 cells not only increased intracellular levels of SPP, it also increased the proportion of cells in the S phase of the cell cycle measured as incorporation of BrdU into nascent DNA (Fig. 3 A). The growth promoting effects of sphingosine kinase were further enhanced by suboptimal concentrations of PDGF (1 ng/ml) and serum (0.1%) (Fig. 3 A), which are known to stimulate sphingosine kinase (Olivera and Spiegel 1993, and Table ). However, addition of exogenous SPP increased BrdU incorporation in empty vector-transfected cells twofold, but had no effect on sphingosine kinase–transfected cells, suggesting that these cells are already maximally stimulated by their intracellular SPP. It should be pointed out that these experiments were carried out in the presence of 2 μg/ml insulin, which is a known survival factor for these cells and was used to prevent cell death caused by serum deprivation.
Figure 3.
Expression of sphingosine kinase stimulates BrdU incorporation into nascent DNA. (A) NIH 3T3 fibroblasts were transiently transfected with empty vector (pCMV-SPORT2, filled bars) or pCMV-SPORT2-SPHK1a (open bars) together with pCEFL-GFP. Cells were serum-starved for 18 h and incubated in serum-free media supplemented with insulin (2 μg/ml) without (None) or with PDGF (1 ng/ml), FBS (0.1%), or SPP (10 μM). After 16 h, BrdU was added for an additional 3 h. Double immunofluorescence was used to visualize transfected cells and BrdU incorporation, and the proportion of cells incorporating BrdU among total transfected cells (expressing GFP) was determined. Data are means ± SD of duplicate cultures from a representative experiment. At least three different fields were scored with a minimum of 100 cells scored per field. Similar results were obtained in three independent experiments. For comparison, in medium containing 10% FBS, 42.3 ± 4.9% of the vector-transfected cells incorporated BrdU. (B) Representative images of cells treated with a suboptimum concentration of PDGF (1 ng/ml). Vector- and sphingosine kinase–transfected NIH 3T3 cells expressing GFP (left) and incorporating BrdU (right) were visualized by double immunofluorescence. Arrows indicate cells that are positive for both.
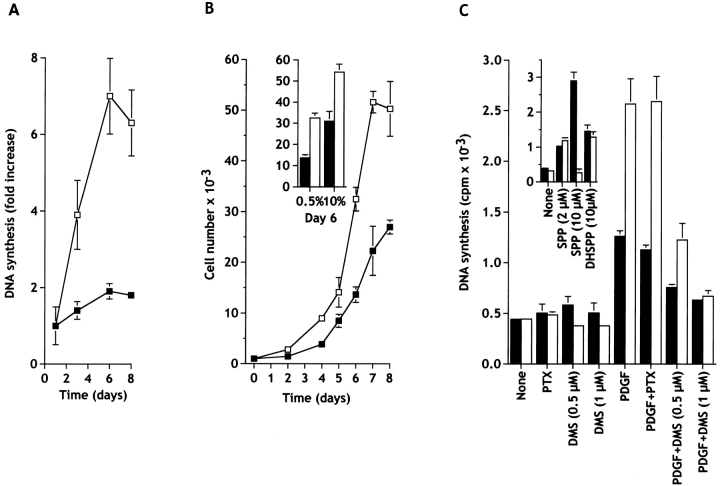
Since transient expression of sphingosine kinase increased the proportion of cells in the S phase, it was of interest to determine whether there was a corresponding increase as a result of stable transfection. Nonclonal pools of stably transfected cells were studied to avoid potential phenotypic changes due to selection and propagation of clones derived from single individual cells. Stable expression of sphingosine kinase had a dramatic effect on [3H]thymidine incorporation into DNA in cells cultured in low serum media, while DNA synthesis was low in empty vector-transfected cells over the course of 8 d in 0.5% serum (Fig. 4 A).
Figure 4.
Expression of sphingosine kinase stimulates growth of NIH 3T3 fibroblasts cultured in low serum. (A) Cells stably transfected with c-myc–pcDNA3 (▪) or with c-myc–SPHK-pcDNA3 (□) were plated at low density, washed after 24 h, and cultured in 0.5% calf serum for the indicated days. Media was replaced every 2 d, the cells were pulsed at the indicated times with [3H]thymidine for an additional 6 h, and DNA synthesis measured as described in Materials and Methods. Data are means ± SD of three independent determinations and are expressed as fold increase of the value determined after 1 d. Similar results were obtained in three additional experiments. (B) Sphingosine kinase–transfected NIH 3T3 fibroblasts proliferate more rapidly than control cells. Cells stably transfected with c-myc–pcDNA3 (▪) or with c-myc–SPHK-pcDNA3 (□) were plated in 24-well tissue culture plates (1,000 per well), cultured for the indicated days in the presence of 0.5% CS (B) or for 6 d in 10% CS (insert), stained with crystal violet, and quantitated as described in Materials and Methods. Media was replaced every 2 d. Similar results were obtained in at least two additional experiments. (C) N,N-Dimethylsphingosine, but not pertussis toxin, inhibits DNA synthesis induced by sphingosine kinase overexpression. Cells stably transfected with c-myc–pcDNA3 (filled bars) or with c-myc–SPHK-pcDNA3 (open bars) were plated at 2 × 104 cells per well, washed after 24 h, and cultured in serum-free DMEM containing 2 μg/ml transferrin and 20 μg/ml BSA, in the absence or presence of the indicated agents. After 16 h, cells were pulsed with 1.0 μCi of [3H]thymidine for 8 h, and incorporation of [3H]thymidine into trichloroacetic acid–insoluble material was measured. Values are the means ± SD of triplicate determinations, and similar results were found in three independent experiments. Agents (concentration): pertussis toxin (PTX; 50 ng/ml); DMS (0.5 or 1 μM); PDGF (20 ng/ml); SPP (2 or 10 μM); dihydro-SPP (DHSPP, 10 μM).
DNA flow cytometry was used to characterize the distribution of cells in the cell cycle. FACS® analysis revealed that after 2 d in 0.5% serum, >95% of the vector-transfected NIH 3T3 fibroblasts were in G0/G1 phase and only a small fraction were in S and G2/M phases (Table ). Overexpression of sphingosine kinase reduced the fraction of cells in G0/G1 and, in agreement with the DNA synthesis data, increased the proportion in the S phase and, to a lesser extent, in the G2/M phase. Even in the presence of 10% serum, which markedly increased the proportion of cells in the S phase and G2/M phase, sphingosine kinase transfection further increased the proportion of cells in the S phase (Table ). This data suggests that either a greater proportion of sphingosine kinase–transfected cells are cycling and/or that the duration of the G1 phase is shortened compared with vector-transfected cells.
Table 3.
Flow Cytometric Analysis of Cell
| Cell cycle distribution | |||
|---|---|---|---|
| Cells | G0/G1 | S | G2/M |
| Percentage of cells | |||
| Vector, 0.5% CS | 96.4 ± 0.8 | 1.6 ± 0.4 | 1.9 ± 0.4 |
| SPHK, 0.5% CS | 90.4 ± 0.7* | 6.4 ± 0.1* | 3.2 ± 0.4* |
| Vector, 10% CS | 65.4 ± 0.1* | 25.4 ± 0.2* | 9.5 ± 0.2* |
| SPHK, 10% CS | 57.5 ± 0.7* | 32.1 ± 0.6* | 10.4 ± 0.1‡ |
NIH 3T3 cells stably transfected with c-myc–pcDNA3 (Vector) or c-myc–pcDNA3-SPHK1a (SPHK) were plated at 500,000 cells/150-mm dish. After 24 h, cells were washed twice with DMEM and incubated in media containing 0.5 or 10% CS for 2 d. Cell-cycle analysis was then performed by flow cytometry as described in Materials and Methods. *Significant differences from vector-transfected values as determined by Student's t test (P ≤ 0.01). ‡Not significantly different.
Growth curves in low serum of vector and sphingosine kinase–transfected 3T3 fibroblasts diverged after 2 d and growth of the sphingosine kinase–transfected cells was markedly enhanced thereafter, demonstrating that the alteration in cell-cycle distribution resulted in increased growth rate (Fig. 4 B). Moreover, even in the presence of 10% serum, sphingosine kinase expression was still able to significantly increase proliferation (Fig. 4 C), and to increase the saturation density (not shown).
Analyses of the growth curves during the exponential growth phase revealed that sphingosine kinase expression decreased the doubling time in 0.5% calf serum from 34 ± 1.4 to 27 ± 1.8 h, although no differences were observed in the doubling times in full serum (vector, 17 ± 2.2 h; SPHK, 16 ± 1.2 h). Furthermore, computation of the parameters of the cell cycle during exponential growth revealed that sphingosine kinase markedly shortened the duration of G1 phase of the cell cycle by 31 ± 2% in low serum, suggesting that sphingosine kinase is important for nontransformed cells to progress through the G1-S boundary. However, in cells growing in full serum, although overexpression of sphingosine kinase increases the proportion of cells in S phase with a corresponding decrease in G0/G1 (Table ), there were no significant changes in the duration of either of these phases of the cell cycle.
To further investigate the possibility that the EDG family of SPP receptors might be involved in the proliferative response induced by overexpression of sphingosine kinase, stably transfected 3T3 fibroblasts were treated with pertussis toxin, as these receptors are known to act through pertussis toxin-sensitive G proteins (Lee et al. 1998; Van Brocklyn et al. 1998; Kon et al. 1999; Sato et al. 1999). In contrast to many other biological responses, including the mitogenic effect of exogenous SPP, which are inhibited by pertussis toxin (Goodemote et al. 1995), it did not abrogate the increase in DNA synthesis induced by expression of sphingosine kinase in the presence of PDGF, suggesting that sphingosine kinase acts independently of Gi proteins (Fig. 4 C). In contrast, treatment of cells with a specific inhibitor of sphingosine kinase, N,N-dimethylsphingosine (DMS), at a concentration that inhibits sphingosine kinase and decreases SPP levels, blocked the effects of sphingosine kinase overexpression (Fig. 4 C). Similar results were obtained in cells transiently expressing sphingosine kinase (data not shown). Moreover, although 2 μM SPP stimulated proliferation in vector and sphingosine kinase–transfected cells (Fig. 4 C, inset), 10 μM SPP markedly stimulated DNA synthesis of vector-transfected cells while inhibiting proliferation of sphingosine kinase–transfected cells. Interestingly, 10 μM dihydro-SPP, which lacks the trans double bond present in SPP and binds and signals through Edg-1, -3, and -5 (Van Brocklyn et al. 1998, Van Brocklyn et al. 1999), only slightly stimulated DNA synthesis in both vector- and kinase-transfected cells (Fig. 4 D, inset), suggesting that SPP receptors are equally responsive in vector as well as sphingosine kinase–transfected cells.
Effect of Sphingosine Kinase Overexpression on Programmed Cell Death
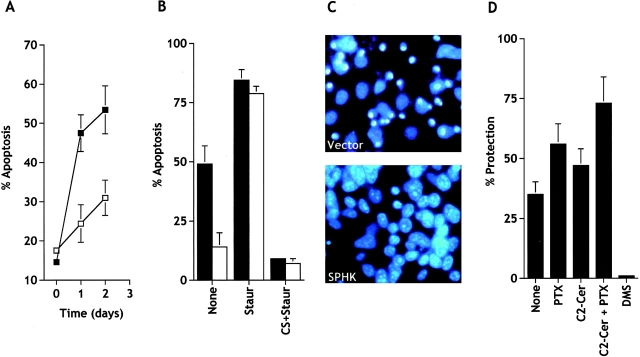
Exogenous SPP has been shown to suppress apoptosis induced by cytokines, such as TNF and Fas ligand (Cuvillier et al. 1996), serum deprivation (Edsall et al. 1997; Van Brocklyn et al. 1998), and to be important in the survival effects of NGF (Edsall et al. 1997), vitamin D3 (Kleuser et al. 1998), and cAMP (Machwate et al. 1998). In contrast, more recently it has been suggested that SPP protected human T lymphoblastoma cells from apoptosis induced by antibodies to Fas, CD2, and CD3/CD28, or C6-ceramide by binding to EDG-3 and EDG-5 GPCRs (Goetzl et al. 1999). However, if the intracellular level of SPP is a critical factor that determines cell survival, then it is expected that overexpression of sphingosine kinase should suppress apoptosis. In agreement with previous studies (Brancolini et al. 1997), prolonged serum deprivation induced apoptosis in NIH 3T3 fibroblasts (Fig. 5 A), where shrinkage and condensation of nuclei were clearly evident (Fig. 5 C). Transient and stable expression of sphingosine kinase in NIH 3T3 fibroblasts suppressed the appearance of apoptotic nuclei induced by serum starvation (Fig. 5, A–C). In contrast, sphingosine kinase expression had almost no effect on apoptosis resulting from treatment with staurosporine (Fig. 5 B), a broad spectrum protein kinase inhibitor that is known to induce apoptosis in normal and neoplastic cells (Jacobsen et al. 1996). Moreover, DMS, but not pertussis toxin, inhibited the cytoprotective effect of sphingosine kinase overexpression in stably (Fig. 5 D) as well as transiently transfected NIH 3T3 fibroblasts.
Figure 5.
Expression of sphingosine kinase in NIH 3T3 fibroblasts reduces apoptosis induced by serum deprivation. (A) NIH 3T3 fibroblasts were transiently transfected with vector (▪) or SPHK1a (□), together with pCEFL-GFP, and serum starved for the indicated times. Total GFP-expressing cells and GFP-expressing cells displaying fragmented nuclei indicative of apoptosis were counted as described in Materials and Methods. A minimum of 500 cells in each field were scored. Data are mean ± SEM of three independent experiments, each one done in duplicate or triplicate. (B) Stably vector-transfected (filled bars) or c-myc–tagged SPHK1a (open bars)-transfected NIH 3T3 cells were serum starved in the absence (None) or presence of 50 nM staurosporine (Staur) or 10% serum plus 250 nM staurosporine (CS+Staur) for 24 h. Percentages of apoptotic cells were determined after Hoechst staining by fluorescence microscopy. (C) Note the typical condensed fragmented nuclei of apoptotic cells in vector but not in SPHK-overexpressing cells after serum deprivation. (D) DMS, but not pertussis toxin, inhibits the protective effect of sphingosine kinase. Stably vector- or c-myc–tagged SPHK1a-transfected NIH 3T3 cells were serum starved in the absence (None) or presence of 5 μM DMS, 20 ng/ml pertussis toxin (PTX), 25 μM C2-ceramide (C2-Cer) or both for 24 h, and the number of viable cells was determined as described in Materials and Methods. Percent protection from apoptosis by sphingosine kinase expression = 100× [(percent viable sphingosine kinase cells − percent of viable vector cells)/(percent of viable vector-transfected cells)].
It should be pointed out that serum withdrawal markedly increases ceramide levels in many cell types, and it has been proposed that ceramide mediates, at least in part, serum deprivation–induced cell death (Hannun 1996). Thus, it was of interest to examine the effect of sphingosine kinase expression on apoptosis of other types of cells. Expression of sphingosine kinase in HEK293 cells also markedly inhibited apoptosis induced by serum deprivation and by the cell permeable ceramide analogue, C2-ceramide, although to a lesser extent (Fig. 6 A). These cytoprotective effects of sphingosine kinase overexpression were also not inhibited by pertussis toxin treatment. In contrast, apoptosis induced by staurosporine, a well recognized inducer of apoptosis in HEK293 cells (Ozawa et al. 1999), was not suppressed by expression of sphingosine kinase (Fig. 6 A).
Figure 6.
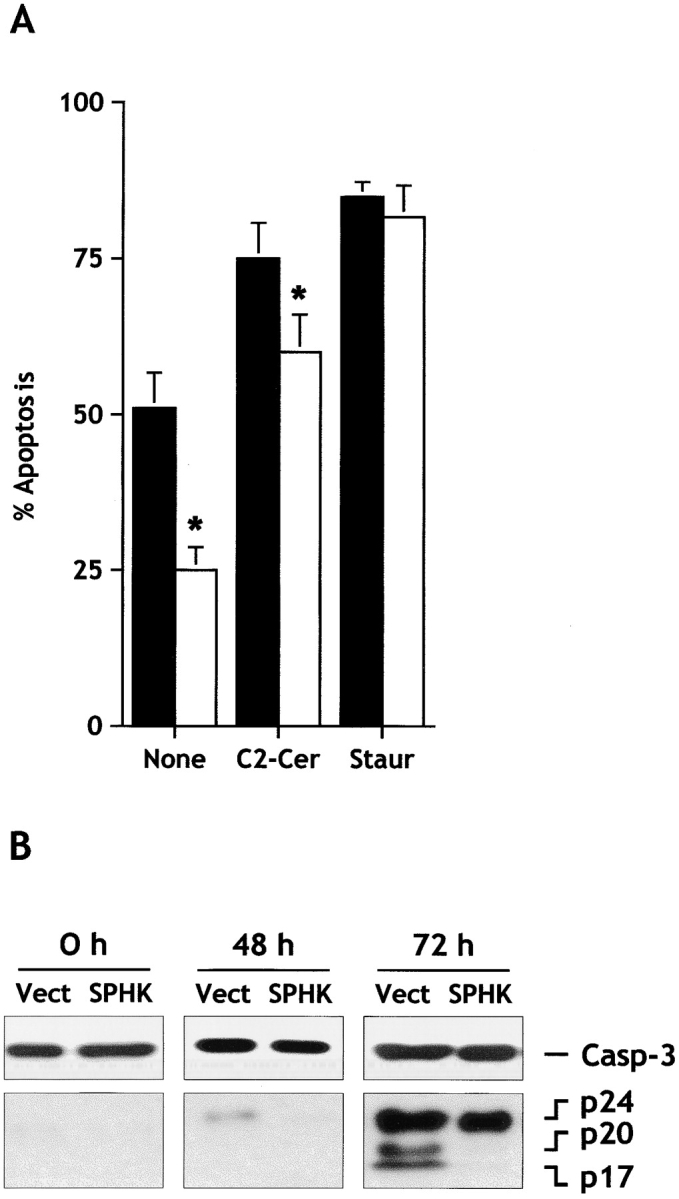
Sphingosine kinase inhibits serum-deprivation– and C2-ceramide–induced apoptosis, but not staurosporine-induced apoptosis, in HEK293 cells. (A) HEK293 cells stably expressing empty vector (filled bars) or c-myc–tagged SPHK1a (open bars) were incubated in serum-free medium for 30 h, and then treated in the absence (None) or presence of 25 μM C2-ceramide (C2-Cer) or 100 nM staurosporine (Staur) for 24 h. Percentages of apoptotic cells were determined after Hoechst staining by fluorescence microscopy. Data are means ± SEM of four independent experiments, each done in triplicate. *Significant differences from vector-transfected values (P ≤ 0.05). (B) Expression of sphingosine kinase inhibits activation of caspase-3. HEK293 cells expressing empty vector (Vect) or c-myc–tagged SPHK1a (SPHK) were incubated in serum-free medium and cytosolic extracts were prepared at the indicated times. Proteins were resolved by 15% SDS-PAGE, blotted, and probed with anti–caspase-3. Migrations indicated are: full-length caspase-3, the cleavage forms p24, p20, and p17.
Caspases, a family of aspartate-specific cysteine proteases, play a critical role in the execution phase of apoptotic cell death by cleavage of a specific set of cytosolic and nuclear proteins leading to disassembly of the cell (Nicholson et al. 1995; Thornberry and Lazebnik 1998). Fig. 6 B demonstrates that serum withdrawal induced activation of caspase-3, as shown by the appearance of the p20 and p17 subunits, which correlated with the onset of apoptosis. Overexpression of sphingosine kinase reduced processing of caspase-3 (Fig. 6 B).
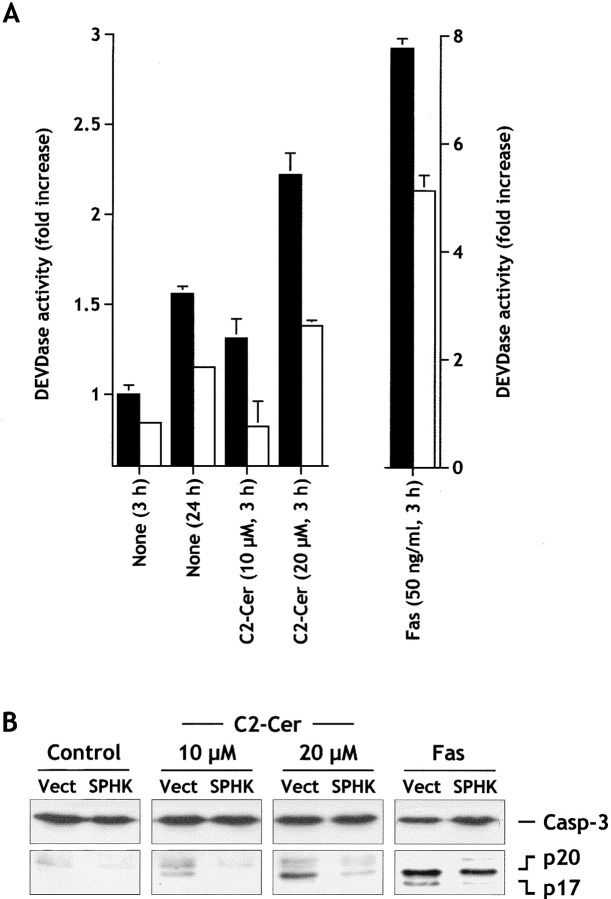
Human leukemic Jurkat T cells are a well-characterized model system that readily undergo apoptosis and are sensitive to C2-ceramide–induced apoptosis (Cuvillier et al. 1998). Moreover, exogenous SPP suppressed Fas- and ceramide-mediated apoptosis in these cells (Cuvillier et al. 1998). Serum deprivation or brief treatment of Jurkat T cells (3 h) with Fas monoclonal antibody or C2-ceramide caused extensive cell death, as measured by the appearance of nuclear fragmentation, which was reduced by expression of sphingosine kinase (data not shown). The fluorogenic substrate Ac-DEVD-AMC, which corresponds to the cleavage site found in executioner caspases targets, was used to measure the activation of these caspases. Indeed, serum deprivation, Fas ligation, and C2-ceramide treatment of Jurkat cells resulted in a time-dependent increase in DEVDase proteolytic activity (Fig. 7 A), which preceded the appearance of fragmented nuclei. Overexpression of sphingosine kinase blocked the increase in DEVDase activity induced by serum deprivation and C2-ceramide (Fig. 7 A) and had a lesser yet still significant effect on Fas ligation–induced DEVDase activation. In agreement, sphingosine kinase effectively prevented activation of caspase-3, as measured by the decrease of its processing to p20 and p17 forms following serum deprivation and C2-ceramide addition, but had a smaller effect on Fas-induced activation (Fig. 7 B).
Figure 7.
Expression of sphingosine kinase suppresses DEVDase activity and inhibits caspase-3 activation induced by serum starvation, C2-ceramide and Fas ligation in Jurkat T cells. (A) Jurkat T cells transfected with vector (filled bars) or SPHK1a (open bars) were serum starved for 3 or 24 h or incubated for 3 h in serum-free media in the presence of 10 or 20 μM C2-ceramide (C2-Cer) or agonistic Fas antibody (50 ng/ml). Activation of DEVD-specific caspases was measured by the cleavage of the fluorogenic substrate Ac-DEVD-AMC. All values for sphingosine kinase–transfected cells were significantly different from vector-transfected cells as determined by Student's t test (P ≤ 0.01). (B) Cellular extracts from Jurkat cells stably transfected with empty vector (Vect) or SPHK1a expression vector (SPHK) were treated for 3 h without (Control) or with the indicated concentration of C2-ceramide or anti–Fas as described in A, separated by SDS-PAGE and immunoblotted with anti–caspase-3. The migration positions of full-length 32-kD caspase-3 and proteolytically processed intermediate p20 and active subunit p17 are indicated.
Discussion
Apparently contradictory reports describe intra- and extracellular actions of SPP in diverse cell types (Goetzl and An 1998; Spiegel 1999). While much evidence has accumulated recently indicating that SPP functions as a ligand for the EDG family of GPCRs, which are linked to diverse biological responses (Goetzl and An 1998; Spiegel 1999), less is known of the role of SPP as a second messenger. SPP has been proposed as an intracellular mediator of cell growth (Spiegel et al. 1996), mainly based on the use of exogenously added SPP in the high micromolar range, stimulation of sphingosine kinase by growth factors, as well as sphingosine kinase inhibitors. For example, in Swiss 3T3 cells, competitive inhibitors of sphingosine kinase block some of the proliferative signals elicited by PDGF (Olivera and Spiegel 1993), including activation of mitogen-activated protein kinase, cyclin-dependent kinases (Cdc2 and Cdk2 kinases), and activation of the transcription factor AP1 (Rani et al. 1997; Su et al. 1994). However, the proliferative response to exogenously added SPP is partially sensitive to pertussis toxin, suggesting the potential involvement of Gi-coupled cell surface receptors (Goodemote et al. 1995). Thus, it was important to develop another approach to unequivocally determine the role of intracellularly generated SPP. To this end, we transiently and stably transfected cells with sphingosine kinase, which markedly increased intracellular levels of SPP. In contrast to activated platelets that release stored SPP in a PKC-dependent manner (Yatomi et al. 1997b), transfected NIH 3T3 fibroblasts and HEK293 cells did not secrete any detectable SPP, suggesting that only certain cell types are capable of secreting SPP and that these transfected cells should be useful to study the intracellular roles of SPP. Interestingly, SPP levels were increased to the same extent by sphingosine kinase expression in both the cytosol and in membrane fractions, whereas other sphingolipid metabolites, ceramide and sphingosine, are predominantly located in membranes. This observation is very intriguing since it might suggest that, similar to another second messenger, inositol trisphosphate, it can move between intracellular compartments.
BrdU incorporation, cell cycle analysis, and growth curves indicate that sphingosine kinase transfection can significantly increase the proliferative rate of nontransformed NIH 3T3 fibroblasts. Enforced expression of sphingosine kinase not only resulted in higher levels of SPP, it also increased the proportion of cells in the S phase of the cell cycle, expedited the G1/S transition, and reduced the doubling time, especially in low serum conditions, indicating that intracellular SPP is an important regulator of cell growth. In support of this notion, microinjection of SPP (Van Brocklyn et al. 1998) or intracellular generation of SPP by photolysis of incorporated caged SPP (Qiao et al. 1998), stimulated DNA synthesis of Swiss 3T3 fibroblasts. Moreover, in contrast to the sphingosine kinase inhibitor, pertussis toxin did not suppress proliferation induced by sphingosine kinase overexpression. However, pertussis toxin markedly inhibited SPP-induced proliferation of endothelial cells (Wang et al. 1999b). It is possible that there is a complex interplay between cell surface receptor signaling and intracellular targets for SPP, which can contribute to its mitogenic response in certain cell types. Moreover, the discovery of G protein–coupled receptors in the nucleus (Bhattacharya et al. 1998) and the presence of intracellular EDG-1 in distinct perinuclear location (Liu et al. 1999) raises the possibility that intracellular SPP might signal through receptors located inside cells.
Sequence analyses identified homologues of sphingosine kinase in numerous widely disparate organisms, including yeast (Kohama et al. 1998; Nagiec et al. 1998), demonstrating that sphingosine kinase is a member of a novel but highly conserved gene family and is distinct from almost all other known lipid kinases. Interestingly, of all the lipid kinases, sphingosine kinase shares the highest degree of homology to diacylglycerol kinase ζ (DGK-ζ) (Kohama et al. 1998). However, overexpression of DGK-ζ, which regulated diacylglycerol levels in the nucleus (Topham et al. 1998), had opposite effects to sphingosine kinase on the cell cycle, increasing the doubling time and the fraction of cells in the G1/G0 phase of the cell cycle (Topham et al. 1998).
The effects of SPP on cell growth appear to be evolutionarily conserved, as phosphorylated long-chain sphingoid bases also regulate proliferation and survival of yeast (Lanterman and Saba 1998; Mandala et al. 1998; Gottlieb et al. 1999; Skrzypek et al. 1999). Spontaneous mutants of Saccharomyces cerevisiae with diminished sphingosine kinase activity had reduced growth rates and failed to pass through the diauxic shift and successfully initiate respiratory growth (Lanterman and Saba 1998), suggesting that yeast sphingosine kinase might play a role in mediating growth responses to changes in nutrients. Because exogenously added dihydro-SPP was unable to affect yeast growth (Lanterman and Saba 1998), these results further substantiate a role for intracellular SPP in cell growth regulation. In agreement, deletion of SPP lyase, which results in accumulation of phosphorylated sphingoid bases in yeast, causes unregulated proliferation on approach to the stationary phase (Gottlieb et al. 1999). Moreover, expression of G1 cyclin, whose downregulation may be directly involved in mediating the growth arrest induced by starvation, was maintained at a higher level throughout the post-diauxic phase in these lyase-deficient yeast (Gottlieb et al. 1999). This growth advantage was associated with a failure to arrest cells in G1 and a marked increase in stationary phase DNA content per cell. In agreement, overexpression of LBP1 and LBP2, which are specific SPP phosphatases (Mandala et al. 1998), reduced levels of long-chain phosphorylated sphingoid bases and arrested cells prematurely at the G1 phase of the cell cycle (Mao et al. 1999). These effects on the yeast cell cycle are mirror images of the effects of overexpression of sphingosine kinase and increased SPP levels in mammalian cells that result in shortening of the G1 phase of the cell cycle and accumulation of cells in the S phase when deprived of growth factors.
Sphingosine kinase activity and SPP levels can be regulated by growth and survival factors (Spiegel and Merrill 1996; Spiegel et al. 1998). Interestingly, the levels of SPP in 3T3 fibroblasts overexpressing sphingosine kinase, despite the enormous increases in in vitro activity, were maximally increased only four- to eightfold. This level was similar to the level of SPP produced in the same cells after stimulation by PDGF and serum, addition of sphingosine, or even SPP itself (Olivera and Spiegel 1993). These observations suggest that cells tightly regulate their levels of SPP, consistent with its role as a second messenger. In addition to sphingosine kinase, rapid degradation by SPP lyase and/or SPP phosphatase may also play an important role in determining the steady state levels of SPP. Another possible explanation for the lack of correlation of sphingosine kinase activity and SPP levels is that the substrate for the overexpressed sphingosine kinase, sphingosine, may be located in a different subcellular compartment. Exogenous SPP (2 μM) stimulated growth of vector-transfected and sphingosine kinase overexpressing cells; however, high concentrations of SPP (10 μM) inhibit, rather than enhance, growth of sphingosine kinase–transfected cells. In agreement, while increases in intracellular levels of long-chain phosphorylated sphingoid bases enhance growth of yeast (Lanterman and Saba 1998; Gottlieb et al. 1999), substantially higher increases reduce their growth rate (Skrzypek et al. 1999).
Paradoxically, although the processes of cell growth and cell death appear to be opposing and mutually contradictory, substantial evidence now suggests that the pathways of apoptosis and mitosis may be mechanistically related or tightly coupled (Harrington et al. 1994; Evan and Littlewood 1998). Mammalian cells overexpressing sphingosine kinase not only had higher growth rates, but also exhibited enhanced survival in serum-free conditions. Previously, it was shown that exogenous SPP can antagonize apoptosis mediated by a number of stress stimuli, including serum withdrawal, TNF-α, Fas ligation, and ceramide elevation (Cuvillier et al. 1996; Edsall et al. 1997). However, the importance of intracellular SPP has been challenged by the recent suggestion that protection from apoptosis may be mediated by binding of SPP to EDG-3 and EDG-5 GPCRs (Goetzl et al. 1999). In contrast, our results indicate that increased cellular SPP levels in various cell types due to overexpression of sphingosine kinase mimics the protective effect of exogenous SPP against apoptosis, especially in response to growth factor withdrawal, supporting the concept that the cytoprotective actions of SPP are mediated intracellularly. In agreement, we previously found that dihydroSPP, which binds to and signals through the EDG family of GPCRs (Van Brocklyn et al. 1998, Van Brocklyn et al. 1999), unlike SPP, did not prevent apoptosis and conversely, the nonhydrolyzable SPP-phosphonate, which does not bind to these receptors, mimicked the effect of SPP on proliferation and survival (Van Brocklyn et al. 1998). Because the sphingolipid metabolite ceramide is a mediator of stress responses (Hannun 1996; Kolesnick and Kronke 1998), both in mammalian cells and in yeast (Dickson et al. 1997; Jenkins et al. 1997; Nickels and Broach 1996), we proposed that the relative intracellular levels of these two sphingolipid metabolites (ceramide and SPP) is an important factor that determines whether cells will survive or die (Cuvillier et al. 1996; Mandala et al. 1998). Consistent with this hypothesis, we found that transient expression of sphingosine kinase modified the sphingolipid metabolite balance, resulting in higher levels of SPP, with concomitant decreases in the levels of sphingosine and ceramide (Kohama et al. 1998). Our present results show that expression of sphingosine kinase in 3T3 fibroblasts, HEK 293, and Jurkat T cells suppressed apoptosis induced by serum deprivation, known to increase ceramide levels, or by the ceramide analogue, C2-ceramide, with concomitant inhibition of activation of the executionary caspase-3. Similarly, transient expression of sphingosine kinase in U937 cells reduced apoptosis induced by both serum starvation and TNF-α treatment, as determined by DNA fragmentation and DEVDase activity assays (Cuvillier, O., and S. Spiegel, unpublished observations), suggesting that the protective effects of intracellularly generated SPP are not restricted to certain cell types. Because the proximal molecular targets by which ceramide activates caspase-3 and related CED-3 subfamily caspases remain largely unidentified, further work is necessary before the target(s) for SPP can be more precisely identified.
This survival function of SPP might also be part of an ancient stress response since, in S. cerevisiae, nutrient deprivation activates sphingosine kinase and yeast mutants, with decreased sphingosine kinase activity also displayed an increased sensitivity to heat stress (Lanterman and Saba 1998). Furthermore, accumulation of phosphorylated long chain sphingoid bases in S. cerevisiae either due to the deletion of LBP1 and LBP2 genes (Mandala et al. 1998), or deletion of DPL1, a gene encoding long-chain–base phosphate lyase (Saba et al. 1997), correlates with increased survival at an elevated temperature (Lanterman and Saba 1998; Mao et al. 1999; Skrzypek et al. 1999), suggesting that long-chain–base phosphates may play a physiological role in heat stress resistance in yeast.
In summary, this study substantiates a role for sphingosine kinase–derived SPP as a second messenger in cell proliferation and survival. Sphingosine kinase belongs to a new class of lipid kinases, different in structure and biochemical properties than other lipid kinases, such as the phosphatidylinositol 3-kinase family, but similar in the broad spectrum of signals and in vital and versatile cell functions that they regulate.
Acknowledgments
This work was supported by research grant RO1 GM43880 from the National Institutes of Health.
Footnotes
1.used in this paper: Ac-DEVD-AMC, acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin; BrdU, bromodeoxyuridine; DMS, N,N-dimethylsphingosine; EDG, endothelial differentiation gene; GFP, green fluorescent protein; GPCR, G protein coupled receptors; SPHK, sphingosine kinase; SPP, sphingosine-1-phosphate; TNF, tumor necrosis factor
References
- An S., Bleu T., Huang W., Hallmark O.G., Coughling S.R., Goetzl E.J. Identification of cDNAs encoding two G protein–coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M., Peri K.G., Almazan G., Ribeiro-da-Silva A., Shichi H., Durocher Y., Abramovitz M., Hou X., Varma D.R., Chemtob S. Nuclear localization of prostaglandin E2 receptors. Proc. Natl. Acad. Sci. USA. 1998;95:15792–15797. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K.E., Graves L.M., Raines E.W., Igarashi Y., Wayman G., Yamamura S., Yatomi Y., Sidhu J.S., Krebs E.G., Hakomori S., Ross R. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cellsspatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Lazarevic D., Rodriguez J., Schneider C. Dismantling cell–cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J. Cell Biol. 1997;139:759–771. doi: 10.1083/jcb.139.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrer B.M., Bardes E.S., Bell R.M. Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim. Biophys. Acta. 1996;1303:233–242. doi: 10.1016/0005-2760(96)00092-6. [DOI] [PubMed] [Google Scholar]
- Buehrer B.M., Bell R.M. Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. J. Biol. Chem. 1992;267:3154–3159. [PubMed] [Google Scholar]
- Choi O.H., Kim J.-H., Kinet J.-P. Calcium mobilization via sphingosine kinase in signalling by the Fc∈RI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Coroneos E., Martinez M., McKenna S., Kester M. Differential regulation of sphingomyelinase and ceramidase activities by growth factors and cytokines. J. Biol. Chem. 1995;270:23305–23309. doi: 10.1074/jbc.270.40.23305. [DOI] [PubMed] [Google Scholar]
- Cuvillier O., Pirianov G., Kleuser B., Vanek P.G., Coso O.A., Gutkind S., Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Cuvillier O., Rosenthal D.S., Smulson M.E., Spiegel S. Sphingosine-1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide-mediated apoptosis in Jurkat T lymphocytes. J. Biol. Chem. 1998;273:2910–2916. doi: 10.1074/jbc.273.5.2910. [DOI] [PubMed] [Google Scholar]
- Dickson R.C., Nagiec E.E., Skrzypek M., Tillman P., Wells G.B., Lester R.L. Sphingolipids are potential heat stress signals in Saccharomyces . J. Biol. Chem. 1997;272:30196–30200. doi: 10.1074/jbc.272.48.30196. [DOI] [PubMed] [Google Scholar]
- Edsall L.C., Pirianov G.G., Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor–mediated neuronal survival and differentiation. J. Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall L.C., Spiegel S. Enzymatic measurement of sphingosine-1-phosphate. Anal. Biochem. 1999;272:80–86. doi: 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- Evan G., Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- Ghosh T.K., Bian J., Gill D.L. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J. Biol. Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- Goetzl E.J., An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- Goetzl E.J., Kong Y., Mei B. Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J. Immunol. 1999;162:2049–2056. [PubMed] [Google Scholar]
- Gonda K., Okamoto H., Takuwa N., Yatomi Y., Okazaki H., Sakurai T., Kimura S., Sillard R., Harii K., Takuwa Y. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem. J. 1999;337:67–75. [PMC free article] [PubMed] [Google Scholar]
- Goodemote K.A., Mattie M.E., Berger A., Spiegel S. Involvement of a pertussis toxin sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J. Biol. Chem. 1995;270:10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- Gottlieb D., Heideman W., Saba J.D. The DPL1 gene is involved in mediating the response to nutrient deprivation in Saccharomyces cerevisiae . Mol. Cell Biol. Res. Commun. 1999;1:66–71. doi: 10.1006/mcbr.1999.0109. [DOI] [PubMed] [Google Scholar]
- Hannun Y. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Harrington E.A., Fanadi A., Evan G.I. Oncogenes and cell death. Curr. Biol. 1994;4:120–129. doi: 10.1016/0959-437x(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Jacobsen M.D., Weil M., Raff M.C. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J. Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G.M., Richards A., Wahl T., Mao C., Obeid L., Hannun Y. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae . J. Biol. Chem. 1997;272:32566–32572. doi: 10.1074/jbc.272.51.32566. [DOI] [PubMed] [Google Scholar]
- Kleuser B., Cuvillier O., Spiegel S. 1α,25-dihydroxyvitamin D3 inhibits programmed cell death in HL-60 cells by activation of sphingosine kinase. Cancer Res. 1998;58:1817–1824. [PubMed] [Google Scholar]
- Kohama T., Olivera A., Edsall L., Nagiec M.M., Dickson R., Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- Kolesnick R.N., Kronke M. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Kon J., Sato K., Watanabe T., Tomura H., Kuwabara A., Kimura T., Tamama K., Ishizuka T., Murata N., Kanda T. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J. Biol. Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- Lanterman M.M., Saba J.D. Characterization of sphingosine kinase (SK) activity in Saccharomyces cerevisiae and isolation of SK-deficient mutants. Biochem. J. 1998;332:525–531. doi: 10.1042/bj3320525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-J., Van Brocklyn J.R., Thangada S., Liu C.H., Hand A.R., Menzeleev R., Spiegel S., Hla T. Sphingosine-1-phosphate as a ligand for the G protein–coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Liu C.H., Thangada S., Lee M.-J., Van Brocklyn J.R., Spiegel S., Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machwate M., Rodan S.B., Rodan G.A., Harada S.I. Sphingosine kinase mediates cyclic AMP suppression of apoptosis in rat periosteal cells. Mol. Pharmacol. 1998;54:70–77. doi: 10.1124/mol.54.1.70. [DOI] [PubMed] [Google Scholar]
- Mandala S., Thornton R., Tu Z., Kurtz M., Nickels J., Broach J., Menzeleev R., Spiegel S. Sphingoid base 1-phosphate phosphatase, a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. USA. 1998;95:150–155. doi: 10.1073/pnas.95.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C., Saba J.D., Obeid L.M. The dihydrosphingosine-1-phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses. Biochem. J. 1999;342:667–675. [PMC free article] [PubMed] [Google Scholar]
- Mazurek N., Megidish T., Hakomori S.-I., Igarashi Y. Regulatory effect of phorbol esters on sphingosine kinase in BALB/C 3T3 fibroblasts (variant A31)demonstration of cell type-specific response. Biochem. Biophys. Res. Commun. 1994;198:1–9. doi: 10.1006/bbrc.1994.1001. [DOI] [PubMed] [Google Scholar]
- Melendez A., Floto R.A., Gillooly D.J., Harnett M.M., Allen J.M. FcγRI coupling to phospholipase D initiates sphingosine kinase–mediated calcium mobilization and vesicular trafficking. J. Biol. Chem. 1998;273:9393–9402. doi: 10.1074/jbc.273.16.9393. [DOI] [PubMed] [Google Scholar]
- Meyer zu Heringdorf D., Lass H., Alemany R., Laser K.T., Neumann E., Zhang C., Schmidt M., Rauen U., Jakobs K.H., van Koppen C.J. Sphingosine kinase–mediated Ca2+ signalling by G-protein–coupled receptors. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2830–2837. doi: 10.1093/emboj/17.10.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiec M.M., Skrzypek M., Nagiec E.E., Lester R.L., Dickson R.C. The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode long chain base kinases. J. Biol. Chem. 1998;273:19437–19442. doi: 10.1074/jbc.273.31.19437. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W., All A., Thornberry N.A., Vaillancourt J.P., Ding C.K., Gallant M., Gareau Y., Griffin P.R., Labelle M., Lazebnik Y.A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nickels J.T., Broach J.R. A ceramide-activated protein phosphatase mediates ceramide-induced G1 arrest of Sacharromyces cerevisiae . Genes Dev. 1996;10:382–394. doi: 10.1101/gad.10.4.382. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takuwa N., Gonda K., Okazaki H., Chang K., Yatomi Y., Shigematsu H., Takuwa Y. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J. Biol. Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- Olivera A., Kohama T., Tu Z., Milstien S., Spiegel S. Purification and characterization of rat kidney sphingosine kinase. J. Biol. Chem. 1998;273:12576–12583. doi: 10.1074/jbc.273.20.12576. [DOI] [PubMed] [Google Scholar]
- Olivera A., Romanowski A., Rani C.S., Spiegel S. Differential effects of sphingomyelinase and cell-permeable ceramide analogs on proliferation of Swiss 3T3 fibroblasts. Biochim. Biophys. Acta. 1997;1348:311–323. doi: 10.1016/s0005-2760(97)00067-2. [DOI] [PubMed] [Google Scholar]
- Olivera A., Rosenthal J., Spiegel S. Sphingosine kinase from Swiss 3T3 fibroblastsa convenient assay for the measurement of intracellular levels of free sphingoid bases. Anal. Biochem. 1994;223:306–312. doi: 10.1006/abio.1994.1589. [DOI] [PubMed] [Google Scholar]
- Olivera A., Spiegel S. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Olivera A., Spiegel S. Sphingosine kinase. Assay and product analysis. In: Bird I.M., editor. Methods in Molecular Biology. Vol. 105. Humana Press Inc; Totawa, NJ: 1998. pp. 233–242. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Kuwabara K., Tamatani M., Takatsuji K., Tsukamoto Y., Kaneda S., Yanagi H., Stern D.M., Eguchi Y., Tsujimoto Y. 150-kDa oxygen-regulated protein (ORP150) suppresses hypoxia-induced apoptotic cell death. J. Biol. Chem. 1999;274:6397–6404. doi: 10.1074/jbc.274.10.6397. [DOI] [PubMed] [Google Scholar]
- Perez G.I., Knudson C.M., Leykin L., Korsmeyer S.J., Tilly J.L. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat. Med. 1997;3:1228–1232. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- Postma F.R., Jalink K., Hengeveld T., Moolenaar W.H. Sphingosine-1-phosphate rapidly induces rho-dependent neurite retractionaction through a specific cell surface receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- Pyne S., Chapman J., Steele L., Pyne N.J. Sphingomyelin-derived lipids differentially regulate the extracellular signal-regulated kinase 2 (ERK-2) and c-Jun N-terminal kinase (JNK) signal cascades in airway smooth muscle cells. Eur. J. Biochem. 1996;237:819–826. doi: 10.1111/j.1432-1033.1996.0819p.x. [DOI] [PubMed] [Google Scholar]
- Qiao L., Kozikowski A.P., Olivera A., Spiegel S. Synthesis and evaluation of a photolyzable derivative of sphingosine 1-phosphate-caged SPP. Bioorg. Med. Chem. 1998;8:711–714. doi: 10.1016/s0960-894x(98)00112-7. [DOI] [PubMed] [Google Scholar]
- Rani C.S., Berger A., Wu J., Sturgill T.W., Beitner-Johnson D., LeRoith D., Varticovski L., Spiegel S. Divergence in signal transduction pathways of PDGF and EGF receptorsinvolvement of sphingosine-1-phosphate in PDGF but not EGF signaling. J. Biol. Chem. 1997;272:10777–10783. doi: 10.1074/jbc.272.16.10777. [DOI] [PubMed] [Google Scholar]
- Rius R.A., Edsall L.C., Spiegel S. Activation of sphingosine kinase in pheochromocytoma PC12 neuronal cells in response to trophic factors. FEBS Lett. 1997;417:173–176. doi: 10.1016/s0014-5793(97)01277-5. [DOI] [PubMed] [Google Scholar]
- Saba J.D., Nara F., Bielawska A., Garrett S., Hanun Y.A. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J. Biol. Chem. 1997;272:26087–26090. doi: 10.1074/jbc.272.42.26087. [DOI] [PubMed] [Google Scholar]
- Sato K., Tomura H., Igarashi Y., Ui M., Okajima F. Exogenous sphingosine 1-phosphate induces neurite retraction possibly through a cell surface receptor in PC12 cells. Biochem. Biophys. Res. Commun. 1997;240:329–334. doi: 10.1006/bbrc.1997.7666. [DOI] [PubMed] [Google Scholar]
- Sato K., Tomura H., Igarashi Y., Ui M., Okajima F. Possible involvement of cell surface receptors in sphingosine 1-phosphate–induced activation of extracellular signal-regulated kinase in C6 glioma cells. Mol. Pharmacol. 1999;55:126–133. doi: 10.1124/mol.55.1.126. [DOI] [PubMed] [Google Scholar]
- Skrzypek M.S., Nagiec M.M., Lester R.L., Dickson R.C. Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces . J. Bacteriol. 1999;181:1134–1140. doi: 10.1128/jb.181.4.1134-1140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S. Sphingosine 1-phosphatea prototype of a new class of second messengers. J. Leukoc. Biol. 1999;65:341–344. doi: 10.1002/jlb.65.3.341. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Cuvillier O., Edsall L., Kohama T., Menzeleev R., Olivera A., Thomas D., Tu Z., Van Brocklyn J., Wang F. Roles of sphingosine-1-phosphate in cell growth, differentiation, and death. Biochemistry (Mosc.) 1998;63:69–73. [PubMed] [Google Scholar]
- Spiegel S., Foster D., Kolesnick R.N. Signal transduction through lipid second messengers. Curr. Opin. Cell Biol. 1996;8:159–167. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Merrill A.H., Jr. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Hellenbroich B., Heimann G. Properties and specificities of sphingosine kinase from blood platelets. Hoppe-Seyler's Z. Physiol. Chem. 1973;354:1311–1316. doi: 10.1515/bchm2.1973.354.2.1311. [DOI] [PubMed] [Google Scholar]
- Su Y., Rosenthal D., Smulson M., Spiegel S. Sphingosine 1-phosphate, a novel signaling molecule, stimulates DNA binding activity of AP-1 in quiescent Swiss 3T3 fibroblasts. J. Biol. Chem. 1994;269:16512–16517. [PubMed] [Google Scholar]
- Thornberry N.A., Lazebnik Y. Caspasesenemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Topham M.K., Bunting M., Zimmerman G.A., McIntyre T.M., Blackshear P.J., Prescott S.M. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-ζ. Nature. 1998;394:697–700. doi: 10.1038/29337. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn J.R., Lee M.J., Menzeleev R., Olivera A., Edsall L., Cuvillier O., Thomas D.M., Coopman P.J.P., Thangada S., Hla T., Spiegel S. Dual actions of sphingosine-1-phosphateextracellular through the Gi-coupled orphan receptor edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brocklyn J.R., Tu Z., Edsall L., Schmidt R.R., Spiegel S. Sphingosine 1-phosphate–induced cell rounding and neurite retraction are mediated by the G protein–coupled receptor H218. J. Biol. Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- van Koppen C.J., Meyer zu Heringdorf D., Laser K.T., Zhang C., Jakobs K.H., Bünnemann M., Pott L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J. Biol. Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P.P., Mannaerts G.P. Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver. J. Biol. Chem. 1991;266:12502–12507. [PubMed] [Google Scholar]
- Van Veldhoven P.P., Mannaerts G.P. Sphinganine 1-phosphate metabolism in cultured skin fibroblastsevidence for the existence of a sphingosine phosphatase. Biochem. J. 1994;299:597–601. doi: 10.1042/bj2990597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Nohara K., Olivera O., Thompson E.W., Spiegel S. Involvement of focal adhesion kinase in inhibition of motility of human breast cancer cells by sphingosine 1-phosphate Exp. Cell Res. 247 1999. 17 28a [DOI] [PubMed] [Google Scholar]
- Wang F., Van Brocklyn J.R., Hobson J.P., Movafagh S., Zukowska-Grojec Z., Milstien S., Spiegel S. Sphingosine-1-phosphate stimulates cell migration through a G0-coupled cell surface receptorpotential involvement in angiogenesis J. Biol. Chem In press 1999. b [DOI] [PubMed] [Google Scholar]
- Xia P., Gamble J.R., Rye K.A., Wang L., Hii C.S.T., Cockerill P., Khew-Goodall Y., Bert A.G., Barter P.J., Vadas M.A. Tumor necrosis factor-α induces adhesion molecule expression through the sphingosine kinase pathway. Proc. Natl. Acad. Sci. USA. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura S., Yatomi Y., Ruan F., Sweeney E.A., Hakomori S., Igarashi Y. Sphingosine 1-phosphate regulates melanoma cell motility through a receptor-coupled extracellular action and in a pertussis toxin-insensitive manner. Biochemistry. 1997;36:10751–10759. doi: 10.1021/bi970926s. [DOI] [PubMed] [Google Scholar]
- Yatomi Y., Igarashi Y., Yang L., Hisano N., Qi R., Asazuma N., Satoh K., Ozaki Y., Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum J. Biochem. 121 1997. 969 973a [DOI] [PubMed] [Google Scholar]
- Yatomi Y., Ruan F., Hakomori S., Igarashi Y. Sphingosine-1-phosphatea platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- Yatomi Y., Yamamura S., Ruan F., Igarashi Y. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid J. Biol. Chem. 272 1997. 5291 5297b [DOI] [PubMed] [Google Scholar]
- Zhang H., Desai N.N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Saba J.D. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem. Biophys. Res. Commun. 1998;242:502–507. doi: 10.1006/bbrc.1997.7993. [DOI] [PubMed] [Google Scholar]
- Zondag G.C.M., Postma F.R., Etten I.V., Verlaan I., Moolenaar W.H. Sphingosine 1-phosphate signalling through the G-protein–coupled receptor Edg-1. Biochem. J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]