Abstract
Pericentrin is a conserved protein of the centrosome involved in microtubule organization. To better understand pericentrin function, we overexpressed the protein in somatic cells and assayed for changes in the composition and function of mitotic spindles and spindle poles. Spindles in pericentrin-overexpressing cells were disorganized and mispositioned, and chromosomes were misaligned and missegregated during cell division, giving rise to aneuploid cells. We unexpectedly found that levels of the molecular motor cytoplasmic dynein were dramatically reduced at spindle poles. Cytoplasmic dynein was diminished at kinetochores also, and the dynein-mediated organization of the Golgi complex was disrupted. Dynein coimmunoprecipitated with overexpressed pericentrin, suggesting that the motor was sequestered in the cytoplasm and was prevented from associating with its cellular targets. Immunoprecipitation of endogenous pericentrin also pulled down cytoplasmic dynein in untransfected cells. To define the basis for this interaction, pericentrin was coexpressed with cytoplasmic dynein heavy (DHCs), intermediate (DICs), and light intermediate (LICs) chains, and the dynamitin and p150Glued subunits of dynactin. Only the LICs coimmunoprecipitated with pericentrin. These results provide the first physiological role for LIC, and they suggest that a pericentrin–dynein interaction in vivo contributes to the assembly, organization, and function of centrosomes and mitotic spindles.
Keywords: pericentrin, centrosomes, mitotic spindle, cytoplasmic dynein light intermediate chains, aneuploidy
The centrosome is the major microtubule nucleating organelle in animal cells (Kellogg et al. 1994; Zimmerman et al. 1999). It is usually composed of a pair of centrioles surrounded by a protein matrix from which microtubules are nucleated (Szollosi et al. 1972; Gould and Borisy 1990). The centrosome proteins, pericentrin and γ tubulin, are localized to the matrix material where they form a unique lattice-like network (Dictenberg et al. 1998). The lattice appears to represent the higher order organization of γ tubulin rings, structures comprised of γ tubulin and several other proteins that appear to provide the templates for nucleation of microtubules at the centrosome (Moritz et al. 1995; Zheng et al. 1995; Schnackenberg et al. 1998). γ Tubulin and pericentrin are also part of a large cytoplasmic protein complex that may represent the fundamental subunit of microtubule nucleation before its assembly at the centrosome (Dictenberg et al. 1998). In addition, the Drosophila melanogaster protein, Asp (abnormal spindle protein), has been shown to play a role in the centrosomal recruitment of γ tubulin (Avides and Glover 1999). However, the precise role of this protein and others in the assembly, organization, and activity of centrosomes is unknown (see Zimmerman et al. 1999).
The assembly and molecular organization of the centrosome is important for bipolar spindle assembly during mitosis (for review see Waters and Salmon 1997). Functional abrogation or depletion of pericentrin or γ tubulin disrupts centrosome assembly and organization, and creates structural defects in microtubule asters and spindles (Doxsey et al. 1994; Felix et al. 1994; Stearns and Kirschner 1994). Alternative pathways for assembly of microtubule asters and spindles in the absence of centrosomes have been described (Gaglio et al. 1997; Merdes and Cleveland 1997; Waters and Salmon 1997; Hyman and Karsenti 1998). In these acentrosomal spindle assembly systems, the molecular motor cytoplasmic dynein and the nuclear mitotic apparatus protein (NuMA)1 play key roles in the organization and focusing of the spindle poles (Heald et al. 1996; Merdes et al. 1996; Gaglio et al. 1997). These proteins are also involved in the organization of spindle poles in the presence of centrosomes (Merdes and Cleveland 1997; Karki and Holzbaur 1999).
The precise role of pericentrin in spindle function is currently unknown. The protein has been shown to contribute to the organization of microtubule arrays in both interphase and mitosis. Pericentrin antibodies introduced into mouse oocytes and Xenopus laevis embryos disrupt the organization of centrosomes and meiotic and mitotic spindles (Doxsey et al. 1994). Moreover, when added to Xenopus extracts, the antibodies inhibit assembly of microtubule asters. Recently, it has been shown that pericentrin levels are elevated in human tumor cells that exhibit defects in centrosome structure, spindle organization, and chromosome segregation (Pihan et al. 1998; Pihan, G., and S. Doxsey, unpublished observations). This suggests that pericentrin may contribute to tumorigenesis through the organization of dysfunctional spindles that missegregate chromosomes and generate aneuploid cells (for review see Doxsey 1998; Pihan and Doxsey 1999).
To further examine the role of pericentrin in spindle organization, we overexpressed the protein in somatic cells. Cells with excess pericentrin formed aberrant mitotic spindles, missegregated chromosomes, and became aneuploid. We found that cytoplasmic dynein was displaced from centrosomes and kinetochores, and the dynein-mediated organization of the Golgi complex was impaired. An interaction between cytoplasmic dynein and pericentrin was identified and shown to be mediated specifically by light intermediate chain (LIC) subunits (Gill et al. 1994; Hughes et al. 1995) of the motor protein. These results indicate that pericentrin and dynein act together to ensure proper organization and function of centrosomes and spindles.
Materials and Methods
cDNA Constructs
A full-length mouse pericentrin was constructed using a three piece cloning strategy. Pericentrin clone λpc1.2 (Doxsey et al. 1994) was excised with restriction enzymes PvuI and EcoRV. The 5′ end of the final clone was amplified by PCR using VENT polymerase from clone PCR 1 (Doxsey et al. 1994) using a 5′ primer (5′-CCGATATCAGATGGAAGACG-3′) with an EcoRV restriction enzyme site and a 3′ primer (5′-GTTTGGGAGGTAGAGGCT-3′) with a PvuI site. The amplified PCR product was digested with EcoRV and PvuI. Plasmid pcDNAI/Amp (Invitrogen Corp.) was used to construct a vector with 13 amino acids of hemagglutinin (HA) protein (MAYPYDVPCYASL, pHAI; Wilson et al. 1984) inserted at the HindIII site in the polylinker (a gift of Michael Green, UMass Medical School, Worcester, MA). The vector was linearized with EcoRV and ligated to form the full-length pericentrin, as described (Sambrook et al. 1989). The correct orientation of the fragments was confirmed by PCR using the T7 vector primer and the 5′-directed pericentrin primer. The sequence of the clone was confirmed using an automated sequencer (Bio-Rad Laboratories). The preparation of cDNAs encoding full-length rat p150Glued (Vaughan et al. 1999), the human dynamitin (Echeverri et al. 1996), rat myc-tagged cytoplasmic dynein intermediate chain (DIC) 2C (IC-2C; Vaughan and Vallee 1995), and rat FLAG-tagged cytoplasmic heavy chain (Mazumdar et al. 1996) have been described previously.
Cell Culture, DNA Transfection, Cell Viability, and Growth
COS-7 cells were cultured as described (American Type Culture Collection) with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma Chemical Co.). Cells were grown on 12-mm round glass coverslips in 35-mm culture dishes (Falcon Plastics) and transfected with 2 μg of plasmid DNA (HA-tagged pericentrin [HA-Pc], β-galactosidase, pHAI, or no DNA) using lipofectamine (GIBCO BRL); transfection efficiency was ∼15%. Cells were fixed 35–42 h after transfection and processed for immunofluorescence staining, immunoprecipitation, metabolic labeling, or Western blotting. Cell viability was determined using mitotracker (Sigma Chemical Co.), which measures energy-dependent electron transport in mitochondria. Cell growth was determined by measuring the ratio of transfected cells to the total cell population; there was little change in this ratio over a 50 h time period.
Antibodies
Affinity-purified rabbit IgG was prepared from sera raised against the COOH terminus of pericentrin (Doxsey et al. 1994) and used at 1:1,000 for immunofluorescence microscopy and Western blotting. Anti-HA mAbs (12CA5) were obtained from Berkeley Antibody Co., Inc., and anti-HA polyclonal antibodies were a gift from Joanne Buxton (UMass Medical School, Worcester, MA; Meisner et al. 1997). Antibodies to α and γ tubulin, mouse IgG, and rabbit IgG were obtained from Sigma Chemical Co. Antibodies to β-galactosidase were from Boehringer Mannheim Corp. Antibodies to the following proteins were also used in these studies under conditions described in the accompanying references: dynein heavy chain (DHC; JR-61, Asai et al. 1994), DIC L5 (Vaughan and Vallee 1995), 74.1 (Dillman and Pfister 1994), dynamitin (Echeverri et al. 1996), p150glued (Vaughan and Vallee 1995; Vaughan et al. 1999), anti-p58 Golgi protein (Bloom and Brashear 1989), and CENP-E (Lombillo et al. 1995). Fluorescein (FITC) and cyanine (cy3)-conjugated IgGs were obtained from Jackson ImmunoResearch Laboratories, Inc. HRP-conjugated IgGs were from Nycomed Amersham Inc. Antibodies were used alone or in combination as described in the text.
Immunofluorescence Microscopy and Quantification of Protein and DNA
Immunofluorescence microscopy was performed essentially as described (Doxsey et al. 1994; Dictenberg et al. 1998). Unless otherwise stated, COS-7 cells expressing HA-Pc, β-galactosidase, pHAI, or mock transfected were fixed in 100% methanol at −20°C. Where indicated, cells were detergent-extracted to remove cytoplasmic protein before fixation (0.5% TX-100 in 80 mM Pipes, pH 6.8, 5 mM EGTA, 1 mM MgCl2, for 1 min). In most cases, monoclonal or polyclonal HA antibody was detected with FITC-labeled secondary antibody, and antibodies used in colabeling experiments were detected with cy3 secondary antibodies. In all cases, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) to detect chromatin. Cells were observed using an Axiophot fluorescence microscope with a 100× objective (Carl Zeiss Inc.).
Quantification of centrosomal staining in mitotic cells and DNA (DAPI) was performed as described (Dictenberg et al. 1998). In brief, the total fluorescence from centrosomes and nuclei in individual cells was determined. Background values from three positions in the cytoplasm and camera noise (dark current) were subtracted (<10% of total). For centrosome staining, fluorescence signals were obtained from only one centrosome per mitotic cell, to avoid photobleaching. Cells with low, intermediate, and high expression levels were included in all analyses.
For coexpression studies (see Fig. 8), HA-Pc and dynein, or dynactin cDNAs were cotransfected into COS-7 cells and processed 38–46 h later. Cells were washed in PBS, lysed in modified RIPA buffer at 4°C for 20 min (150 mM NaCl, 50 mM Tris, pH 8.0, 1 mM EGTA, 1% IGEPAL) with leupeptin, aprotinin, and AEBSF (Boehringer Mannheim Corp.), and precleared. Monoclonal anti-HA bound to protein G beads (Pharmacia Biotech) was added to lysates at 4°C for 12 h, and beads were collected and washed five times with modified RIPA buffer. Proteins were exposed to SDS-PAGE and transferred to PVDF membranes (Millipore Corp.). The presence of dynein/dynactin subunits was assayed by Western blot with anti-myc, anti-p50, and anti-p150 antibodies.
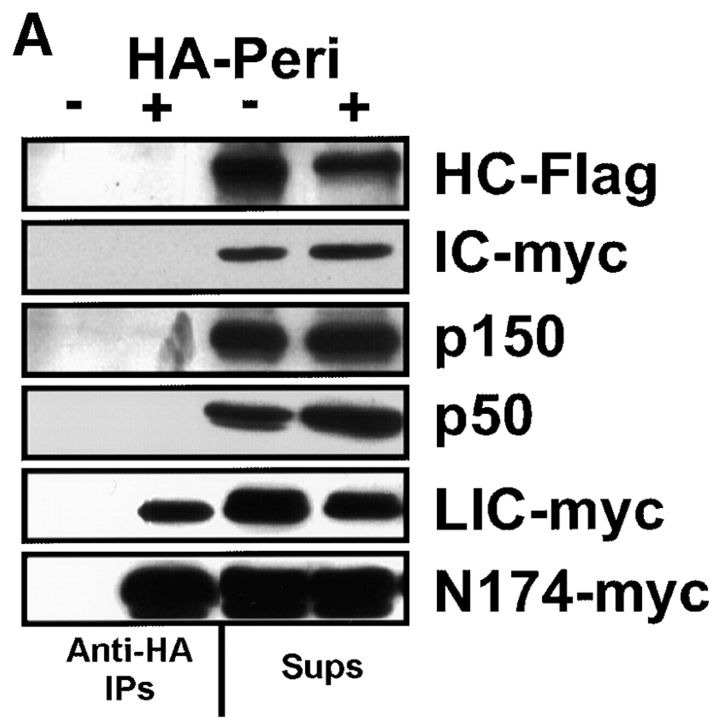
Figure 8.
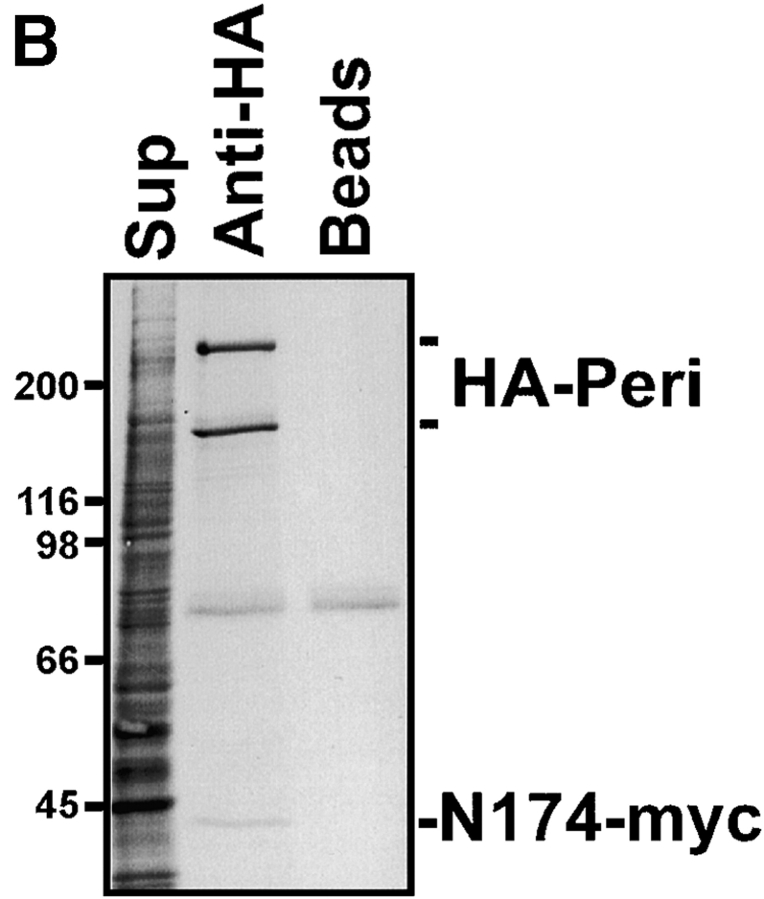
HA-Pc interacts directly with the light intermediate chain of dynein. A, Dynein and dynactin components were expressed alone or together with HA-Pc in COS-7 cells (+ or −). Cells were lysed, HA-Pc was immunoprecipitated, and blots were probed with myc, Flag, or dynactin antibodies, as indicated on right. LIC (LIC-myc) and a COOH-terminal fragment of LIC (N174-myc) coprecipitated with HA-Pc, whereas other dynein components, DHC (HC-Flag), DIC (IC-myc), and dynactin components (p50 and p150glued) did not coprecipitate. Supernatants (Sups) from immunoprecipitations are shown on right. HA-Pc expression was similar in all samples, as confirmed by Coomassie blue staining (data not shown). B, [35S]methionine-labeled COS-7 cells coexpressing HA-Pc and N174-myc were used for immunoprecipitation with anti-HA antibodies or no antibody (beads). HA-Pc (HA-Peri) and N174-myc coprecipitated specifically with HA antibodies. The identity of N174 was confirmed by immunoblotting (data not shown). The ∼60-kD band represents a nonspecific protein that precipitates with HA antibodies in COS-7 cells.
35S-Labeling of Cells
COS-7 cells were transferred to methionine- and serum-deficient DME (GIBCO BRL) containing 50–100 uCi of [35S]methionine (New England Nuclear). They were labeled for 4 (see Fig. 8 B) or 18 h (see Fig. 7 B), washed in PBS, and lysed in 50 mM Tris, pH 7.5, 137 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 4 μg/ml aprotinin, 4 μg/ml leupeptin, 4 μg/ml antipain, 12.5 μg/ml chymostatin, 5 mM iodoacetamide, 130 μg/ml caproic acid, 12 μg/ml pepstatin, 200 μg/ml P-amino-benzamidine, and 1 mg/ml BSA. Protein G beads preblocked with COS-7 cell extract made from untransfected, unlabeled cells (4 h at 4°C), were added to precleared 35S-labeled extracts with primary antibody, and immunoprecipitates were processed as described above. Dried gels (see Fig. 7) or membranes (see Fig. 8) were exposed to X-OMAT film (Kodak) for 24–48 h.
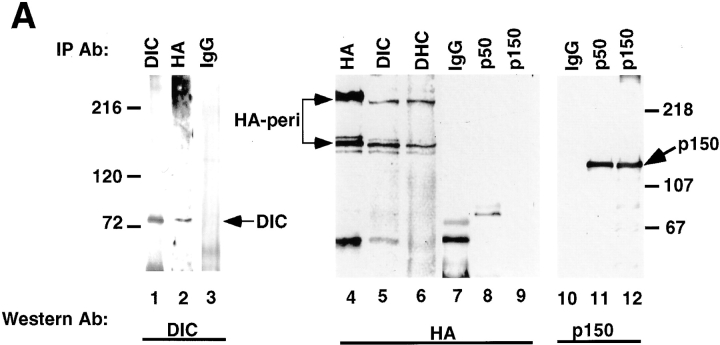
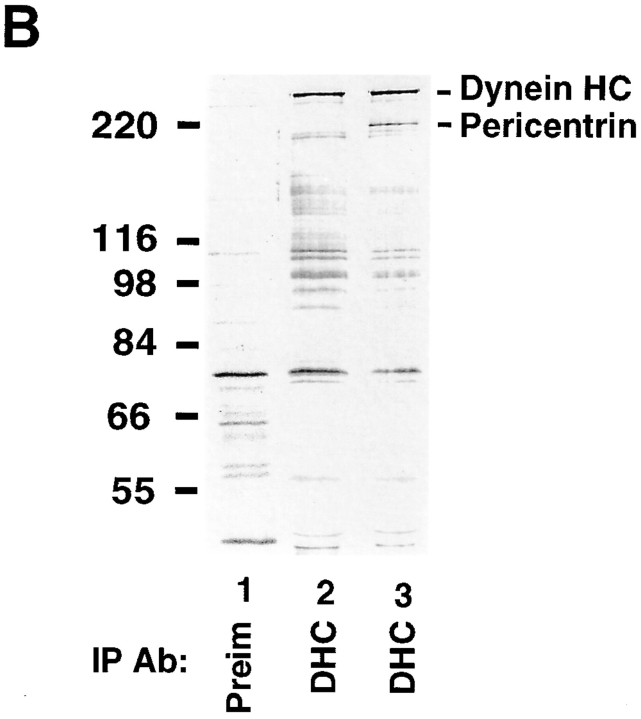
Figure 7.
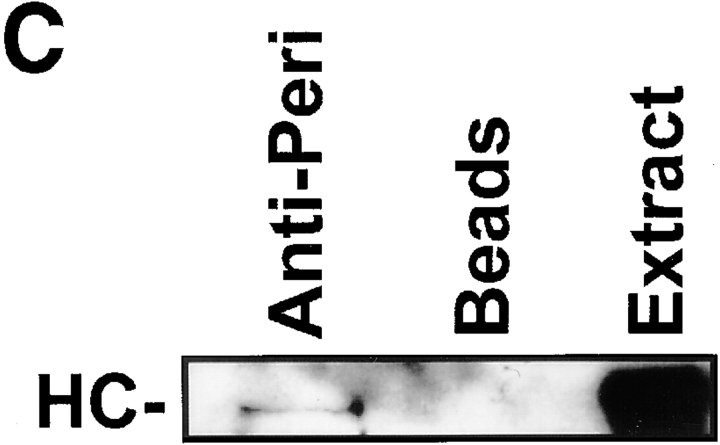
Pericentrin and cytoplasmic dynein coimmunoprecipitate. A, Detergent lysates of HA-Pc–expressing COS-7 cells were used for immunoprecipitations with antibodies to DIC, DHC (polyclonal anti-rat heavy chain1, Mikami, A., and R. Vallee, unpublished results), p50, p150, HA, or nonspecific IgG, as indicated. Immunoprecipitates were immunoblotted for DIC (lanes 1–3), HA (lanes 4–9), or p150 (lanes 10–12) as indicated. Dynein coimmunoprecipitates with HA-Pc (lane 2), but not with control IgG (lane 3). Conversely, HA-Pc coimmunoprecipitates with dynein components (lanes 5 and 6), but not with control IgG or dynactin components (lanes 7–9). HA-Pc was partially degraded in some cases (lower band in lanes 4–6), but not others (see Fig. 1 A and 7 B). An ∼60-kD band is nonspecifically precipitated by IgG (lanes 4, 5, and 7). B, [35S]methionine-labeled cells expressing HA-Pc (lanes 1 and 3) or mock transfected (lane 2) immunoprecipitated with antibodies to DHC or preimmune sera (Preim) as indicated. HA-Pc is only detected in HA-Pc–expressing cells after DHC immunoprecipitation (lane 3). C, Lysates from nontransfected control cells were used for immunoprecipitation with antipericentrin antibodies (lane 1) or no antibody (beads, lane 2), and proteins were immunoblotted with anti-DHC antibody. Molecular mass markers are indicated (in kD × 10−3).
Microtubule Nucleation
COS-7 cells expressing HA-Pc or mock transfected were treated with nocodazole (10 μg/ml) for 1 h at 37°C to depolymerize microtubules. After removal of the drug, cells were incubated for 3 min to allow microtubules to regrow, then fixed in methanol and stained with α tubulin to reveal nucleated microtubules, as described previously (Brown et al. 1996; Dictenberg et al. 1998).
Results
Previously, we demonstrated that functional abrogation of pericentrin disrupts centrosome and spindle organization in several systems (Doxsey et al. 1994). Based on these observations, we reasoned that an artificial elevation of pericentrin levels would provide additional information on protein function and interaction. To this end, we constructed and expressed an HA-Pc in COS-7 cells, and examined centrosome and spindle composition and function.
As expected, HA-Pc had an electrophoretic mobility of ∼220 kD and was found in both Triton X-100 soluble and insoluble fractions (Fig. 1 A). Immunofluorescence analysis demonstrated that the more abundant detergent soluble fraction was distributed throughout the cytoplasm (Fig. 1 D, inset), whereas the detergent insoluble fraction colocalized with γ tubulin at centrosomes (Fig. 1B and Fig. C). Centrosome localization of HA-Pc was unaltered when microtubules were depolymerized, suggesting that the protein was an integral component of centrosomes and not simply bound there by microtubules (data not shown).
Figure 1.
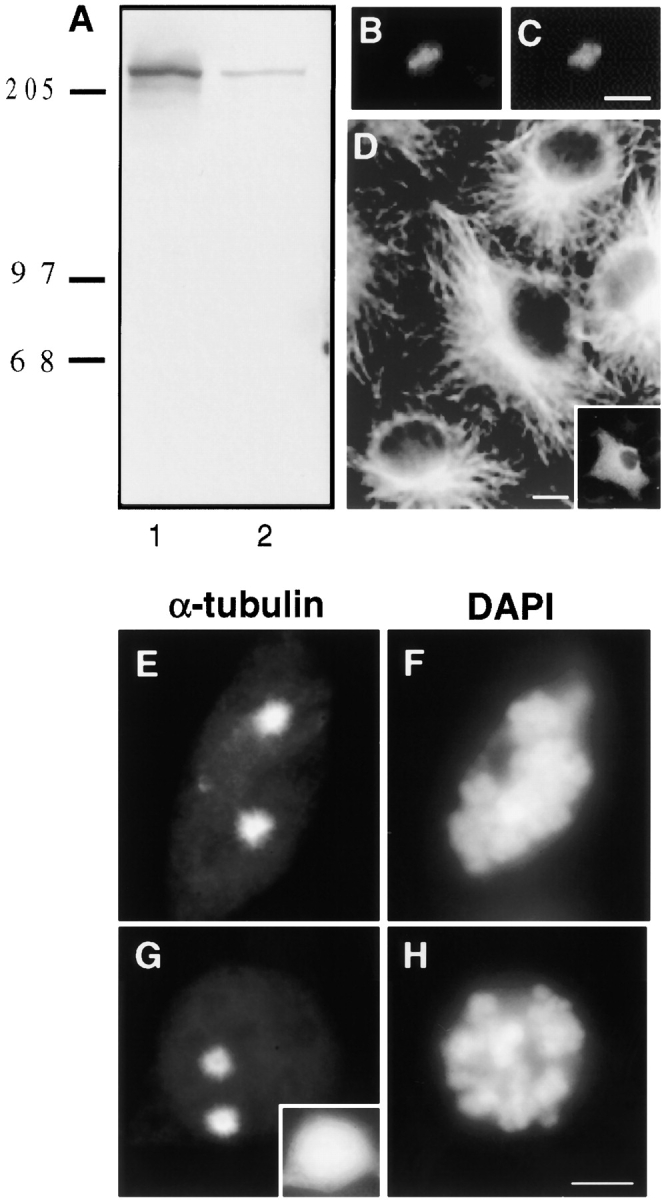
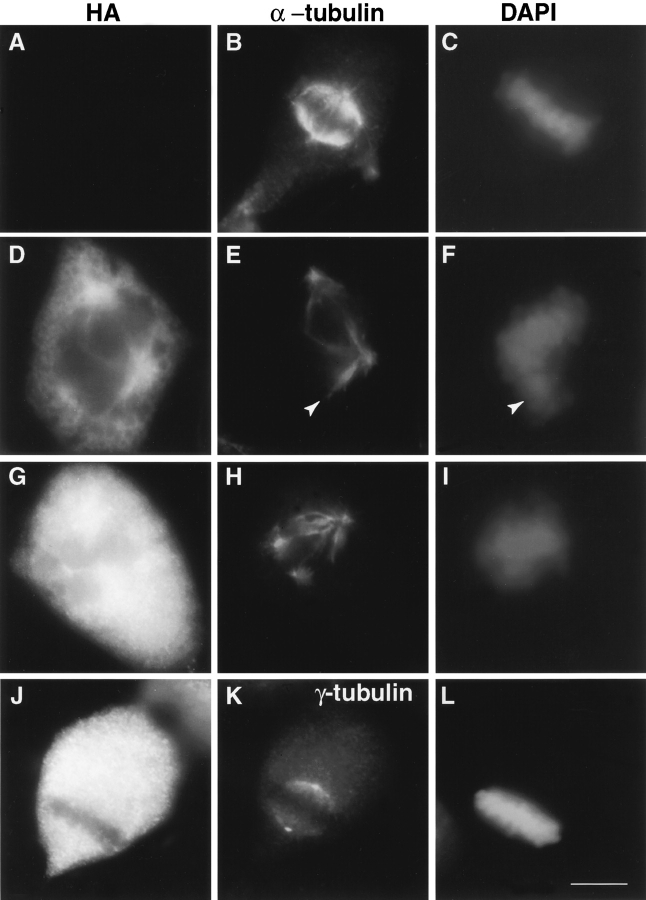
HA-Pc overexpression has no detectable effect on microtubule nucleation or organization. A, Triton X-100 soluble (lane 1) and insoluble fractions (lane 2) of HA-Pc–expressing COS-7 cells immunoblotted with anti-HA antibodies. Detergent extracted COS-7 cell showing centrosome-associated HA-Pc (B), which colocalizes with γ tubulin (C). Microtubule organization in a pericentrin-expressing interphase cell (D, inset) is similar to surrounding control cells. The extent of microtubule regrowth from prometaphase centrosomes after nocodazole-induced depolymerization is similar in an HA-Pc–expressing cell (G and H) and a control cell (E and F). Inset in G, HA stain. DAPI staining shows prometaphase chromosomes (F and H). Note that individual microtubules are not easily observed (E and G) after short periods of microtubule regrowth. Bars: (C, for B and C) 1 μm; (D) 5 μm; (H, for E–H) 10 μm.
Mitotic Spindles Are Structurally and Functionally Disrupted in Pericentrin-overexpressing Cells
The organization of microtubules in interphase HA-Pc expressing cells was indistinguishable from control cells (Fig. 1 D). Moreover, there was no detectable difference in microtubule nucleation from centrosomes (Fig. 1, E–H). The most dramatic consequence of HA-Pc expression was disruption of mitotic spindle organization (Fig. 2 and Fig. 3). A significant fraction of mitotic COS-7 cells at all expression levels exhibited spindle defects (75.7 ± 6.1%, n = 423), compared with nontransfected cells (2.5 ± 1.5%, n = 598) and vector DNA transfected cells (3.0 ± 1.0, n = 201). Three categories of spindle defects were observed. Spindles with structural defects were detected in 36.2% of transfected cells and included multipolar, monopolar, and distorted spindles (Fig. 2, D–I, also see Fig. 5L, Fig. M, Fig. Q, and Fig. R). Mispositioned spindles were observed in 22.0% of the cells, and were often positioned far from the cell center (Fig. 2, J–L). Spindles with misaligned, missegregated, and mono-oriented chromosomes were commonly observed (42.5%; Fig. 3, also see Fig. 5L, Fig. M, Fig. Q, and Fig. R). Spindle defects occurred alone or in combination.
Figure 2.
Mitotic spindle organization and positioning is impaired in HA-expressing cells. Immunofluorescence staining of microtubules (or γ tubulin; K) in nontransfected (A–C) and HA-Pc–overexpressing COS-7 cells (D–L). HA-Pc–expressing cell with a spindle elongated in the pole to pole dimension (D–F) and a subset of chromosomes misaligned on the metaphase plate (arrowheads). A spindle with multiple poles is seen in G–I (also see Fig. 5L, Fig. M, Fig. Q, and Fig. R). A mispositioned spindle located adjacent to the plasma membrane is shown in J–L. Spindles were considered mispositioned if the metaphase chromosomes did not contact the intersection of two lines drawn in the cell at its shortest and longest dimensions. Horizontal series are of the same cell. Bar, 10 μm.
Figure 3.
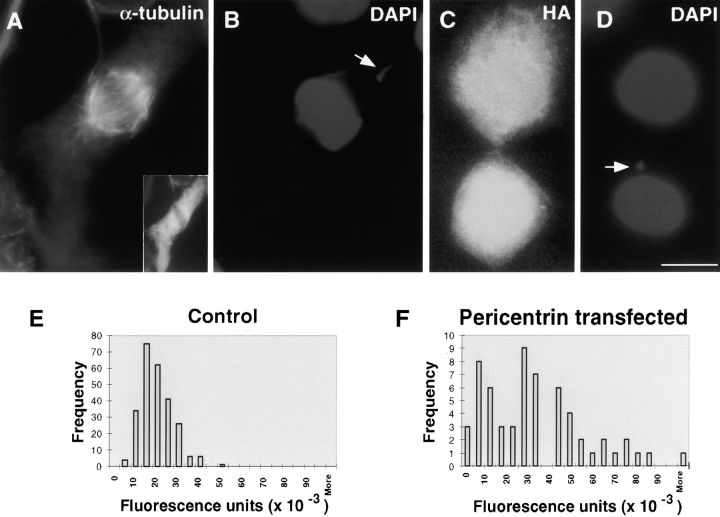
Chromosomes in pericentrin-overexpressing cells are misaligned and missegregated, creating aneuploidy. A pericentrin-overexpressing COS-7 cell (A) with a chromosome that is not aligned on the metaphase plate (B, arrow). Note metaphase DNA overexposed to highlight misaligned chromosome. Inset in A, HA staining. A late telophase cell (C) with chromosome(s) excluded from a reforming nucleus (D, arrow). Quantification of DAPI-stained chromatin in nuclei of pericentrin-overexpressing cells (F) reveals significant variability in DNA content, compared with control cell nuclei (E). Bar, 10 μm.
Figure 5.
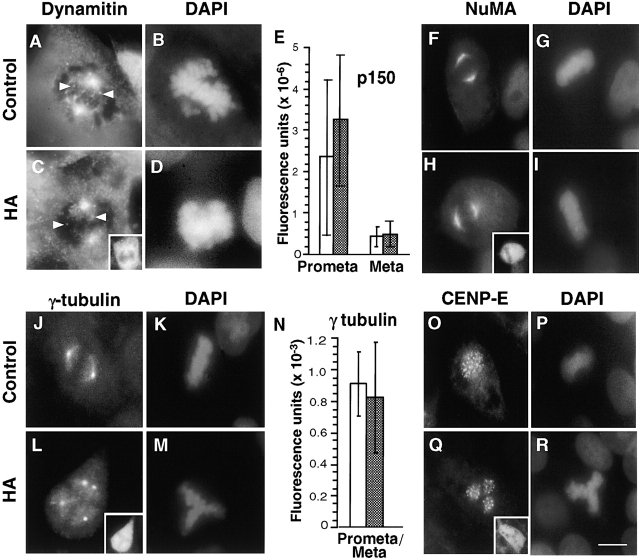
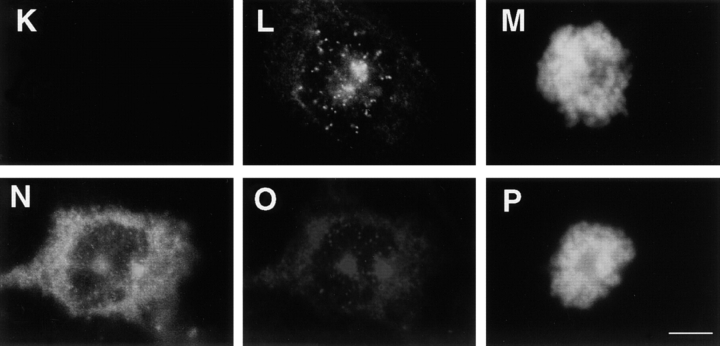
Localization of several centrosome and kinetochore proteins are unaltered in pericentrin-expressing cells. HA-expressing COS-7 cells were immunolabeled for dynactin subunits (dynamitin, A–D; p150glued, E) or proteins involved in spindle pole integrity (NuMA, F–I), microtubule nucleation (γ tubulin, J–N), and kinetochore function (CENP-E, O–R). The distribution and levels of these proteins in HA-Pc–expressing cells (HA panels, white bars) did not appear to be significantly different from nonexpressing control cells (control panels, filled bars). HA stained cells shown in insets in C, H, L, and Q. Horizontal series are of the same cell. Bar, 10 μm. In E and N the fluorescence intensity of individual centrosomes/spindle poles is shown (n > 40 centrosomes/bar). Cells with low, intermediate, and high expression levels were included in the analysis.
Despite the presence of improperly attached chromosomes, HA-Pc cells progressed through mitosis and were frequently observed in later stages of mitosis with missegregated chromosomes (Fig. 3C and Fig. D). The percentage of mitotic figures in the population of HA-Pc–expressing cells (3.1 ± 0.9%, n = 3,490) was not significantly different from control cells transfected with other constructs or mock transfected cells (2.9 ± 1.0 to 4.4 ± 2.1%, n = 5,002), and the cell viability and growth rate appeared unchanged. Nuclei exhibited a remarkably wide variation in DNA content. Values ranged from zero to five times those of controls (Fig. 3E and Fig. F), demonstrating that the cells were becoming aneuploid. From this analysis, we conclude that pericentrin overexpression causes multiple mitotic spindle defects leading to chromosome missegregation and aneuploidy.
Cytoplasmic Dynein Is Dissociated from Multiple Cellular Sites in HA-Pc–expressing Cells
The spindle defects in pericentrin-overexpressing cells were similar to those previously observed in cells overexpressing the dynamitin subunit of dynactin (Echeverri et al. 1996; Burkhardt et al. 1997). Dynactin is a protein complex which regulates the function of cytoplasmic dynein, a minus end microtubule motor protein involved in numerous physiological processes (reviewed in Vallee and Sheetz 1996; Karki and Holzbaur 1999). Dynein and dynactin have been localized to prometaphase kinetochores, centrosomes, spindle poles, and the plasma membrane (Pfarr et al. 1990; Steuer et al. 1990; Clark and Meyer 1992; Echeverri et al. 1996; Busson et al. 1998). Overexpression of dynamitin disrupts the dynactin complex, releases cytoplasmic dynein from mitotic kinetochores, disrupts mitosis, and alters the distribution of membranous organelles, including the Golgi complex (Echeverri et al. 1996; Burkhardt et al. 1997).
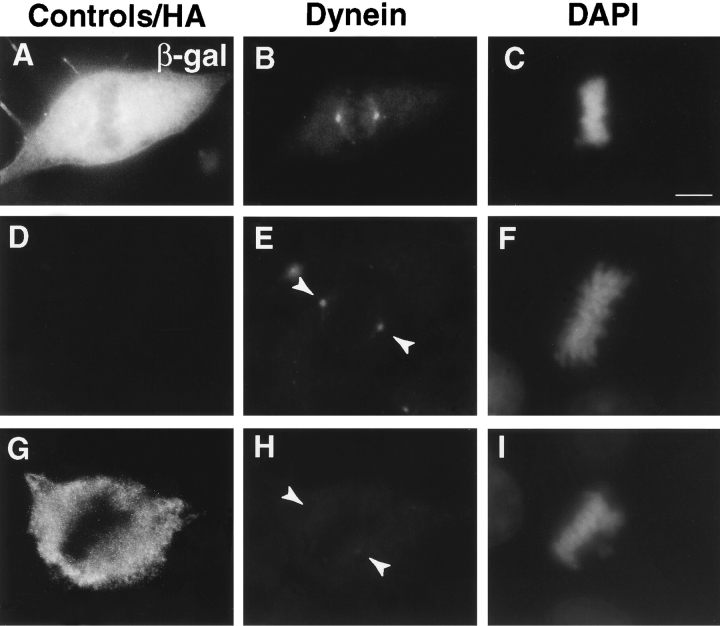
To test the possibility that cytoplasmic dynein or dynactin contributed to the pericentrin overexpression phenotype, we examined the distribution of these protein complexes in pericentrin-overexpressing cells. The level of cytoplasmic dynein immunoreactivity in mitotic cells was dramatically reduced at spindle poles (8–12-fold; Fig. 4, G–J). The motor appeared to be specifically displaced from spindle poles and not simply masked from antibody access for several reasons. First, diminished dynein staining was detected with two independent antibody preparations raised against the DIC (L5, polyclonal and 74.1, monoclonal). Second, control cells expressing β-galactosidase (Fig. 4, A–C) or untransfected cells (Fig. 4, D–F) had normal levels of dynein at their poles (Fig. 4 J). Third, there was no detectable change in the distribution and abundance of several other centrosome and spindle pole components. The centrosome localization and levels of the dynactin subunits, dynamitin (Fig. 5, A–D) and p150glued, did not appear to be altered, although there was some variability in p150glued levels in prometaphase (Fig. 5 E). There was no apparent change in the level of γ tubulin at individual spindle poles, even in cells with multiple poles (Fig. 5, J–N). This suggests that multipolar spindles have normal centrosomes at their poles, each with the appropriate amount of γ tubulin (see Discussion). The spindle pole protein, NuMA, also appeared to be localized normally to poles of mitotic spindles (Fig. 5, F–I).
Figure 4.
Dynein immunofluorescence is reduced at spindle poles in mitotic HA-Pc–expressing cells. HA-Pc–expressing metaphase cells stained with antidynein antibody (74.1) show significantly reduced levels of dynein immunofluorescence at spindle poles (G–I), compared with β-galactosidase–expressing control cells (A–C) or nontransfected control cells (D–F). Quantification of dynein immunofluorescence at spindle poles (J). Open bars, mock (vector) transfected cells; filled bars, pericentrin-transfected cells. Each bar represents an average value obtained from at least 65 cells. The dynein level on kinetochores is reduced in a prometaphase HA-Pc–expressing cell (N–P), compared with a nontransfected control cell (K–M). Horizontal series are of the same cell. Bars, 10 μm (bar in C for A–C, bar in P for D–P). Images in all panels were exposed and processed similarly.
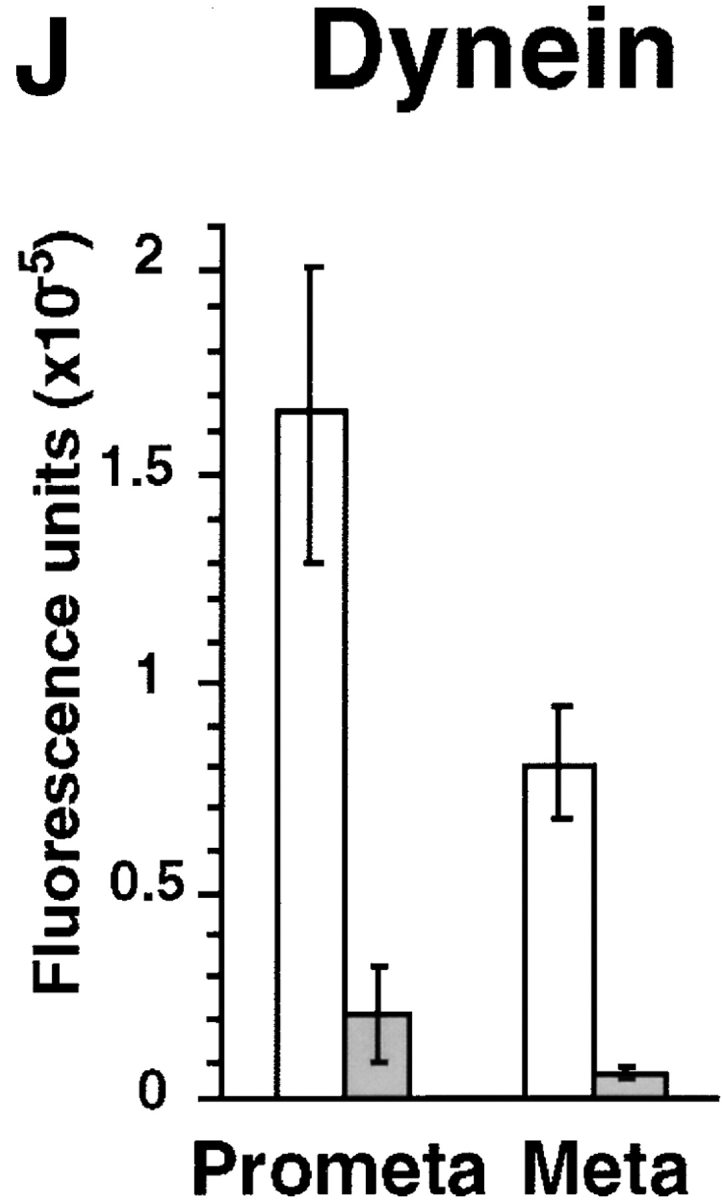
Cytoplasmic dynein was also dramatically reduced at kinetochores (Fig. 4, K–P). In contrast, kinetochore localization and levels of dynactin (Fig. 5, A–D, arrowheads; and data not shown) and the kinesin-related protein, CENP-E (Fig. 5, O–R; Yen et al. 1992; Lombillo et al. 1995), both appeared unchanged.
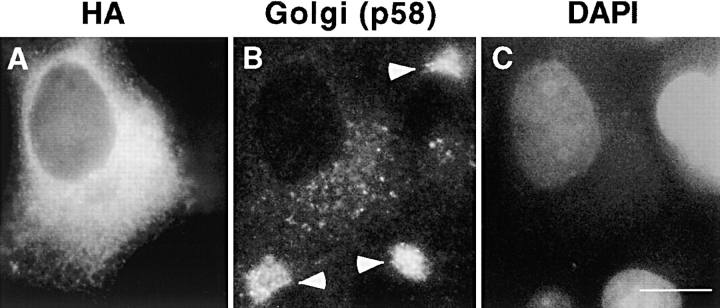
Consistent with defects in the Golgi complex induced by overexpression of the dynamitin subunit of dynactin (Burkhardt et al. 1997), HA-Pc overexpression caused dispersal of Golgi elements. This was observed by immunostaining with antibodies to the Golgi protein, p58 (Bloom and Brashear 1989; Fig. 6; 77 ± 3.3%, n = 251). In adjacent nontransfected control cells, Golgi complexes had the characteristic tightly focused appearance and were found in the perinuclear region of the cells (Fig. 6 B, arrowheads; 95.6%, n = 497). Disruption of the Golgi complex was also observed using a green fluorescent protein (GFP)-tagged N-acetylglucosamine transferase in cotransfection experiments with pericentrin (data not shown). Golgi complex dispersal did not appear to result from impaired microtubule integrity, as no detectable changes in the microtubule network were observed (see Fig. 1 D).
Figure 6.
Golgi complexes are disrupted in pericentrin-overexpressing cells. An HA-Pc COS-7 cell (A) showing dispersal of the Golgi complex as revealed by staining with anti-p58 antibodies (B, center). In adjacent nontransfected cells, the Golgi complexes are well organized (B, arrowheads) and found in the typical juxtanuclear region (C, DAPI). Bar, 10 μm.
Pericentrin Interacts Directly with Cytoplasmic Dynein through the Light Intermediate Chain
The loss of cytoplasmic dynein from spindle poles and kinetochores, and the abrogation of cellular functions mediated by dynein (spindle positioning, Golgi complex organization) suggested that overexpressed pericentrin sequestered the motor in the cytoplasm. This was tested directly by coimmunoprecipitation assays. Antibodies to both DIC and DHC precipitated HA-Pc (Fig. 7 A, lanes 5 and 6), whereas a control IgG preparation did not (Fig. 7 A, lane 7). Conversely, antibodies to HA, but not to control IgGs, precipitated DIC (Fig. 7 A, lanes 1–3). Under the same conditions, antibodies to dynactin components (dynamitin and p150glued) did not precipitate detectable amounts of HA-Pc (Fig. 7 A, lanes 8 and 9), although they immunoprecipitated other proteins of the dynactin complex (Fig. 7 A, lanes 11, 12). In cells metabolically labeled with [35S]methionine, HA-Pc was specifically immunoprecipitated with antibodies to DHC, but not to preimmune sera (Fig. 7 B). Moreover, despite very low levels of endogenous pericentrin in nontransfected control cells (Doxsey et al. 1994), we were able to specifically detect DHC after immunoprecipitation of pericentrin from lysates prepared from large numbers of cells (Fig. 7 C). These results suggest that overexpressed pericentrin binds to and sequesters dynein in the cytoplasm, and prevents it from associating with its cellular targets.
To determine whether the dynein–pericentrin interaction was direct or indirect, we cotransfected cells with HA-Pc and individual dynein and dynactin subunits, and performed a series of immunoprecipitation and immunoblot analyses. Immunoprecipitation of HA-pericentrin failed to pull down the DHCs and DICs or the dynactin subunits p150Glued and dynamitin (Fig. 8 A). However, a myc-tagged rat cytoplasmic dynein light intermediate chain (Hughes, S., A. Purohit, S. Doxsey, and R. Vallee, manuscript in preparation) and its COOH-terminal fragment N174 clearly coimmunoprecipitated with HA-pericentrin (Fig. 8 A). When cells cotransfected with the LIC N174 fragment and HA-Pc were labeled with [35S]methionine, the only bands specifically immunoprecipitated with anti-HA antibodies were HA-Pc and N174 (Fig. 8 B). Dynactin did not appear to be required for the pericentrin–LIC interaction since overexpression of dynamitin had no effect on the ability of the proteins to coimmunoprecipitate (data not shown). These results provide strong evidence for a direct interaction between HA-Pc and the light intermediate chain of cytoplasmic dynein.
Discussion
We have found that pericentrin overexpression has profound effects on the organization, positioning, and function of mitotic spindles, and on the organization of the Golgi complex. Several studies show that cytoplasmic dynein is involved in processes affected by pericentrin overexpression (for reviews see Holzbaur and Vallee 1994; Vallee and Sheetz 1996; Karki and Holzbaur 1999). Consistent with a role for cytoplasmic dynein in mediating the pericentrin overexpression phenotype is the reduction of dynein staining intensity at the prometaphase kinetochore and the centrosome/spindle pole. Our immunoprecipitation data further support an interaction between pericentrin and cytoplasmic dynein. Our data indicate that the interaction is direct and specifically mediated by the light intermediate chains of the motor protein complex. Thus, this study provides the first evidence for a dynein–pericentrin interaction, and identifies the first functional role for LICs.
Mechanism of Dynein–Pericentrin Interaction
The function of the light intermediate chains has been obscure. They have only been identified in cytoplasmic forms of dynein and contain well-conserved P-loop elements of unknown function near their NH2 termini (Paschal et al. 1987; Gill et al. 1994; Hughes et al. 1995). Previous studies have implicated a different class of dynein subunit, the intermediate chains, in subcellular targeting. The intermediate chains reside at the base of the dynein complex and interact with the p150Glued subunit of the dynactin complex (Karki and Holzbaur 1995; Vaughan and Vallee 1995). Dissociation of the dynactin complex by dynamitin overexpression was found to release dynein from prometaphase kinetochores. Together, these data supported a role for dynactin in anchoring dynein to at least one form of subcellular cargo through the intermediate chains (Echeverri et al. 1996). This mechanism has received further support from evidence that mutations in zw10, a dynactin-anchoring kinetochore component, also release dynein from the kinetochore (Starr et al. 1998).
The current studies identify an additional and previously unsuspected mechanism for linking dynein to its cargo. The presence of cytoplasmic dynein, but not dynactin, in pericentrin immunoprecipitates, strongly suggests that dynactin is not necessary for the pericentrin/dynein interaction. Coexpression of recombinant dynein and dynactin subunits with pericentrin reveal a direct interaction with the light intermediate chains, further supporting a dynactin-independent mechanism. Thus, these results identify the light intermediate chains as an additional class of dynein-anchoring or -targeting subunit. Whether these polypeptides serve in a subset of dynein-mediated processes, such as interactions with soluble protein complexes versus membranous organelles or kinetochores, remains to be determined.
Whether light intermediate chain-mediated dynein interactions are completely independent of dynactin also remains to be resolved. Examination of the behavior of GFP-pericentrin in living cells has revealed clear centripetal transport of pericentrin-containing particles to the centrosome (Young, A., R. Tuft, J. Dictenberg, A. Purohit, and S. Doxsey, manuscript submitted for publication). This behavior is correlated with a cell cycle-dependent accumulation of pericentrin and γ tubulin at the centrosome, which is strongly inhibited by nocodazole, antibody to cytoplasmic DIC, or overexpressed dynamitin. These data, together with the identification of a pericentrin–dynein interaction (this study), demonstrates that recruitment of pericentrin and γ tubulin to centrosomes involves dynein-mediated transport. Since pericentrin previously has been shown to interact with the γ tubulin complex (Dictenberg et al. 1998), and more recently with protein kinase A (Diviani, D., L. Langeberg, A. Purohit, A. Young, S. Doxsey, and J. Scott, manuscript submitted for publication), we currently believe that pericentrin functions as a molecular scaffold that transports important activities to the centrosome and anchors them at this site.
The ability of dynamitin overexpression to inhibit centrosome protein recruitment suggests a role for dynactin in pericentrin-mediated transport, despite the lack of evidence in the current study for a role for dynactin in the dynein–pericentrin interaction. It is conceivable that dynactin disruption affects pericentrin accumulation via a mechanism unrelated to direct pericentrin transport, such as the disruption of the microtubule cytoskeleton. Alternatively, dynactin could regulate dynein-mediated pericentrin motility independent of a role in linking pericentrin to dynein. Such a model contrasts with an obligatory role for dynactin in the attachment of dynein to kinetochores (Echeverri et al. 1996), but is consistent with our current evidence for an involvement of alternative dynein targeting mechanisms in different cellular processes. Finally, it is possible that pericentrin interacts with dynein by a bivalent mechanism involving both the light intermediate chains and dynactin, but that the latter interaction is poorly preserved in vitro.
Molecular Basis for the Pericentrin Overexpression Phenotype
Our data support a cytoplasmic dynein sequestration model to explain the effects of pericentrin overexpression. Dynein is removed from at least two of the sites where it is normally found, the kinetochore and the spindle pole (Fig. 4). The association of dynein with membranous structures is more difficult to assess because of the profusion of such structures in the cytoplasm, but the dispersal of the Golgi apparatus that we observe is strongly consistent with a loss of dynein from this organelle as well. Thus, we imagine that soluble pericentrin binds to the light intermediate chains and interferes with normal dynein targeting interactions. Interference of light intermediate chain localization or function by overexpressed pericentrin could result from competition with other light intermediate chain interactions in the cell. Alternatively, it could be due to steric interference by overexpressed pericentrin with the intermediate chain/dynactin interaction. Mapping studies have, in fact, shown the binding sites for the intermediate and light intermediate chains to be in close proximity within the DHCs (Tynan, S., and R. Vallee, unpublished results). Further work will be required to identify the full range of light intermediate chain functions.
One distinction between the pericentrin and dynamitin overexpression effects is that there is no detectable change in the mitotic index of pericentrin-overexpressing cells. This result is puzzling in view of the similarity in mitotic defects observed in the two cases, including the production of multipolar mitotic spindles. The latter structures are suggestive of mitotic failure (i.e., cytokinesis failure) which typically occurs after a delay in mitosis. Although pericentrin-overexpressing cells do not exhibit a mitotic delay, they appear to grow and divide normally. This suggests a defect in the checkpoint that regulates the transition from metaphase to anaphase (Rudner and Murray 1996), an idea we are currently testing.
Pericentrin previously has been shown to be part of a large protein complex that includes γ tubulin (Dictenberg et al. 1998). Thus, it is possible that disruption of γ tubulin in pericentrin-overexpressing cells contributes to the spindle defects. However, we believe this is unlikely because recruitment of γ tubulin to spindle poles is not noticeably different than in control cells. Moreover, the ability of individual mitotic spindle poles to nucleate microtubules, a function thought to be mediated by γ tubulin, appears unchanged in pericentrin-overexpressing cells. Some pericentrin-overexpressing cells have multiple γ tubulin staining structures that seem to contribute to the formation of multipolar spindles. Since each of the multiple poles has approximately the same amount of γ tubulin as normal spindle poles, we believe that they represent bona fide centrosomes (with centrioles). We are currently investigating how these multiple foci of γ tubulin are generated and whether they contribute to aneuploidy in pericentrin-overexpressing cells.
It is unclear why recruitment of γ tubulin and NuMA to spindle poles appear to be unaffected by the HA-Pc–induced dynein disruption since the evidence suggests that both proteins may also interact with, or be under the control of, cytoplasmic dynein. One possibility is that cell cycle variability in the localization and levels of these proteins (Compton and Cleveland 1993; Dictenberg et al. 1998), together with variability in the level of pericentrin overexpression, make it difficult to detect significant differences. Another possibility is that the proposed HA-Pc–induced sequestration of dynein may be less than complete, allowing some dynein-mediated transport to occur. This may be sufficient to localize the spindle pole proteins examined in this study, but insufficient to maintain Golgi complex organization or localize dynein to spindle poles and kinetochores. Alternatively, dynein may interact with many different cargoes (e.g. vesicles, protein complexes) whose localization is differentially affected by pericentrin overexpression. This could explain why NuMA and dynactin, which form a discrete complex with dynein in Xenopus extracts (Merdes et al. 1996), appear to accumulate to normal levels at spindle poles.
A final interesting feature of the pericentrin-overexpressing phenotype is the generation of aneuploid cells. In fact, pericentrin-overexpressing cells have chromatin levels both below and above diploid, suggesting that they undergo persistent chromosome missegregation as described (Lengauer et al. 1997). Since little is known about how aneuploid cells are generated, this cell system provides a powerful model to study this phenomenon. This system may also prove useful in understanding human tumorigenesis since pericentrin levels are elevated in most aneuploid tumors (Doxsey 1998; Pihan et al. 1998; Pihan, G., and S. Doxsey, unpublished observations). For these reasons, it is important to determine the precise contributions of dynein and other pericentrin-interacting molecules in the generation of aneuploidy and spindle defects in pericentrin-overexpressing cells.
Acknowledgments
We thank the following individuals for reagents: T. Yen (Fox Chase Cancer Center, Philadelphia, PA) for antibodies to CENP-E, C. Sparks (University of Massachusetts, Worcester, MA) for antibodies to NuMA, J. Buxton (University of Massachusetts Medical School, Worcester, MA) for polyclonal anti-HA antibodies, G. Bloom (University of Texas, Southwestern Medical Center, TX) for anti-p58 Golgi antibodies, D. Asai (Purdue University, IN) for antibodies to DHC, K. Pfister (University of Virginia, VA) for antibodies to DIC (74.1) and David Shima for GFP-NAGT cDNA. For useful discussions and critical reading of the manuscript, we thank Y.-L. Wang, G. Sluder, and W. Theurkauf.
S.J. Doxsey is the recipient of an Established Investigator award from the American Heart Association. This work was supported by funding from the National Institutes of Health (GM51994 to S.J. Doxsey and GM47434 to R. Vallee).
Footnotes
1.used in this paper: DAPI, 4′,6-diamidino-2-phenylindole; DHC, dynein heavy chain; DIC, dynein intermediate chain; GFP, green fluorescent protein; HA, hemagglutinin; HA-Pc, hemagglutinin-tagged pericentrin; LIC, dynein light intermediate chain; NuMA, nuclear mitotic apparatus protein
Reprint requests can be made to either S.J. Doxsey or R.B. Vallee.
References
- Asai D.J., Beckwith K.A., Kandl H.H., Keating H., Tjandra H., Forney J.D. The dynein genes of Paramecium tetraureliasequences adjacent to the catalytic P-loop identify cytoplasmic and axonemal heavy chain isoforms. J. Cell Sci. 1994;107:839–847. doi: 10.1242/jcs.107.4.839. [DOI] [PubMed] [Google Scholar]
- Avides M., Glover D.M. Abnormal spindle protein, ASP, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 1999;283:1733–1735. doi: 10.1126/science.283.5408.1733. [DOI] [PubMed] [Google Scholar]
- Bloom G.S., Brashear T.A. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J. Biol. Chem. 1989;264:16083–16092. [PubMed] [Google Scholar]
- Brown R., Doxsey S.J., Hong-Brown L., Martin R.L., Welsh W. Molecular chaperones and the centrosomea role for TCP-1 in microtubule nucleation. J. Biol. Chem. 1996;271:824–832. doi: 10.1074/jbc.271.2.824. [DOI] [PubMed] [Google Scholar]
- Burkhardt J.K., Echeverri C.J., Nilsson T., Vallee R.B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson S., Dujardin D., Moreau A., Dompierre J., De Mey J.R. Dynein and dynactin are localized to astral microtubules at cortical sites in mitotic epithelial cells. Curr. Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- Clark S.W., Meyer D.I. Centracin is an actin homologue associated with the centrosome. Nature. 1992;359:246–250. doi: 10.1038/359246a0. [DOI] [PubMed] [Google Scholar]
- Compton D.A., Cleveland D.W. NuMA is required for the proper completion of mitosis. J. Cell Biol. 1993;120:947–957. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg J., Zimmerman W., Sparks C., Young A., Carrington W., Zheng Y., Vidair C., Fay F., Doxsey S.J. Pericentrin and gamma tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman J.F., Pfister K. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J. Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S.J. The centrosomea tiny organelle with big potential. Nat. Genetics. 1998;20:104–106. doi: 10.1038/2392. [DOI] [PubMed] [Google Scholar]
- Doxsey S.J., Stein P., Evans L., Calarco P., Kirschner M. Pericentrin, a highly conserved protein of centrosomes involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Echeverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M.-A., Antony C., Wright M., Maro B. Centrosome assembly in vitrorole of γ-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T., Dionne M.A., Compton D. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.R., Cleveland D.W., Schroer T.A. Characterization of DLC-A and DLC-B, two families of cytoplasmic dynein light chain subunits. Mol. Biol. Cell. 1994;5:645–654. doi: 10.1091/mbc.5.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R.R., Borisy G.G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J. Cell Biol. 1990;73:601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Holzbaur E.L.F., Vallee R.B. Dyneinsmolecular structure and cellular function. Annu. Rev. Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- Hughes S.M., Vaughan K.T., Herskovits J.S., Vallee R.B. Molecular analysis of a cytoplasmic dynein light intermediate chain reveals homology to a family of ATPases. J. Cell Sci. 1995;108:17–24. doi: 10.1242/jcs.108.1.17. [DOI] [PubMed] [Google Scholar]
- Hyman A., Karsenti E. The role of nucleation in patterning microtubule networks. J. Cell Sci. 1998;111:2077–2083. doi: 10.1242/jcs.111.15.2077. [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E.L.F. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E.L.F. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Kellogg D.R., Moritz M., Alberts B.M. The centrosome and cellular organization. Annu. Rev. Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Kinzler K.W., Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- Lombillo V.A., Nislow C., Yen T.J., Gelfand V.I., McIntosh J.R. Antibodies to the kinesin motor domain of CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J. Cell Biol. 1995;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar M., Mikami A., Gee M.A., Vallee R.B. In vitro motility from recombinant dynein heavy chain. Proc. Natl. Acad. Sci. USA. 1996;93:6552–6556. doi: 10.1073/pnas.93.13.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner H., Daga A., Buxton J., Fernandez B., Chalwa A., Banerjee U., Czech M.P. Interactions of Drosophila Cbl with epidermal growth factor receptors and role of Cbl in R7 photoreceptor cell development. Mol. Cell. Biol. 1997;17:2217–2225. doi: 10.1128/mcb.17.4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Cleveland D.W. Pathways of spindle pole formationdifferent mechanisms; conserved components. J. Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Ramyar K., Vechio J.D., Cleveland D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Moritz M., Braunfeld M.B., Sedat J.W., Alberts B., Agard D.A. Microtubule nucleation by gamma-tubulin–containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Paschal B.M., Shpetner H.S., Vallee R.B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J. Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr C.M., Coue M., Grisson P.M., Hays T.S., Porter M.E., McIntosh J.R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Pihan G.A., Doxsey S.J. The mitotic machinery as a source of genetic instability in cancer. Semin. Cancer Biol. 1999;9:289–302. doi: 10.1006/scbi.1999.0131. [DOI] [PubMed] [Google Scholar]
- Pihan G.A., Purohit A., Knecht H., Woda B., Quesenberry P., Doxsey S.J. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998;58:3974–3985. [PubMed] [Google Scholar]
- Rudner A.D., Murray A.W. The spindle assembly checkpoint. Curr. Opin. Cell Biol. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Schnackenberg B.J., Khodjakov A., Rieder C.L., Palazzo R.E. The disassembly and reassembly of functional centrosomes in vitro. Proc. Natl. Acad. Sci. USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D.A., Williams B.C., Hays T.S., Goldberg M.L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Kirschner M. Reconstitution of centrosome assembly, role of gamma tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Steuer E.R., Wordeman L., Schroer T.A., Sheetz M.P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Szollosi D., Calarco P., Donahue R.P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Vallee R.B., Sheetz M.P. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Vaughan K.T., Vallee R.B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150glued . J. Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan K.T., Tynan S.H., Echeverri C.J., Faulkner N.F., Vallee R.B. Colocalization of dynactin and cytoplasmic dynein with CLIP-170 at microtubule distal ends. J. Cell Sci. 1999;112:1437–1447. doi: 10.1242/jcs.112.10.1437. [DOI] [PubMed] [Google Scholar]
- Waters J.C., Salmon E.D. Pathways of spindle assembly. Curr. Opin. Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Wilson I.A., Niman H.L., Houghton R.A., Cherenson A.R., Connolly M.L., Lerner R.A. The structure of an intigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Yen T.J., Li G., Schaar B.T., Szilak I., Cleveland D.W. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992;359:536–539. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wong M.L., Alberts B., Mitchison T. Nucleation of microtubule assembly by a gamma tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman W., Sparks C.A., Doxsey S.J. Amorphous no longerthe centrosome comes into focus. Curr. Opin. Cell Biol. 1999;11:122–128. doi: 10.1016/s0955-0674(99)80015-5. [DOI] [PubMed] [Google Scholar]