Abstract
Sodium/calcium(-potassium) exchangers (NCX and NCKX) are critical for the rapid extrusion of calcium, which follows the stimulation of a variety of excitable cells. To further understand the mechanisms of calcium regulation in signaling, we have cloned a Drosophila sodium/calcium-potassium exchanger, Nckx30C. The overall deduced protein topology for NCKX30C is similar to that of mammalian NCKX, having five membrane-spanning domains in the NH2 terminus separated from six at the COOH-terminal end by a large intracellular loop. We show that NCKX30C functions as a potassium-dependent sodium/calcium exchanger, and is not only expressed in adult neurons as was expected, but is also expressed during ventral nerve cord development in the embryo and in larval imaginal discs. Nckx30C is expressed in a dorsal–ventral pattern in the eye-antennal disc in a pattern that is similar to, but broader than that of wingless, suggesting that large fluxes of calcium may be occurring during imaginal disc development. Nckx30C may not only function in the removal of calcium and maintenance of calcium homeostasis during signaling in the adult, but may also play a critical role in signaling during development.
Keywords: calcium, development, Drosophila, photoreceptor, signal transduction
In resting cells, intracellular calcium concentration is maintained at 10–100 nM, and can rise up to tens of μM during stimulation (Peretz et al. 1994b; Bootman and Berridge 1995; Hardie 1996b). The precise control of spatial and temporal profiles of calcium is essential for cellular function, and prolonged elevation of cytosolic calcium can be toxic, leading to cell death (Berridge 1998; Berridge et al. 1998). Hence, proper calcium removal or sequestration after a transient rise in cytoplasmic calcium is vital to all cells. Calcium extrusion from cells is carried out by two classes of membrane proteins, ATP-driven calcium pumps and sodium/calcium exchangers (NCX).1 The latter are thought to be particularly important in cells that handle a large flux of calcium across their plasma membrane, such as cardiac myocytes and many neurons, including vertebrate photoreceptors (for review see Blaustein and Lederer 1999).
Exchangers function to lower and maintain intracellular calcium at or below 100 nM by using the transmembrane (TM) sodium gradient as an energy source. Of the two well-characterized families of mammalian sodium/calcium (Na+/Ca2+) exchangers, one family, NCX, utilizes a stoichiometry of 3Na+/1Ca2+ and has three isoforms (Nicoll et al. 1996b; Philipson et al. 1996). NCX-type exchangers are expressed in a variety of tissues including heart, kidney, brain, as well as smooth and skeletal muscle (Nicoll et al. 1996b). The second family, called potassium-dependent sodium/calcium exchangers (NCKX), uses both the inward sodium gradient and the outward potassium gradient to extrude calcium at a stoichiometry of 4Na+/1Ca2+,1K+ (Cervetto et al. 1989; Schnetkamp et al. 1989). NCKX-type exchangers appear to have a more limited tissue distribution, and have thus far only been characterized extensively in the outer segments of retinal rod photoreceptors (for reviews see Schnetkamp 1989; Lagnado and McNaughton 1990). Retinal rod NCKX1 has been cloned from several mammalian species and shown to be an NCKX after heterologous expression in HEK293 cells (Reiländer et al. 1992; Tucker et al. 1998; Cooper et al. 1999). It has been localized to the plasma membrane of rod photoreceptor outer segments (Haase et al. 1990; Reiländer et al. 1992). Analysis of the results obtained by the various genomic sequencing projects demonstrate that NCKX homologues are encoded by eukaryotic and prokaryotic genomes (Wilson et al. 1994; Schwarz and Benzer 1997). Although NCKX- and NCX-type exchangers display little overall amino acid sequence identity, they are thought to be evolutionarily related (Nicoll et al. 1996a; Schwarz and Benzer 1997).
Calcium plays an important role in phototransduction in both vertebrates and invertebrates. Both utilize rhodopsin-mediated, G protein–coupled cascades, but with some important differences. Illumination of vertebrate rod photoreceptors results in the closing of cGMP-gated cation channels, and thus hyperpolarization of the photoreceptor cell (Stryer 1986; Yau 1994). A dark equilibrium between calcium influx via cGMP-gated channels and calcium extrusion via NCKX1 is disrupted, and net extrusion of calcium through NCKX1 results. Cytosolic calcium then falls from a dark value of 500–600 nM to a value of <50 nM in bright light (Gray-Keller and Detwiler 1994; Sampath et al. 1998). This process mediates the process of light adaptation in both retinal rods and cones (Matthews et al. 1988; Nakatani and Yau 1988a).
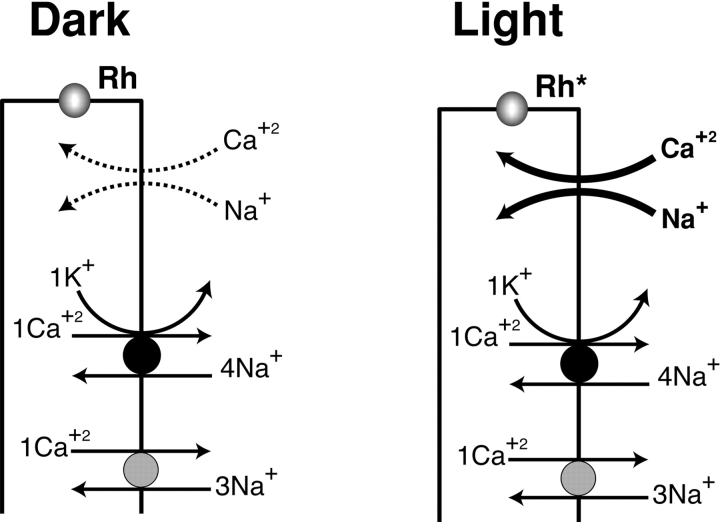
In Drosophila, calcium changes in the opposite direction (see Fig. 1). Phototransduction occurs via a phospholipase C–mediated cascade, and illumination triggers the opening of the cation-selective channels. The photoreceptors depolarize and intracellular calcium rises to tens of μM (Peretz et al. 1994a; Ranganathan et al. 1994; Hardie 1995, Hardie 1996a,Hardie 1996b; Ranganathan et al. 1995; Zuker 1996). The mechanism that reduces calcium back to resting levels (100 nM) after illumination has not yet been identified, but may utilize an electrochemical exchanger which couples calcium release from the cell to the inward sodium gradient, as is the case in vertebrate rod photoreceptors (Yau and Nakatani 1984; Schnetkamp 1986; Lagnado et al. 1988; Lagnado and McNaughton 1990). In fact, sodium/calcium exchange has been measured in Drosophila as well as in other invertebrate photoreceptors, and is also thought to be critical in light-adaptation (Lisman and Brown 1972; Minke and Armon 1984; O'Day and Gray-Keller 1989; O'Day et al. 1991; Ranganathan et al. 1994; Hardie 1995; Bauer et al. 1999).
Figure 1.
The phototransduction cascade in Drosophila. Absorption of a photon photoactivates rhodopsin, leading to the opening of the cation-selective channels (Light). Extracellular calcium (Ca+2) and sodium (Na+) enter the cell via the light-activated channels, causing a depolarization of the photoreceptor cells. It is estimated that calcium levels rise from 100 nM to >10 μM upon light stimulation (Hardie 1996b). Calcium entering through the light-sensitive channels is thought to play a key role in deactivation of the light response and light adaptation. Rapid removal of calcium from the photoreceptor cells is key to the recovery from the light response. Removal of calcium following light-stimulation may occur via NCKX (filled circle) and NCX (shaded circle). NCX utilizes a stoichiometry of 3Na+/1Ca2+ to extrude calcium, and NCKX uses both the inward sodium gradient and the outward potassium gradient to extrude calcium at a stoichiometry of 4Na+/1Ca2+,1K+. Broken arrows indicate that the cation influx through the light-sensitive channel does not occur in the dark (Dark). Rh, rhodopsin molecule; Rh*, photoexcited rhodopsin molecule. This figure is an adaptation of one drawn by Yau 1994.
To further understand the molecular basis of calcium homeostasis during signaling, we have cloned and characterized a Drosophila potassium-dependent sodium/calcium exchanger, Nckx30C. We show that it functions as an NCKX when expressed in High Five cells (an insect cell line). Nckx30C is distinct from Calx, an NCX-type exchanger in Drosophila (Hryshko et al. 1996; Ruknudin et al. 1997; Schwarz and Benzer 1997; Dyck et al. 1998; Omelchenko et al. 1998). Both Nckx30C and Calx are expressed in the adult brain and in photoreceptor cells as well as during development. We hypothesize that these exchangers are not only critical to the proper functioning of the photoreceptor cells, and other cells in adult flies, but may also play critical roles in the removal of calcium generated during signaling processes in embryogenesis and in patterning of the imaginal discs of Drosophila.
Materials and Methods
Molecular Cloning and DNA Sequencing of Ncxk30C
A cDNA clone was constructed, corresponding to the bovine rod photoreceptor NCKX1 that encoded a fusion between the highly conserved TM1–TM5 and TM6–TM11 regions. The clone was 32P-radiolabeled using the Prime-It II Random Primer Labeling Kit (Stratagene). We used this 32P-radiolabeled DNA probe to screen a Drosophila genomic library (a gift of K. Moses, Emory University, Atlanta, GA, and G.M. Rubin, University of California, Berkeley, Berkeley, CA). The genomic library was screened according to the methods described in Sambrook et al. 1989. We obtained several genomic clones. A 1.3-kb EcoRI and XbaI genomic fragment was subcloned into pBluescriptII KS (Stratagene), 32P-radiolabeled, and used to screen a Drosophila eye-enriched lambda ZAPII cDNA library (gift of C.S. Zuker, University of California, San Diego, San Diego, CA) (Shieh et al. 1989). A set of overlapping cDNAs encompassing the entire Nckx30C transcript was isolated and sequenced. No other cDNAs were identified. The full-length Nckx30C cDNA was constructed and the DNA sequence was determined by fluorescent-based sequencing methods (Applied Biosystems). The BLAST search program (Altschul et al. 1990) (http://www.ncbi.nlm.nih.gov/BLAST) and the CLUSTAL W multiple sequence alignment program were used for sequence similarity analysis (Thompson et al. 1994) (http://pbil.ibcp.fr/NPSA/npsa_clustalw.html). The sequence data have been submitted to the DDBJ/EMBL/GenBank databases under accession number AF190455.
Functional Analysis
The full-length Nckx30C cDNA was cloned into the novel insect cell vector pEIA as an EcoRI fragment, and this was used for the stable transfection of High Five insect cells as described (Farrell et al. 1998). High Five cells (BTI-TN-5B1-4) derived from Trichoplusia ni egg cell homogenates were purchased from Invitrogen. These cells do not display endogenous exchanger activity.
Potassium-dependent sodium/calcium exchange activity was measured in High Five cells transformed with Nckx30C cDNA. The cells were loaded with sodium via the sodium-potassium ionophore, monensin, in a medium containing high sodium, 150 mM NaCl, 80 mM sucrose, 0.05 mM EDTA, and 20 mM Hepes, pH 7.4, according to the methods described for rod outer segments (Schnetkamp et al. 1995). The ionophore was removed and the sodium-loaded cells were washed with and resuspended in 150 mM LiCl, 80 mM sucrose, 0.05 mM EDTA, and 20 mM Hepes, pH 7.4. The cells were diluted 10-fold in media containing 80 mM sucrose, 20 mM Hepes, pH 7.4, and either 150 mM KCl, 150 mM NaCl, or 150 mM LiCl. 45Ca uptake was initiated by addition of 35 μM CaCl2 and 1 μCi 45Ca. 45Ca uptake in High Five cells was measured with a rapid filtration method with the use of borosilicate glass fibers over a time-course of 5 min as described previously (Schnetkamp et al. 1991b). The ice-cold washing medium contained 140 mM KCl, 80 mM sucrose, 5 mM MgCl 2, 1 mM EGTA, and 20 mM Hepes, pH 7.4.
Northern Analysis
Total RNA was prepared from the heads and bodies of 0–7-d-old Drosophila w1118 and eya lines using the Ultraspec RNA isolation system (Biotecx). PolyA+ RNA was isolated by affinity chromatography on oligo(dT) cellulose columns using the FastTrack 2.0 System (Invitrogen, Inc.). PolyA+ RNA from third instar larvae and 0–24-h embryos (Canton S strain) was purchased from Clontech. PolyA+ RNA (10 μg) from each sample was run on a denaturing 0.9% agarose gel for 3 h 130V/cm. The gel was stained with ethidium bromide and photographed on a UV transilluminator. The mRNA was transferred overnight by capillary action in 20× saline sodium citrate (SSC) to a positively charged nylon membrane (Nytran Plus), and fixed by UV cross-linking. The membrane was probed with [32P]UTP antisense riboprobes (Maxiscript In Vitro Transcription kit; Ambion, Inc.). Four subclones of Nckx30C were constructed and used for riboprobe production; 1.3 kb BamHI-BamHI (5′ end), 500 bp BamHI-ClaI (TM1–TM5), ClaI-ClaI (loop region), and ClaI-EcoRV (TM6–TM11) (see Fig. 4 A). The antisense and sense riboprobes were incubated with the membranes using the NorthernMax hybridization buffer (Ambion, Inc.), the membranes were washed with 0.2× SSC, 1% SDS at 65°C, and exposed to Kodak X-OMAT X-ray film (Eastman Kodak). All four antisense probes displayed the same labeling pattern. No signal was detected with the sense probes. A 250-bp mouse β-actin antisense riboprobe was used as a control for the amount of RNA loaded (Maxiscript In Vitro Transcription kit; Ambion, Inc.).
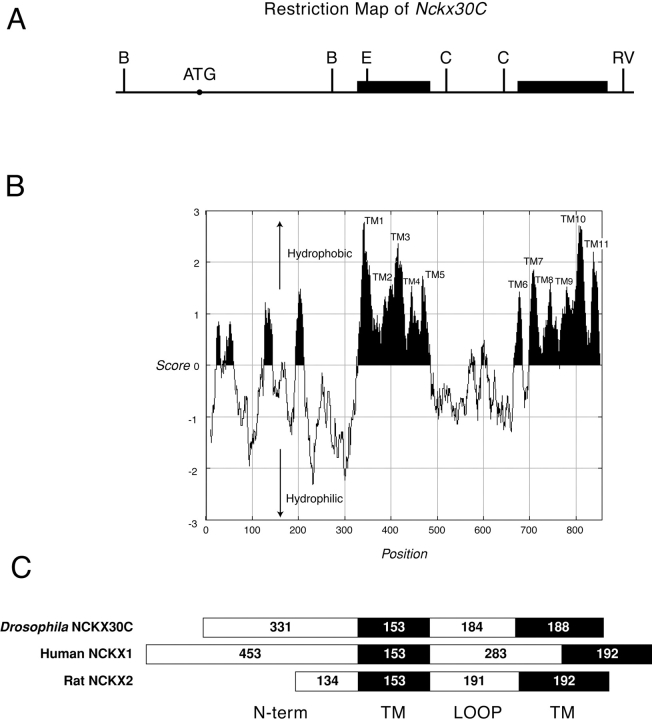
Figure 4.
Hydropathy plot of the conceptual NCKX30C protein. (A) Map of Nckx30C cDNA. The black boxes represent the TM regions of the deduced amino acid sequence. Cleavage sites for BamHI (indicated by B), EcoRI (indicated by E), Cla I (indicated by C), and EcoRV (indicated by RV) are shown. (B) Hydropathy plot of the conceptual NCKX30C protein, analyzed by the Kyte-Doolittle algorithm (Kyte and Doolittle 1982). Hydrophobic regions are designated in black. (C) Domain structure of Drosophila NCKX30C, human rod photoreceptor NCKX1, and rat brain NCKX2. Filled boxes represent the two clusters of TM segments (TM1–TM5 and TM6–TM11) that display high identity among the three sequences. Numbers represent the number of amino acids per segment.
In Situ Hybridization to Polytene Chromosomes
Polytene chromosome squashes (w m f strain) were prepared as described (Lim 1993). A 1.3-kb EcoRI and XbaI genomic fragment was nick-translated using biotin-16-UTP (Boehringer Mannheim Corp.). Hybridization with the biotinylated probe was carried out in 600 mM sodium chloride, 1× Denhardt's, 50 mM magnesium chloride, and 50 mM sodium phosphate, pH 6.8, at 37°C overnight. Samples were washed with 2× SSC, 0.1% Triton X-100, and PBS, pH 7.2 (Lim 1993). Hybrids were detected using Vectastain ABC (Vector Laboratories, Inc.). Labeling was identified at a single cytological position on chromosome 2L, 30C5-7.
In Situ Hybridization
Heads from the w1118 strain of flies were embedded in Tissue-Tek OCT Compound (Miles, Inc.) and frozen on dry ice. Eyes were fixed in 4% paraformaldehyde, infiltrated with 2.3 M sucrose, and frozen in liquid nitrogen according to methods described previously (Colley et al. 1991). In situ hybridizations were carried out on 14-μm head sections and 0.5-μm eye sections in 50% formamide, 5× SSC, 0.5 mg/ml sheared herring sperm DNA, 0.05 mg/ml heparin, 0.25% CHAPS, 0.1% Tween 20, 1 mg/ml yeast torula RNA, 1× Denhardt's, at 55°C overnight. Embryo and imaginal disc in situ hybridizations were carried out essentially as described in Panganiban et al. 1994. In brief, embryos (Canton S strain) were collected, dechorionated, fixed, and processed for in situ hybridization as described by Panganiban et al. 1994. Proteinase K was not used. Embryos were hybridized in 50% formamide, 5× SSC, 500 μg/ml sheared salmon sperm DNA, 0.1% Tween 20, 1 mg/ml glycogen. Hybridization was carried out at 55°C for 24–30 h. Probe concentrations were 90 ng/300 μl. The larval imaginal discs were dissected from crawling third-instar larvae, but left attached to the cuticle. The discs were fixed, permeabilized with proteinase K at 25 μg/ml for 3 min at room temperature, and postfixed. Probe concentrations were 120 ng/300 μl.
Digoxigenin-labeled sense and antisense riboprobes were generated by in vitro transciption of five DNA templates as recommended by the digoxigenin/UTP supplier (Boehringer Mannheim Corp.). Five Nckx30C cDNA probes were used: 1.3 kb BamHI-BamHI (5′ end), 500 bp BamHI-ClaI (TM1–TM5), ClaI-ClaI (loop region), ClaI-EcoRV (TM6–TM11), and the full-length Nckx30C cDNA clone (see Fig. 4 A). Calx cDNA was provided by E. Schwarz (Columbia University, New York, NY) (Schwarz and Benzer 1997). Chaoptin cDNA was provided by D. Van Vactor (University of California, Berkeley) and S.L. Zipursky (University of California, Los Angeles, Los Angeles, CA) (Reinke et al. 1988). After transcription, the reaction mix was treated with DNase and the riboprobes were hydrolyzed in carbonate buffer for 2 min at 80°C. All riboprobes were quantified by analysis in denaturing 0.8% agarose gels and by dot blot analysis. All detection steps were as described in Tautz and Pfeifle 1989. All Nckx30C antisense probes showed the same labeling pattern. No signal was detected with the sense probes.
Results
Isolation of a Drosophila NCKX
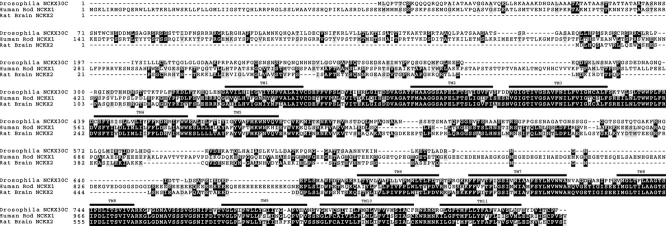
The NCKX-type exchangers are a novel group of calcium extrusion proteins. NCKX1 function was initially described in the outer segments of retinal rod photoreceptor cells (Fig. 1) (Cervetto et al. 1989; Schnetkamp et al. 1989). Antibodies directed to NCKX1 were used to screen a bovine retinal expression library, and NCKX1 cDNA was cloned and sequenced (Reiländer et al. 1992). A second isoform, NCKX2, was cloned from rat brain, and was shown on the basis of in situ hybridization to be widely expressed in various regions of the brain (Tsoi et al. 1998). To isolate Drosophila Nckx30C, we constructed a cDNA probe from the bovine rod photoreceptor NCKX1 that encoded a fusion between the highly conserved TM1–TM5 and TM6–TM11 regions. We used this probe to screen the Drosophila genomic library and obtained a 1.3-kbp EcoRI-XbaI fragment that corresponds to an EcoRI site at the 5′ end of the gene. The XbaI site is located in the intron. This 1.3-kbp genomic fragment was used to screen the cDNA library. A set of overlapping cDNAs encompassing the entire Nckx30C transcript was isolated and sequenced. The composite cDNA has a single open reading frame that encodes a protein of 856 amino acids (Fig. 2). We compared the derived amino acid sequence of Nckx30C with the human NCKX1 and rat NCKX2 (Fig. 3). There is 66 and 71% identity in two clusters of amino acids that corresponds to the predicted TM domains of human rod photoreceptor cell NCKX1 and rat brain NCKX2, respectively. A partial restriction map for Nckx30C is shown in Fig. 4 A. The hydropathy analysis confirms that Drosophila NCKX contains 11 hydrophobic regions that correspond to the 11 predicted TM helices in NCXK1 and NCKX2 (Fig. 4 B). As with NCKX1 and NCKX2, there is a large cytoplasmic loop located between TM5 and TM6 in Nckx30C. This large cytoplasmic loop displays very little amino acid identity with the corresponding cytoplasmic loops of NCKX1 or NCKX2 (Fig. 3). The number of amino acids that make up the hydrophilic loops and the two sets of membrane-spanning segments are shown in Fig. 4 C. One feature distinguishes NCKX30C from other members of the NCKX superfamily. Hydropathy analysis revealed that NH2-terminal to TM1 is a region that may contain two additional membrane-spanning segments not observed in either NCKX1 or NCKX2. The correct identity of this NH2-terminal sequence for Nckx30C was confirmed by 5′ rapid amplification of cDNA ends (RACE) starting with a primer corresponding to sequence of TM1, and by PCR using first-strand cDNA.
Figure 2.
Complementary DNA and protein sequence. Numbers for nucleotides are indicated to the left and for amino acids to the right. TM domains are underlined.

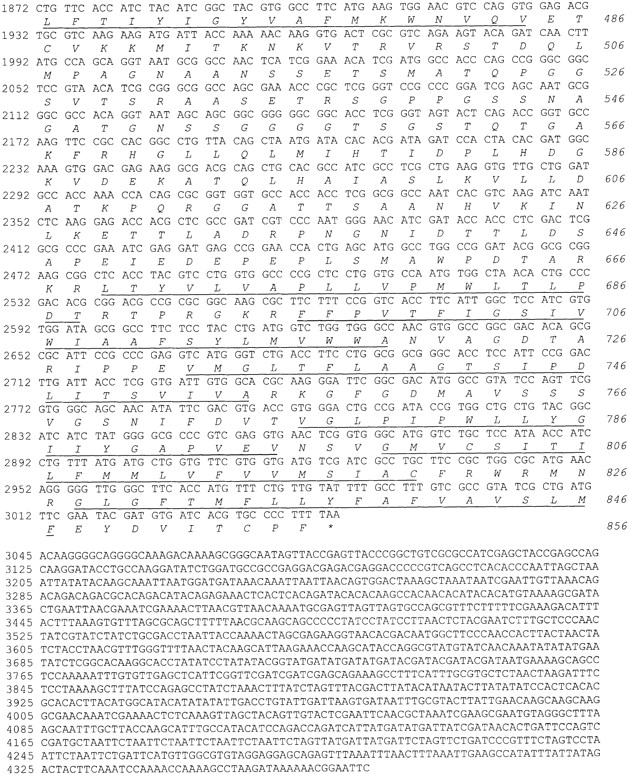
Figure 3.
Nckx30C encodes a novel Drosophila NCKX. CLUSTAL W alignment between Drosophila NCKX30C, human rod NCKX1, and rat brain NCKX2 (Tsoi et al. 1998; Tucker et al. 1998). Dark shading indicates identity and light shading indicates similarity. The 11 TM domains of NCKX30C are indicated by lines above each row.
We cytologically mapped Nckx to 2L at 30C5-7 by in situ hybridization to polytene chromosomes, and based on the chromosomal location have named the gene Nckx30C.
NCKX30C Is an NCKX
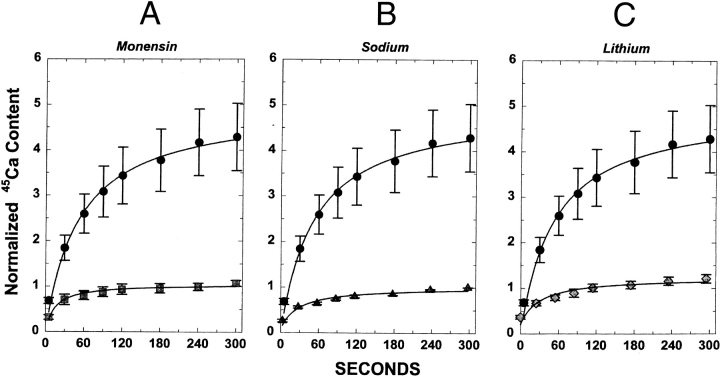
We examined the functional properties of NCKX30C in High Five cells. The strategy for these experiments takes advantage of properties of both NCX and NCKX. First, NCX and NCKX are bidirectional in their ability to mediate both calcium efflux (forward exchange) and calcium influx (reverse exchange). The direction of transport is dictated by the direction of the TM sodium gradient. Normally, the inward sodium gradient removes calcium from the cell (forward exchange). However, when external sodium is removed, the outward sodium gradient drives calcium into the cell (reverse exchange). Therefore, we measured the properties of reverse exchange of NCKX30C by measuring 45Ca uptake in sodium-loaded cells. We tested for NCKX activity by using three different manipulations of the cation gradient that are known to prevent NCKX activity. The cells were loaded with sodium via the sodium-potassium ionophore, monensin, in a medium containing high sodium, according to the methods described for rod outer segments (Schnetkamp et al. 1995). The ionophore was removed and the sodium-loaded cells were washed with and resuspended in low sodium buffer as described in the Materials and Methods. The cells were diluted 10-fold in media containing 80 mM sucrose, and 20 mM Hepes, pH 7.4, and either 150 mM KCl, 150 mM NaCl, or 150 mM LiCl. 45Ca uptake was initiated by addition of 35 μM CaCl2 and 1 μCi 45Ca. The first condition causes the release of internal sodium by the action of monensin, a sodium(-potassium) selective ionophore. Fig. 5 A shows that 45Ca uptake by NCKX30C was strongly inhibited by the addition of monensin, thus demonstrating a requirement for intracellular sodium for calcium transport.
Figure 5.
NCKX30C is an NCKX. Reverse exchange was measured as Nainside-dependent 45Ca uptake in High Five cells transformed with Nckx30C in the presence of external KCl (A–C, filled circles). Mean ± SEM results are shown in A–C (filled circles). (A) Reverse sodium/calcium exchange requires intracellular sodium. 45Ca uptake was measured in KCl medium with 20 μM monensin present (shaded squares) or without monensin (filled circles). Monensin was added 30 s before addition of 45Ca, and causes the release intracellular sodium to the external medium. Average values ± SEM are shown for 13 experiments conducted in KCl medium, and 4 experiments conducted in media containing KCl plus monensin. (B) Reverse sodium/calcium exchange is inhibited by extracellular sodium. 45Ca uptake was measured in KCl medium (filled circles) or NaCl medium lacking potassium (triangles). Average values ± SEM are shown for 13 experiments conducted in KCl medium, and 10 experiments conducted in media containing NaCl. (C) Reverse sodium/calcium exchange requires extracellular potassium. 45Ca uptake was measured in KCl medium (filled circles) or LiCl medium lacking potassium (diamonds). Average values ± SEM are shown for 13 experiments conducted in KCl medium, and 8 experiments conducted in media containing LiCl.
A second property of both NCK and NCKX is that calcium uptake by reverse exchange is inhibited by high extracellular sodium (Schnetkamp et al. 1991a). This is thought to occur because sodium and calcium compete for a common binding site (Schnetkamp et al. 1991a). We demonstrate that 45Ca uptake by NCKX30C is strongly inhibited by high extracellular sodium, consistent with its identity as an NCX (Fig. 5 B).
A critical distinction between NCKX and NCX is that calcium influx via NCKX requires the presence of potassium, and lithium cannot substitute for potassium (Tsoi et al. 1998). In contrast, calcium influx via reverse exchange in NCX is the same in media containing potassium or lithium (Tsoi et al. 1998; Cooper et al. 1999). 45Ca uptake by NCKX30C requires potassium and was not observed in media containing lithium (Fig. 5 C). This result demonstrates that NCKX30C functions as an NCKX.
As negative controls, we subjected nontransformed High Five cells to the same 45Ca uptake experimental protocols described above: 45Ca uptake in media containing potassium was indistinguishable from that in media containing either sodium or lithium, showing that there is no endogenous NCX or NCKX activity in the cells (data not shown). The above results demonstrate that NCKX30C mediates potassium-dependent sodium/calcium exchange similar to that observed for NCKX1 and NCKX2 (Tsoi et al. 1998; Cooper et al. 1999).
Northern Blot Analysis
Northern blot analysis revealed an 8-kb transcript that is expressed in embryos and larvae (Fig. 6). In the adult, two transcripts of 8 kb and 10 kb were detected. Expression was detected predominately in the head. Transcripts present in the body may represent the thoracic ganglia or other components of the nervous system (Demerec 1994). The 8-kb and 10-kb transcripts were also detected in eya-1 mutant flies, which lack eyes, indicating that expression is not restricted to the eyes (Fig. 6).
Figure 6.
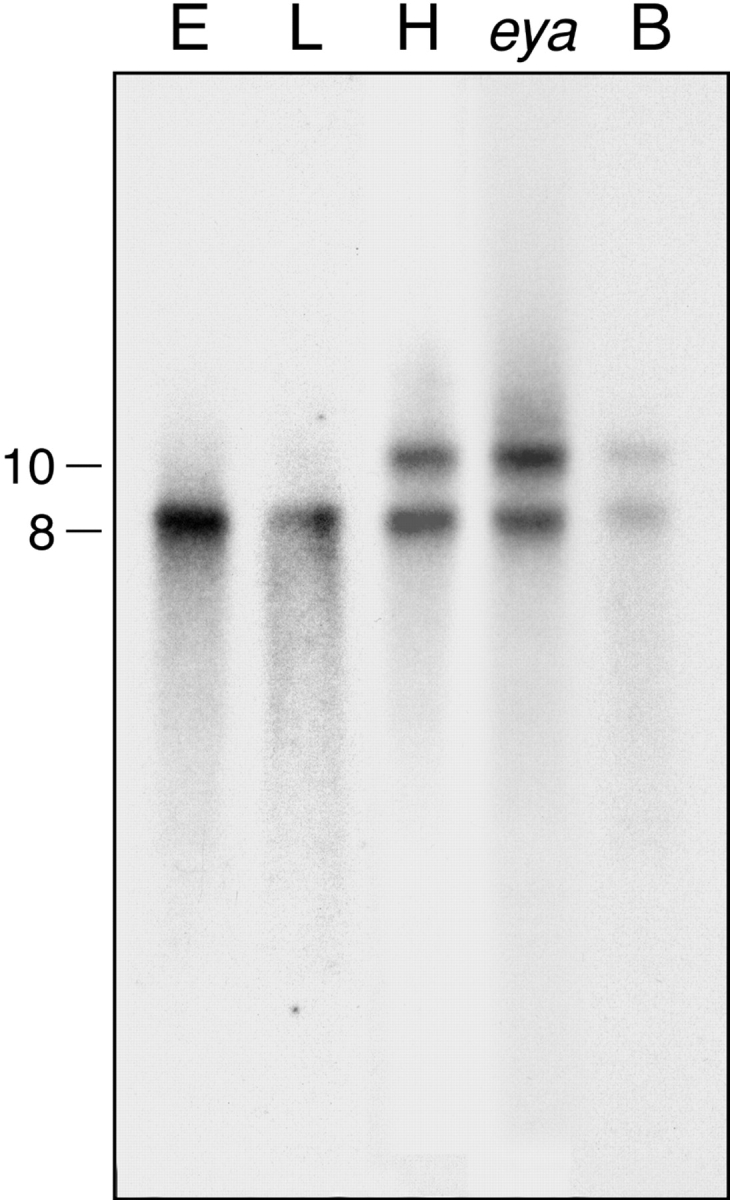
Nckx30C encodes 8-kb and 10-kb transcripts. Northern blot analysis reveals two transcripts of 8 kb and 10 kb expressed in adult heads (H) and adult bodies (B). Mutant flies lacking eyes (eya) also express both transcripts, whereas only the lower 8-kb transcript is detected in embryos (E) and larvae (L). We loaded 10 μg of polyA+ RNA in each lane. Identical results were obtained with four different radiolabeled riboprobes corresponding to various regions of the exchanger. Specifically, we used a 1.1-kbp BamHI-BamHI and 500 nucleotide BamHI-ClaI, ClaI-ClaI loop region, and ClaI-EcoRV (see Fig. 4 A).
Nckx30C and Calx Are Expressed in the Adult Eye and Brain of Drosophila
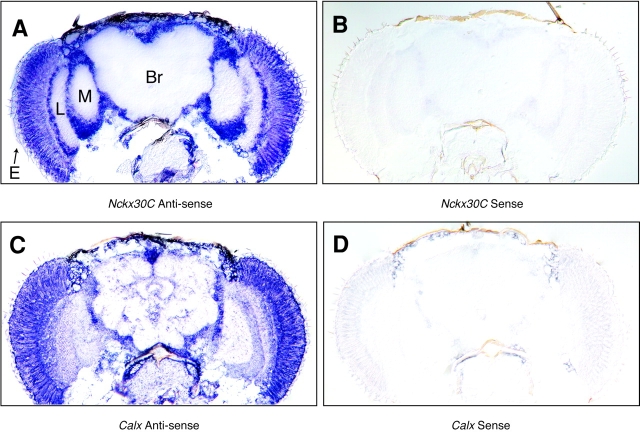
Previous work has identified a Drosophila NCX, Calx, which encodes a functional ortholog of the NCX-type exchangers (Hryshko et al. 1996; Ruknudin et al. 1997; Schwarz and Benzer 1997). Expression patterns for Calx were reported in Schwarz and Benzer 1997. In situ hybridization analysis reveals that both Nckx30C and Calx are expressed in the adult nervous system. Fig. 7 A shows that Nckx30C is expressed in the photoreceptor cells as well as in the lamina, medulla, and optic lobes of the brain. Fig. 7 C shows that Calx is also expressed in the photoreceptors as well as in the brain. Analysis of many images did not reveal a convincing difference in expression patterns between Nckx30C and Calx. In situ analysis at higher resolution, on 0.5-μm cryosections, confirmed that expression is detected in the photoreceptor cells (data not shown). However, this does not exclude the possibility that the subcellular distribution of the proteins could be very different. No signal was detected with the sense probes (Fig. 7B and Fig. D).
Figure 7.
Nckx30C and Calx are expressed in the adult eye and the brain of Drosophila. (A) Shown are in situ hybridizations to 14-μm cryostat sections of adult heads hybridized with digoxigenin-labeled riboprobes. (A) Antisense riboprobe for Nckx30C; (B) sense probe for Nckx30C; (C) antisense riboprobe for Calx; and (D) sense riboprobe for Calx. E, eye; L, lamina; M, medulla; and Br, brain.
Nckx30C and Calx Are Expressed in the Developing Embryonic Nervous System
Beginning at embryonic stage 13–14, Nckx30C transcripts were detected in a discrete pattern of unpaired cells at the ventral midline of the central nervous system (Fig. 8 E). Nckx30C transcripts were not detected in the preblastoderm or blastoderm stages (Fig. 8A and Fig. C). Unlike Nckx30C, Calx expression is robust in the preblastoderm before and during nuclear migration from the interior to the cortex (Fig. 8 B). Since this is before activation of zygotic transcription, Calx transcripts are likely to be maternally inherited. Calx transcripts disappear in the cellular blastoderm (Fig. 8 D) and then reappear in the stage 11 embryo (Fig. 8 F). By stage 12, Calx expression is noted in two cells per hemisegment, one on either side of the ventral midline (Fig. 8 H). In embryonic stage 15, Nckx30C expression was detected in several neurons within the ventral nerve cord (Fig. 8 G). By stage 16, when many neurons within the ventral nerve cord and the embryonic brain express Nckx30C (Fig. 8 I), Calx expression is restricted to a much smaller subset of neurons in the ventral nerve cord (Fig. 8 J). Outside the central nervous system, Nckx30C and Calx transcripts were observed in some cells, which may represent parts of the peripheral nervous system (Fig. 8I and Fig. J).
Figure 8.
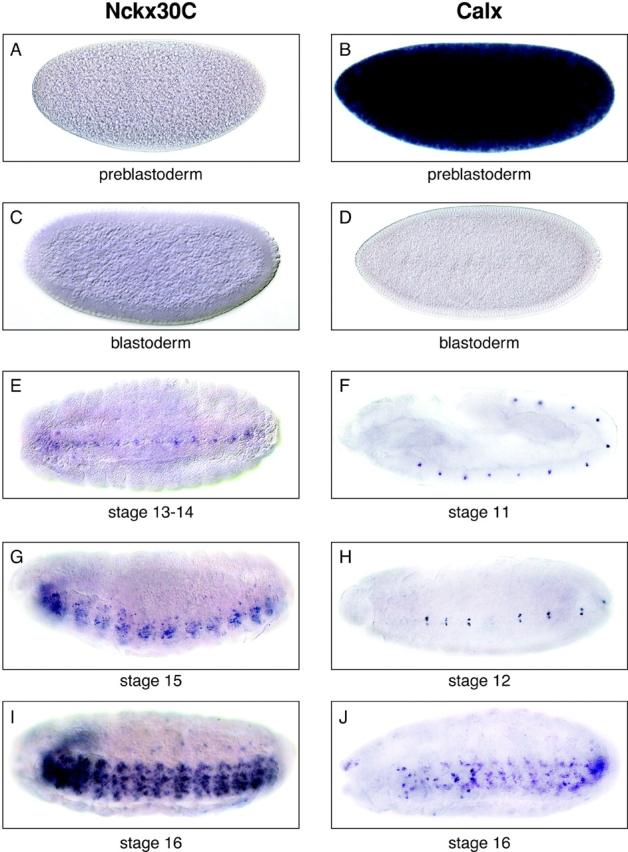
Nckx30C and Calx are expressed in the ventral nerve cord of the Drosophila embryo. Shown are in situ hybridizations to whole-mount wild-type embryos (Canton S strain) hybridized with digoxigenin-labeled antisense riboprobes for Nckx30C and Calx. (A) Lateral view, preblastoderm, Nckx30C; (B) lateral view, preblastoderm, Calx; (C) lateral view, blastoderm, Nckx30C; (D) lateral view, blastoderm, Calx; (E) ventral view, stage 13-14, Nckx30C; (F) lateral view, stage 11, Calx; (G) ventrolateral view, stage 15, Nckx30C; (H) ventral view, stage 12, Calx; (I) ventrolateral view, stage 16, Nckx30C; and (J) ventrolateral view, stage 16, Calx. Note the labeling of the developing ventral nerve cord. Control hybridizations with sense probes did not produce signals (data not shown). All embryos are orientated anterior to the left. In lateral views, all embryos are orientated dorsal side up.
Nckx30C Is Expressed in the Larval Imaginal Discs
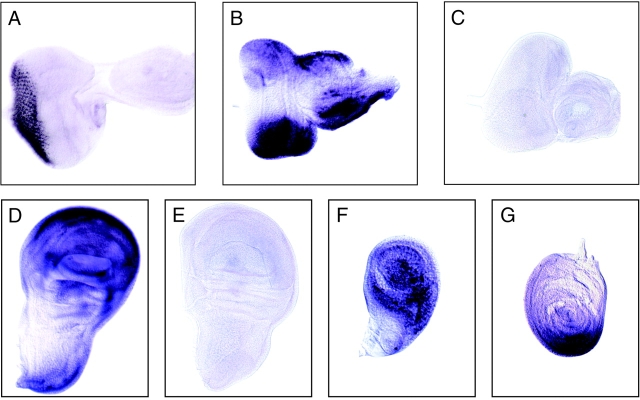
Drosophila appendages develop from imaginal discs in a highly orchestrated series of events that divide the discs into distinct subregions during patterning. Retinal differentiation begins at the posterior end of the eye disc, which coincides with the dorsal–ventral midline (Treisman and Rubin 1995; Heberlein et al. 1998; Treisman and Heberlein 1998), and proceeds as a wave across the eye disc from posterior to anterior (Ready et al. 1976; Tomlinson and Ready 1987; Tomlinson 1988). The morphogenetic furrow is a dorsoventral indentation in the eye disc, with the region anterior to the furrow comprising actively dividing and unpatterned cells, and the region posterior to the furrow containing differentiating photoreceptor cells assembling into ommatidia. (Tomlinson 1988; Ready 1989; Banerjee and Zipursky 1990). Fig. 9 A shows expression of chaoptin, which encodes a photoreceptor cell surface glycoprotein (Reinke et al. 1988), in photoreceptor cells located posterior to the furrow. Calx expression was not detected in any of the imaginal discs. Nckx30C transcripts were detected both anterior and posterior to the morphogenetic furrow in a dorsal–ventral pattern with no labeling in the midline (Fig. 9B and Fig. C). This expression pattern is similar to but broader than that observed for wingless (wg), which is known to play an important role in dorsal–ventral patterning in the eye disc (Heberlein and Moses 1995; Ma and Moses 1995; Treisman and Rubin 1995; Heberlein et al. 1998; Treisman and Heberlein 1998). In vertebrates, one of the two apparently distinct Wnt pathways may act through a G protein–mediated phosphatidylinositol signaling cascade that results in an increase in intracellular calcium via inositol 1,4,5 trisphosphate (InsP3)-sensitive stores (Moon et al. 1997; Slusarski et al. 1997a,Slusarski et al. 1997b). It is intriguing to hypothesize that NCKX30C may be playing a role in modulating calcium in this patterning pathway.
Figure 9.
Nckx30C is expressed in the third instar imaginal discs of larvae. Shown are in situ hybridizations to imaginal discs from wild-type larvae with digoxigenin-labeled riboprobes for Nckx30C and chaoptin. (A) Eye-antennal disc, antisense riboprobe for chaoptin; (B) eye-antennal disc, antisense riboprobe for Nckx30C; (C) eye-antennal disc, control sense riboprobe for Nckx30C; (D) wing disc, antisense riboprobe for Nckx30C; (E) wing disc, sense riboprobe for Nckx30C; (F) haltere disc, antisense riboprobe for Nckx30C; and (G) leg disc, antisense riboprobe for Nckx30C. Note that control hybridizations with sense probes did not produce signals. The eye discs are oriented posterior to the left and dorsal up. The wing and haltere discs are orientated with posterior to the left and ventral up. The leg disc is oriented ventral down.
Nckx30C is expressed strongly throughout most of the wing disc and throughout most of the haltere disc (Fig. 9, D–F). In the leg disc, Nckx30C is expressed in a ventral wedge in the cells that will give rise to the ventral and/or proximal structures (Fig. 9 G). This pattern of expression is similar to that observed for wingless in the leg discs (Baker 1988; Couso et al. 1993; Theisen et al. 1994). Given what we know about the role of NCKX in retinal rod and cone photoreceptors, it is tempting to speculate that cells that express Nckx30C may be experiencing large and sustained rises in cytosolic calcium.
Discussion
We have cloned Nckx30C from Drosophila and have shown that it is expressed in the adult nervous system and also during development in the embryo and the imaginal discs. Heterologous expression in cultured insect High Five cells demonstrates that NCKX30C functions as an NCKX. Calx, a member of the NCX gene family, is distantly related to Nckx30C (Hryshko et al. 1996; Ruknudin et al. 1997; Schwarz and Benzer 1997; Dyck et al. 1998; Omelchenko et al. 1998). Both NCX and NCKX function in sodium–calcium exchange in cells experiencing large calcium fluxes. In addition, both Nckx30C and Calx are expressed in Drosophila photoreceptors as well as in other cells, indicating that there may be multiple mechanisms for calcium efflux in these cells.
A puzzling finding is that the photoreceptors in Drosophila express both NCKX30C and Calx exchangers. The NCKX exchangers have several unique features that make them well-suited for calcium extrusion during phototransduction. Illumination of Drosophila photoreceptors activates the phototransduction cascade leading to the opening of the cation-selective channels and an increase in the intracellular calcium (Fig. 1). In both flies and vertebrate photoreceptor cells, sodium influx contributes substantially to the inward current, potentially leading to elevated [Na+]inside (Coles and Orkand 1985; Nakatani and Yau 1988b; Hardie 1996b; Gerster 1997). As the cytosolic sodium concentration increases and reduces the TM sodium gradient, NCX exchangers, like Calx, are expected to reverse direction at much lower cytosolic sodium concentrations when compared with the potassium-dependent NCKX exchangers (Schnetkamp et al. 1991c). Therefore, the potassium-dependent exchangers will be better able to function in phototransduction under these conditions. The apparent redundancy could also be explained if the subcellular distribution of NCKX30C and Calx are very different, with each fulfilling distinct functions. A combination of immunocytochemistry with mutant analysis will assist in resolving these questions.
To date, the central focus of efforts in development of Drosophila has been on the identification of regulatory genes that control development, with the potential contribution of calcium signaling remaining largely unexplored. Notable exceptions include systems responsible for dorsal–ventral positional information, such as the dorsal protein in dorsal–ventral pattern information in the early embryo and the wingless pathway. It has been demonstrated that increased calcium can act as a second messenger in the signal transduction pathway leading to the nuclear localization of dorsal, a maternally inherited transcription factor (Kubota et al. 1993). Another signaling pathway that may utilize calcium is wingless. wingless, a member of the Wnt gene family, encodes a secreted glycoprotein that is involved in a complex variety of signaling events (Nusse and Varmus 1992; Cadigan and Nusse 1997). In vertebrate embryos, one of the two apparently distinct Wnt pathways can act through a G protein–mediated phosphatidylinositol signaling cascade, resulting in a release of intracellular calcium from inositol 1,4,5 trisphosphate (InsP3 )-sensitive stores (Moon et al. 1997; Slusarski et al. 1997a,Slusarski et al. 1997b). Here, we demonstrate that Nckx30C is expressed in a pattern similar to, but broader than that observed for wingless. Nckx30C expression would indicate that, most likely, a substantial calcium flux is occurring in these cells. In this study, we show that exchangers are expressed during development, indicating that they may not only function in the removal of calcium and maintenance of calcium homeostasis during signaling in the adult, but may also play critical roles in signaling events during embryogenesis and patterning of imaginal discs. The isolation of Nckx30C in Drosophila permits a genetic analysis of the in vivo role of calcium in modulating signaling pathways in Drosophila.
Acknowledgments
We thank P.J. Bauer, D. Bownds, C. Doe, V.E. Foe, G. Halder, R. Hardie, B. Minke, K. Moses, R. Moon, G. Panganiban, R. Payne, A. Polans, P. Robinson, E. Schwarz, and C.S. Zuker for valuable discussions and comments on the manuscript. We also thank E. Corse, T. Hoening, T. James, S. Park, and J. Riley for their technical assistance; and R.A. Kreber and J. Lim for expert assistance with the chromosome in situs. E. Schwarz and S. Benzer generously provided Calx and unpublished data. We are grateful to E. Nielsen and J. Loeffelholz for their excellent assistance with the computer graphics. S. Carroll and S. Paddock generously provided their expertise and microscopes.
This work was supported by the Medical Research Council of Canada (P.P.M. Schnetkamp), and National Institutes of Health grant EY08768, Retina Research Foundation, Howard Hughes Medical Institute, Foundation Fighting Blindness, Fight-For-Sight, and the Research to Prevent Blindness Foundation (N.J. Colley).
Footnotes
1.used in this paper: NCKX, potassium-dependent sodium/calcium exchanger(s); NCX, sodium/calcium exchanger(s); SSC, saline sodium citrate; TM, transmembrane
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baker N.E. Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development. 1988;102:489–497. doi: 10.1242/dev.102.3.489. [DOI] [PubMed] [Google Scholar]
- Banerjee O., Zipursky S.L. The role of cell-cell interaction during development of the Drosophila visual system. Neuron. 1990;4:177–187. doi: 10.1016/0896-6273(90)90093-u. [DOI] [PubMed] [Google Scholar]
- Bauer P.J., Schauf H., Schwarzer A., Brown J.E. Direct evidence of Na+/Ca2+ exchange in squid rhabdomeric membranes. Am. J. Physiol. 1999;276:C558–C565. doi: 10.1152/ajpcell.1999.276.3.C558. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Bootman M.D., Lipp P. Calcium—a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Blaustein M.P., Lederer J. Sodium/calcium exchangeits physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Bootman M., Berridge M. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Cadigan K.M., Nusse R. Wnt signalinga common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R., Robinson D., McNaughton P. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Coles J.A., Orkand R.K. Changes in sodium activity during light stimulation in photoreceptors. J. Physiol. 1985;362:415–435. doi: 10.1113/jphysiol.1985.sp015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley N.J., Baker E.K., Stamnes M.A., Zuker C.S. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Cooper C., Winkfein R., Szerencsei R., Schnetkamp P. cDNA cloning and functional expression of the dolphin retinal rod Na-Ca+K exchanger NCKX1comparison with the functionally silent bovine NCKX1. Biochemistry. 1999;38:6276–6283. doi: 10.1021/bi983068o. [DOI] [PubMed] [Google Scholar]
- Couso J.P., Bate M., Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Demerec M. Biology of Drosophila 1994. Cold Spring Harbor Laboratory Press; Plainview, NY: pp. 632 [Google Scholar]
- Dyck C., Maxwell K., Buchko J., Trac M., Omelchenko A., Hnatowich M., Hryshko L.V. Structure-function analysis of CALX1.1, a Na+-Ca2+ exchanger from Drosophila. Mutagenesis of ionic regulatory sites. J. Biol. Chem. 1998;273:12981–12987. doi: 10.1074/jbc.273.21.12981. [DOI] [PubMed] [Google Scholar]
- Farrell P., Lu M., Prevost J., Brown C., Behie L., Iatrou K. High-level expression of secreted glycoproteins in transformed lepidopteran insect cells using a novel expression vector. Biotechnol. Bioeng. 1998;60:656–663. doi: 10.1002/(sici)1097-0290(19981220)60:6<656::aid-bit2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gerster U. A quantitative estimate of flash-induced Ca+- and Na+-influx and Na+/Ca+-exchange in blowfly Callophora photoreceptors. Vision Res. 1997;37:2477–2485. doi: 10.1016/s0042-6989(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Gray-Keller M.P., Detwiler P.B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Haase W., Friese W., Gordon R.D., Müller H., Cook N.J. Immunological characterization and localization of the Na+/Ca2+-exchanger in bovine retina. J. Neurosci. 1990;10:1486–1494. doi: 10.1523/JNEUROSCI.10-05-01486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R.C. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J. Neurosci. 1995;15:889–902. doi: 10.1523/JNEUROSCI.15-01-00889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R.C. Calcium signallingsetting store by calcium channels Curr. Biol. 6 1996. 1371 1373a [DOI] [PubMed] [Google Scholar]
- Hardie R.C. INDO-1 measurements of absolute resting and light-induced Ca2+ concentration in Drosophila photoreceptors J. Neurosci. 16 1996. 2924 2933b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U., Moses K. Mechanisms of Drosophila retinal morphogenesisthe virtues of being progressive. Cell. 1995;81:987–990. doi: 10.1016/s0092-8674(05)80003-0. [DOI] [PubMed] [Google Scholar]
- Heberlein U., Borod E.R., Chanut F.A. Dorsoventral patterning in the Drosophila retina by wingless. Development. 1998;125:567–577. doi: 10.1242/dev.125.4.567. [DOI] [PubMed] [Google Scholar]
- Hryshko L.V., Matsuoka S., Nicoll D.A., Weiss J.N., Schwarz E.M., Benzer S., Philipson K.D. Anomalous regulation of the Drosophila Na+-Ca2+ exchanger by Ca2+ . J. Gen. Physiol. 1996;108:67–74. doi: 10.1085/jgp.108.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K., Keith F.J., Gay N.J. Relocalization of Drosophila dorsal protein can be induced by a rise in cytoplasmic calcium concentration and the expression of constitutively active but not wild-type Toll receptors. Biochem. J. 1993;296:497–503. doi: 10.1042/bj2960497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. A simple model for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., McNaughton P.A. Electrogenic properties of the Na:Ca exchange. J. Membr. Biol. 1990;113:177–191. doi: 10.1007/BF01870070. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P.A. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc. Natl. Acad. Sci. USA. 1988;85:4548–4552. doi: 10.1073/pnas.85.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.K. In situ hybridization with biotinylated DNA. Dros. Inform. Serv. 1993;72:73–77. [Google Scholar]
- Lisman J.E., Brown P.K. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J. Gen. Physiol. 1972;59:701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Moses K. wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995;121:2279–2289. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- Matthews H.R., Murphy R.L.W., Fain G.L., Lamb T.D. Photoreceptor light adaptation is mediated by cytoplamic calcium concentration. Nature. 1988;334:67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Minke B., Armon E. Activation of electrogenic Na-Ca exchange by light in fly photoreceptors. Vision Res. 1984;24:109–115. doi: 10.1016/0042-6989(84)90095-6. [DOI] [PubMed] [Google Scholar]
- Moon R.T., Brown J.D., Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. Calcium and light adaptation in retinal rods and cones Nature. 334 1988. 69 71a [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment J. Physiol. 395 1988. 675 730b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll D., Hryshko L., Matsuoka S., Frank J., Philipson K. Mutation of amino acid residues in the putative transmembrane segments of the cardiac sarcolemmal Na+-Ca2+ exchanger J. Biol. Chem. 271 1996. 13385 13391a [DOI] [PubMed] [Google Scholar]
- Nicoll D.A., Quednau B.D., Qui Z., Xia Y.R., Lusis A.J., Philipson K.D. Cloning of a third mammalian Na+-Ca2+ exchanger, NCX3 J. Biol. Chem. 271 1996. 24914 24921b [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- O'Day P.M., Gray-Keller M.P. Evidence for electrogenic Na+/Ca2+ exchange in Limulus ventral photoreceptors. J. Gen. Physiol. 1989;93:473–494. doi: 10.1085/jgp.93.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day P.M., Gray-Keller M.P., Lonergan M. Physiological roles of Na+/Ca2+ exchange in Limulus ventral photoreceptors. J. Gen. Physiol. 1991;97:369–391. doi: 10.1085/jgp.97.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko A., Dyck C., Hnatowich M., Buchko J., Nicoll D.A., Philipson K.D., Hryshko L.V. Functional differences in ionic regulation between alternatively spliced isoforms of the Na+-Ca2+ exchanger from Drosophila melanogaster . J. Gen. Physiol. 1998;111:691–702. doi: 10.1085/jgp.111.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban G., Nagy L., Carroll S.B. The role of the Distal-less gene in the development and evolution of insect limbs. Curr. Biol. 1994;4:671–675. doi: 10.1016/s0960-9822(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Peretz A., Sandler C., Kirschfeld K., Hardie R., Minke B. Genetic dissection of light induced Ca2+ influx into Drosophila photoreceptors J. Gen. Physiol. 104 1994. 1057 1077a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A., Suss-Toby E., Rom-Glas A., Arnon A., Payne R., Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calciumeffects of specific mutations Neuron. 12 1994. 1257 1267b [DOI] [PubMed] [Google Scholar]
- Philipson K.D., Nicoll D.A., Matsuoka S., Hryshko L.V., Levitsky D.O., Weiss J.N. Molecular regulation of the Na(+)-Ca2+ exchanger. Ann. N.Y. Acad. Sci. 1996;779:20–28. doi: 10.1111/j.1749-6632.1996.tb44766.x. [DOI] [PubMed] [Google Scholar]
- Ranganathan R., Bacskai B.J., Tsien R.Y., Zuker C.S. Cytosolic calcium transientsspatial localization and role in Drosophila photoreceptor cell function. Neuron. 1994;13:837–848. doi: 10.1016/0896-6273(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Ranganathan R., Malicki D.M., Zuker C.S. Signal transduction in Drosophila photoreceptors. Annu. Rev. Neurosci. 1995;18:283–317. doi: 10.1146/annurev.ne.18.030195.001435. [DOI] [PubMed] [Google Scholar]
- Ready D.F. A multifaceted approach to neural development. Trends Neurosci. 1989;12:102–110. doi: 10.1016/0166-2236(89)90166-5. [DOI] [PubMed] [Google Scholar]
- Ready D.F., Hanson T.E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Reiländer H., Achilles A., Freidel U., Maul G., Lottspeich F., Cook N.J. Primary structure and functional expression of the Na/Ca, K-exchanger from bovine rod photoreceptors. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1689–1695. doi: 10.1002/j.1460-2075.1992.tb05219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke R., Krantz D.E., Yen D., Zipursky S.L. Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell. 1988;52:291–301. doi: 10.1016/0092-8674(88)90518-1. [DOI] [PubMed] [Google Scholar]
- Ruknudin A., Valdivia C., Kofuji P., Lederer W.J., Schulze D.H. Na+/Ca2+ exchanger in Drosophilacloning, expression, and transport differences. Am. J. Physiol. 1997;273:C257–C265. doi: 10.1152/ajpcell.1997.273.1.C257. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. Cold Spring Harbor Laboratories; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Sampath A., Matthews H., Cornwall M., Fain G. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. J. Gen. Physiol. 1998;111:53–64. doi: 10.1085/jgp.111.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp P., Li X., Basu D., Szerencsei R. Regulation of free cytosolic Ca2+ concentration in the outer segments of bovine retinal rods by Na-Ca-K exchange measured with fluo-3. I. Efficiency of transport and interactions between cations J. Biol. Chem. 266 1991. 22975 22982a [PubMed] [Google Scholar]
- Schnetkamp P., Szerencsei R., Basu D. Unidirectional Na+, Ca2+, and K+ fluxes through the bovine rod outer segment Na-Ca-K exchanger J. Biol. Chem. 266 1991. 198 206b [PubMed] [Google Scholar]
- Schnetkamp P.P.M. Sodium-calcium exchange in the outer segments of bovine rod photoreceptors. Physiol. J. 1986;373:25–45. doi: 10.1113/jphysiol.1986.sp016033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp P.P.M. Na-Ca or Na-Ca-K exchange in rod photoreceptors. Prog. Biophys. Mol. Biol. 1989;54:1–29. doi: 10.1016/0079-6107(89)90007-2. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P.P.M., Basu D.K., Szerencsei R.T. Na+-Ca2+ exchange in bovine rod outer segments requires and transports K+ . Am. J. Physiol. 1989;257:C153–C157. doi: 10.1152/ajpcell.1989.257.1.C153. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P.P.M., Basu D.K., Szerencsei R.T. The stoichiometry of Na-Ca+K exchange in rod outer segments isolated from bovine retinas Ann. N.Y. Acad. Sci. 639 1991. 10 21c [DOI] [PubMed] [Google Scholar]
- Schnetkamp P.P.M., Tucker J.E., Szerencsei R.T. Ca2+ influx into bovine retinal rod outer segments mediated by Na+/Ca2+/K+ exchange. Am. J. Physiol. 1995;269:C1153–C1159. doi: 10.1152/ajpcell.1995.269.5.C1153. [DOI] [PubMed] [Google Scholar]
- Schwarz E.M., Benzer S. Calx, a Na-Ca exchanger gene of Drosophila melanogaster . Proc. Natl. Acad. Sci. USA. 1997;94:10249–10254. doi: 10.1073/pnas.94.19.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B.-H., Stamnes M.A., Seavello S., Harris G.L., Zuker C.S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989;338:67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Corces V.G., Moon R.T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling Nature. 390 1997. 410 413a [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Yang-Snyder J., Busa W.B., Moon R.T. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A Dev. Biol. 182 1997. 114 120b [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu. Rev. Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Theisen H., Purcell J., Bennett M., Kansagara D., Syed A., Marsh J.L. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL Wimproving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A. Cellular interactions in the developing Drosophila eye. Development. 1988;104:183–193. doi: 10.1242/dev.104.2.183. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Ready D.F. Neuronal differentiation in the Drosophila ommatidium. Dev. Biol. 1987;120:366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Treisman J.E., Rubin G.M. wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development. 1995;121:3519–3527. doi: 10.1242/dev.121.11.3519. [DOI] [PubMed] [Google Scholar]
- Treisman J.E., Heberlein U. Eye development in Drosophilaformation of the eye field and control of differentiation. Curr. Top. Dev. Biol. 1998;39:119–158. doi: 10.1016/s0070-2153(08)60454-8. [DOI] [PubMed] [Google Scholar]
- Tsoi M., Rhee K.H., Bungard D., Li X.F., Lee S.L., Auer R.N., Lytton J. Molecular cloning of a novel potassium-dependent sodium-calcium exchanger from rat brain. J. Biol. Chem. 1998;273:4155–4162. doi: 10.1074/jbc.273.7.4155. [DOI] [PubMed] [Google Scholar]
- Tucker J.E., Winkfein R.J., Cooper C.B., Schnetkamp P.P. cDNA cloning of the human retinal rod Na-Ca + K exchangercomparison with a revised bovine sequence. Invest. Ophthalmol. Vis. Sci. 1998;39:435–440. [PubMed] [Google Scholar]
- Wilson R., Ainscough R., Anderson K., Baynes C., Berks M., Bonfield J., Burton J., Connell M., Copsey T., Cooper J. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. . Nature. 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Yau K.-W. Phototransduction mechanism in retinal rods and cones. Invest. Ophthalmol. Vis. Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- Yau K.-W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984;311:661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Zuker C.S. The biology of vision in Drosophila . Proc. Natl. Acad. Sci. USA. 1996;93:571–576. doi: 10.1073/pnas.93.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]