Abstract
The mitogen-activated protein (MAP) kinase pathway is a critical regulator of cell growth, migration, and differentiation. Growth factor activation of MAP kinase in NIH 3T3 cells is strongly dependent upon integrin-mediated adhesion, an effect that contributes to the anchorage dependence of normal cell growth. We now show that expression of constructs that constitutively activate focal adhesion kinase (FAK) rescued the defect in serum activation of MAP kinase in suspended cells without directly activating MAP kinase. Dominant negative FAK blocked both the rescue of suspended cells by the activated construct and the serum activation of MAP kinase in adherent cells. MAP kinase in FAK−/− mouse embryo fibroblasts was adhesion-insensitive, and reexpression of FAK restored its adhesion dependence. MAP kinase activity in ras-transformed cells is still decreased in suspension, but expression of constructs that constitutively activate FAK enhanced their anchorage-independent growth without increasing adherent growth. V-src, which activates both Ras and FAK, induced MAP kinase activation that was insensitive to loss of adhesion, and that was blocked by a dominant negative FAK. These results demonstrate that FAK mediates the integrin requirement for serum activation of MAP kinase in normal cells, and that bypassing this mechanism contributes to anchorage-independent growth in transformed cells.
Keywords: growth factor regulation, signal transduction, anchorage independence, src transformation, cancer
Mitogen-activated protein (MAP)1 kinases, also known as Erks 1 and 2 (for extracellular-regulated kinases), control many cellular responses including proliferation, migration, and differentiation (for reviews see Crews and Erikson 1993; Blumer and Johnson 1994; M.H. Cobb et al. 1994; Johnson and Vaillancourt 1994; Marshall 1995). The MAP kinase pathway is stimulated by soluble growth factors and cytokines, and in some cases by cell adhesion to extracellular matrix (ECM) proteins (Chen et al. 1994; Morino et al. 1995; Zhu and Assoian 1995). Normal cell growth requires stimulation by both growth factors and adhesion to the ECM. We and others have reported that growth factor activation of the MAP kinase pathway requires cell adhesion (Cybulsky et al. 1994; Inoue et al. 1996; Miyamoto et al. 1996; Cybulsky and McTavish 1997; Lin et al. 1997b; Renshaw et al. 1997). Adding serum or growth factors to nonadherent cells triggers robust activation of early components of the pathway such as Ras, but activation of Raf or MEK1 is substantially diminished (Lin et al., 1997; Renshaw et al. 1997). This effect is distinct from the transient induction of MAP kinase activity when suspended cells are replated on ECM proteins, which involves integrin activation of Ras (Chen et al. 1994; Schlaepfer et al. 1994). We also showed that the effect is specifically mediated by integrins (Renshaw et al. 1997).
Focal adhesion kinase (FAK) is a nonreceptor tyrosine kinase that is ubiquitously expressed throughout development (Furuta et al. 1995), and is widely expressed in adherent cell types in culture (Kornberg et al. 1992; Matsumoto et al. 1994; Zhang et al. 1994). Its phosphorylation and kinase activity are closely regulated by integrin-mediated cell adhesion (Kanner et al. 1990; Guan and Shalloway 1992), suggesting that FAK may be an important mediator of integrin signaling. Deletion of FAK from the mouse genome leads to an early embryonic lethal phenotype indicative of a defect in gastrulation. Cells from FAK−/− mice show decreased migration, which is consistent with deficient gastrulation. Other major defects include impaired vasculogenesis and angiogenesis. The overall phenotype of the FAK−/− mouse is similar to the fibronectin (FN−/−) mouse (George et al. 1993), supporting the hypothesis that FAK lies on an integrin signaling pathway.
FAK has been implicated in cell migration (Ilic et al. 1995; Cary et al. 1996; Gilmore and Romer 1996), growth, and survival (Furuta et al. 1995; Frisch et al. 1996; Gilmore and Romer 1996; Hungerford et al. 1996). It has been shown to bind a variety of adapter and signaling molecules including paxillin (Turner and Miller 1994), src family kinases (M.H. Cobb et al. 1994; Schlaepfer et al. 1994), p130cas (Polte and Hanks 1995), PI 3-kinase (Chen and Guan 1994), and GRB2 (Schlaepfer et al. 1994). However, there is little information available concerning specific biochemical pathways that mediate cellular effects of FAK. FAK has been proposed to mediate the transient integrin-dependent activation of MAP kinase when suspended cells are replated on ECM proteins such as FN (Chen et al. 1994; Schlaepfer et al. 1994; Schlaepfer and Hunter 1997). This pathway was reported to involve GRB2 binding to phosphorylated FAK, followed by recruitment of SOS, and activation of ras. However, other groups showed that activation of MAP kinase by adhesion to ECM does not correlate with FAK phosphorylation (Wary et al. 1996; Lin et al., 1997). Furthermore, FAK−/− fibroblasts still show integrin activation of MAP kinase upon replating (Sieg et al. 1998). It seems likely that FAK can contribute to the direct integrin activation of MAP kinase in replated cells under some experimental conditions, but that there are other pathways that make FAK nonessential.
We have examined the role of FAK, not in the transient integrin-dependent activation of MAP kinase that occurs upon replating of cells on ECM proteins, but in the integrin requirement for growth factor activation of MAP kinase in stably adherent cells. Our data show that FAK activity is both necessary and sufficient for the integrin enhancement of growth factor induction of the MAP kinase pathway. Our results also define the role of FAK in oncogenic transformation by v-src.
Materials and Methods
Reagents and Plasmids
DME, calf serum, and Lipofectamine were purchased from GIBCO BRL. FBS was purchased from Gemini Bio-Products Inc. Myelin basic protein was prepared from spinal cord (Pelfreeze) as previously described (Deibler et al. 1972). Methyl cellulose, agarose, and all other reagents were purchased from Sigma Chemical Co. unless otherwise specified. The plasmids CMV5 HA-ERK2 (Renshaw et al. 1996), IL2Rβ1, IL2Rβ3 (Akiyama et al. 1994; LaFlamme et al. 1994), CD2FAK, CD2FAK K454R, CD2FAK Y397F (Chan et al. 1994), FRNK and pcDNA3 FAK wt (Sieg et al. 1998), pDCR ras G12V, and RSV Hyg (Renshaw et al. 1997) are as described. The src construct containing an activating point mutation at tyrosine 527 was obtained from Dr. Jean Wang (University of California, San Diego).
Cell Culture and Transfection
NIH 3T3 cells were cultured in DME supplemented with 10% bovine calf serum. FAK+/+ and FAK−/− mouse embryo fibroblasts (MEFs) were cultured in DME supplemented with 10% FBS. For transfections, cells were plated at a density of 4 × 105 cells per 6-cm dish 24 h before transfection. Cells were transfected with Lipofectamine (GIBCO BRL) as previously described (Renshaw et al. 1997). 24 h after transfection, cells were transferred to medium containing 0.5% serum for an additional 24 h for adherent cells. Cells that were suspended for 24 h were trypsinized 24 h after transfection and placed in suspension in DME media containing 0.5% methyl cellulose, 0.4% serum over 1% agar-coated dishes as previously described (Renshaw et al. 1997). Cells were stimulated by the addition of serum to 10% for 10 min, extracted in lysis buffer (Renshaw et al. 1997), and assayed for Erk2 and MEK 1 activity.
Transformation
For transformation assays, cells were transfected using Lipofectamine with 0.4 μg of RSVHyg and 0.8 μg of two different cDNAs to give 2.0 μg total DNA. Vectors were the empty control vector, pDCR ras G12V, IL2β1, and CD2FAK. After 24 h, the cells were fed fresh medium and allowed to grow for 2 d. Cells were trypsinized and 1/20 of the total was replated in either normal growth medium, minimal medium, or soft agar to measure foci formation as previously described (Renshaw et al. 1997). Cells were also replated in medium containing 200 μg/ml Hygromycin B (Boehringer Mannheim) to measure transformation efficiency. Soft agar colony volume was determined by visually measuring colony diameter against a scale, and then calculating the volume according to the formula, V = 4/3πr3. Minimal media foci size was determined by trypsinizing the foci and replating them in soft agar. The number of soft agar colonies were counted after 2 wk to determine the number of transformed cells per original minimal medium focus.
Measurement of Erk 2 Activity
For assays of transfected hemagglutinin-tagged (HA) Erk2 activity, anti–HA (12CA5) antibody purified over an HA peptide affinity column was used for immunoprecipitations. Erk activities were immunoprecipitated from 150 μg of cell lysates using 0.5 μg of anti–HA antibody. For all assays, Erk activation was normalized to the amount of Erk2 protein immunoprecipitated. One third of each immunoprecipitation was run on a 10% SDS–polyacrylamide gel that was transferred to Hybond C (Amersham) and immunoblotted using the anti–Erk2 antibody (C-14; Santa Cruz Biotechnology) to measure the amount of Erk2 protein. The remaining two thirds were used to measure Erk2 activity to measure the in-gel kinase assay as described (Renshaw et al. 1997). Activities of endogenous Erks 1 and 2 from FAK+/+ and FAK−/− MEFs were measured by running 5 μg of total cell lysate on gels for assay by the in-gel kinase assay method. In brief, samples were run on 12.5% SDS–polyacrylamide gels containing 0.25 mg/ml myelin basic protein and renatured. Kinase reactions were performed soaking gels in kinase buffer (Kamashita and Fujisawa 1989) containing 25 μCi/ml γ-[32P]ATP and 10 μM cold ATP. Gels were washed exhaustively and analyzed by autoradiography. Autoradiographs were quantitatively analyzed using a model I.S. 1000 digital imaging system from Alpha-Innotech Corp.
Measurement of MEK 1 Activity
Endogenous MEK-1 was immunoprecipitated from 100 μg of cell lysate using anti–MEK-1 (Santa Cruz Biotechnology). Immunoprecipitates were washed three times in lysis buffer and once in kinase buffer (Chen et al. 1996), 10 mM Tris, pH 7.5, 10 mM MgCl2, and 1 mM DTT. One fifth of the samples were used to measure the amount of MEK-1 protein by Western blotting, four fifths were used for kinase assays. MEK kinase activity was measured in kinase buffer containing 25 μM ATP, 5 μCi γ-ATP, and 2 μg of kinase-dead GST-ERK2 (Hipskind et al. 1994), for 30 min at room temperature. Samples were electrophoresed on 10% SDS–polyacrylamide gels, which were dried and analyzed by autoradiography.
Results
Restoration of MAP Kinase Activation in Suspension
Our strategy for identifying components of the pathway that mediates the integrin requirement for growth factor activation of MAP kinase was to screen for activated mutants that could specifically restore MAP kinase in suspended cells. As an initial test of the strategy, HA-tagged Erk2 was coexpressed with a chimera that contains the cytoplasmic tail of the integrin β1 subunit fused to the extracellular and transmembrane domain of the Tac subunit of the IL-2 receptor (IL2Rβ1) (Akiyama et al. 1994). This chimera induces FAK phosphorylation in suspended cells, indicating that it signals constitutively (Akiyama et al. 1994). Previous work showed that cellular responses to serum or the purified mitogen platelet–derived growth factor or lysophosphatidic acid were similarly modulated by cell adhesion (Renshaw et al. 1997). Thus, cells were stimulated with 10% serum for convenience, and because it mimics normal culture conditions.
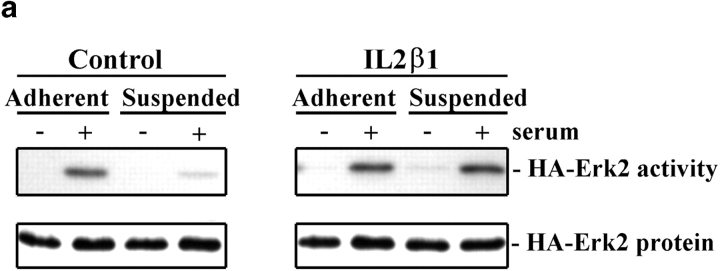
When cotransfected with a control plasmid, the activity of the transiently transfected HA-Erk2 was strongly stimulated by serum in adherent cells, but was minimal in suspended cells (Fig. 1). This behavior mimics the endogenous MAP kinase (Renshaw et al. 1997), indicating that the transfected Erk is regulated normally. By contrast, cells cotransfected with HA-Erk2 and IL2Rβ1 showed strong activation in both the adherent and suspended cells. Expression of IL2Rβ3 also rescued serum induction of MAP kinase activity in suspension, but expression of an IL2α5 construct had no effect (not shown). Neither IL2Rβ1 nor IL2Rβ3 directly induced Erk activity in the absence of serum or altered its activation in adherent cells. These results show that a constitutively activated integrin β1 cytoplasmic tail can substitute for cell adhesion and specifically restore serum activation of MAP kinase in suspended cells.
Figure 1.
IL2Rβ1 restores the serum induction of MAP kinase in suspended cells. (a) NIH 3T3 cells were cotransfected with HA-Erk2 and either IL2β1 or empty control vector. Activity of the immunoprecipitated HA-Erk2 was measured from both adherent or suspended cells that were starved for 24 h in 0.5% serum (−), or stimulated with 10% serum for 10 min (+). The amount of immunoprecipitated HA-Erk2 protein was determined by Western blotting. (b) Quantitation of the Erk kinase assays is shown graphically. Values are normalized to Erk protein levels and represent the means ± SDs from four independent experiments.
FAK Activity Mediates the Integrin Signal
The IL2Rβ1 construct induces phosphorylation of FAK in suspended cells (Akiyama et al. 1994; Lukashev et al. 1994); thus, FAK was an obvious candidate to mediate this effect. To test if FAK was involved, a FAK chimera (CD2FAK) that has constitutively high kinase activity (Chan et al. 1994) was coexpressed with the HA-tagged Erk2 construct. Expression of CD2FAK also completely restored serum activation of Erk2 in suspended cells (Fig. 2), but like the IL2Rβ1, did not directly activate MAP kinase in the absence of serum or alter activation of Erk2 in adherent cells. Kinase-defective CD2FAK (K454R) had no effect on MAP kinase, indicating that tyrosine kinase activity is required.
Figure 2.
Restoration of MAP kinase activation by constitutively active FAK. (a) Activity of transfected MAP kinase was measured as in Fig. 1, in 3T3 cells cotransfected with HA-Erk2 and either constitutively active FAK (CD2FAK) or the kinase-dead variant (CD2FAK K454R). (b) The means ± SDs for specific kinase activity (normalized to protein level) were quantitated from three independent experiments.
It is well established that activity of endogenous FAK is regulated by integrin-mediated adhesion (Guan and Shalloway 1992; Lipfert et al. 1992; Schaller et al. 1992), though soluble mitogens also stimulate FAK in some systems (Zachary et al. 1992; Rankin et al. 1996). We also observed that kinase activity of FAK immunoprecipitated from adherent 3T3 cells was much higher than from suspended cells, and that addition of serum caused only a slight increase in either case (data not shown). Western blotting of whole cell lysates indicated no change in the levels of FAK or appearance of proteolytic fragments after 24 h in suspension (data not shown). These results are in agreement with published data and support the idea that FAK is regulated by cell adhesion.
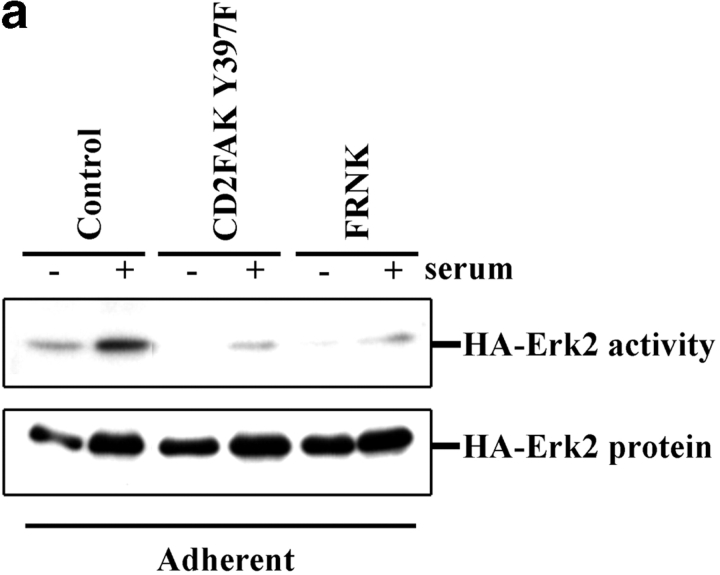
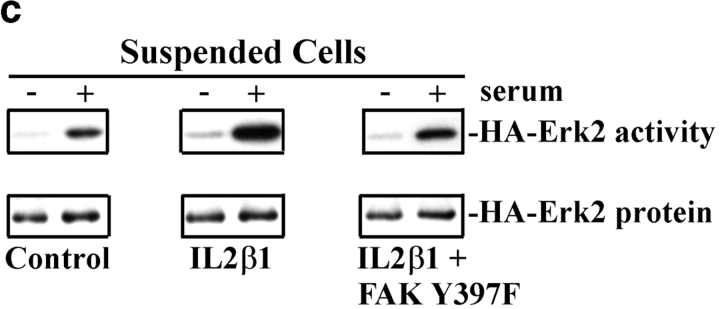
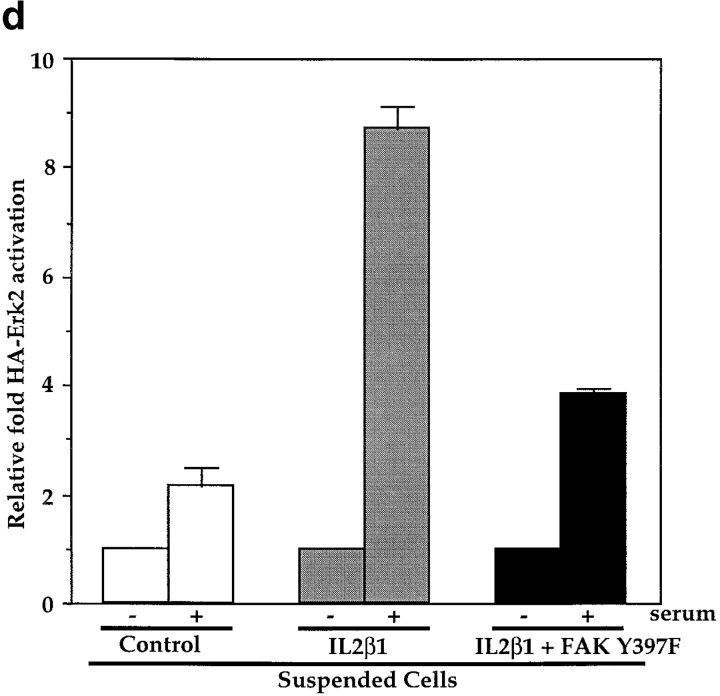
Next, we tested whether FAK was required for the effect of integrins on this pathway. HA-Erk2 was coexpressed with the FAK tyrosine autophosphorylation mutant (CD2FAK Y397F) or the COOH terminus of FAK, termed FRNK, both of which have been shown to function as dominant negatives (Gilmore and Romer 1996; Richardson and Parsons 1996; Richardson et al. 1997). These mutants were tested for their ability to block effects of the IL2β1 chimera and the endogenous integrins that bind to ECM in adherent cells in culture. Results from these experiments showed that the Y397F FAK mutant blocked activation of MAP kinase in adherent cells by 80% (Fig. 3, a and b). A similar result (79% inhibition) was obtained using the dominant negative FAK construct, FRNK. Dominant negative FAK also blocked the rescue of Erk2 activity by IL2β1 in suspended cells (Fig. 3c and Fig. d). These results demonstrate that the integrin-dependent signal that promotes serum activation of MAP kinase requires FAK.
Figure 3.
Inhibition of MAP kinase by dominant negative FAK. (a) HA-Erk2 kinase activity was measured in stably adherent cells cotransfected with HA-Erk2 and either CD2FAK Y397F, FRNK, or the control vector. Cells were starved for 24 h in low serum (−) or starved, and then stimulated with 10% serum (+). (b) Bar graphs depict the means and SDs of data from at least three independent experiments. (c) HA-Erk2 kinase activity was measured in suspended cells cotransfected with HA-Erk2 and either the control vector, IL2Rβ1, or IL2Rβ1 in combination with CD2FAK Y397F. (d) Graphs depict the means ± range from two experiments.
MAP Kinase in FAK−/− Cells Is Adhesion-insensitive
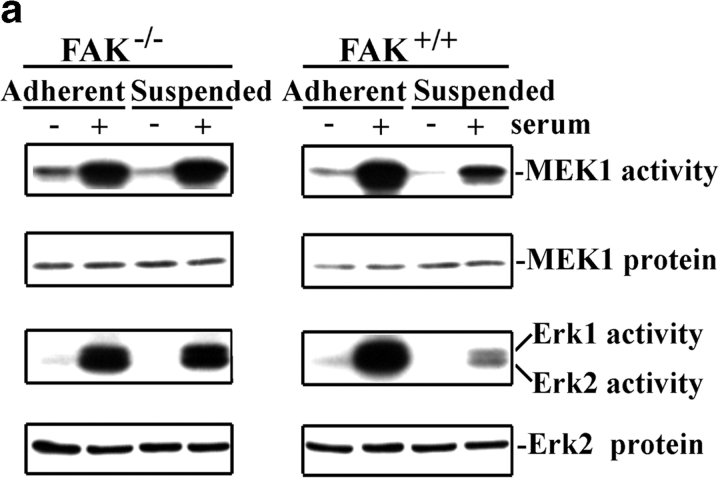
To test the role of FAK in this pathway without relying on overexpression of mutant proteins, we examined adherent and suspended polyclonal fibroblasts from FAK−/− mouse embryos. FAK-positive MEFs behave similarly to 3T3 cells in that the activation of MEK 1, Erk1, and Erk2 was strongly dependent on cell adhesion (Fig. 4). By contrast, the FAK−/− cells showed no adhesion dependence for the activation of the MAP kinase pathway. The absolute level of kinase activation in the FAK−/− cells was lower than in FAK+/+ cells, equivalent to 46% for MEK1 and 61% for Erk activity relative to FAK+/+ cells. However, the baseline for MAP kinase activity in the FAK−/− cells in the absence of serum was 72% higher than in FAK+/+ cells; serum induction of MAP kinase activity was, therefore, 3.4-fold in FAK−/− cells compared with 9.5-fold in FAK+/+ cells. Importantly, no reduction in the activation of MEK or Erks occurred when the FAK−/− cells were placed in suspension. Thus, although these cells may have partially adapted for the loss of FAK, they have completely lost the adhesion dependence for the regulation of MAP kinase by serum. We also noted that Raf in both FAK+/+ and FAK−/− cells showed a strong gel shift upon addition of serum, independent of whether cells were adherent or suspended (not shown). These results agree with our previous data showing that adhesion acts at the step between Raf and MEK (Renshaw et al. 1997).
Figure 4.
MAP kinase activation is adhesion-insensitive in FAK−/− cells. (a) Activation of endogenous MEK-1 and Erks 1 and 2 was measured in both stably adherent and suspended FAK+/+ and FAK−/− MEFs. (b) Graphs depict the means ± SDs for endogenous MEK-1 kinase activity normalized to protein levels from at least three experiments. (c) Graphs represent means ± SD from three experiments measuring endogenous Erks 1 and 2 activation normalized to protein level.
To confirm that FAK was responsible for these differences, FAK−/− cells were transiently cotransfected with wild-type (wt) FAK and HA-Erk2. Expression of wt FAK in the FAK−/− cells completely restored the adhesion dependence for the activation of the HA-Erk2, whereas cotransfection with empty vector had no effect (Fig. 5). These data demonstrate that FAK is absolutely required for the adhesion dependence of MAP kinase activation by serum.
Figure 5.
Reconstitution of FAK−/− cells. (a) FAK−/− cells were cotransfected with HA-Erk2 and either the control vector or wt FAK. Activation of HA-Erk2 and protein levels were measured in adherent and suspended cells as for Fig. 1. (b) Graphs display the mean ± SD of kinase activity normalized to protein levels from three independent experiments.
FAK Promotes Anchorage-independent Growth
The ability of the IL2Rβ1 and CD2FAK chimeras to maintain serum activation of MAP kinase in suspended cells suggests that these constructs should promote anchorage-independent growth. Our previous work showed that oncogenic V12 ras strongly activates MAP kinase, but that this activation is still dramatically decreased in suspended cells (Renshaw et al. 1997). Thus, increasing MAP kinase in suspended ras-transformed cells should enhance colony formation in semisolid medium. To test this hypothesis, ras G12V was cotransfected with either active FAK (CD2FAK) or with the IL2Rβ1 construct. Cell growth was assayed under adherent and nonadherent conditions (Table and Table ). Expression of IL2Rβ1 or CD2FAK did not increase the number of ras transformants (foci), consistent with the idea that activated ras transforms cells with high efficiency (White et al. 1995) (Table .) Nor were these constructs sufficient to induce anchorage independence or foci in low serum in the absence of ras, which is consistent with data showing that activation of MAP kinase alone transforms cells very poorly (Khosravi-Far et al. 1995; White et al. 1995). However, cotransfection of CD2FAK or IL2Rβ1 enhanced the growth rate of ras-transformed cells in suspension (Table II.). Importantly, this effect occurred without altering growth of adherent ras-transformed cells, where endogenous integrins already promote MAP kinase activation by serum. These data are consistent with the hypothesis that these constructs specifically enhance anchorage-independent growth by allowing transforming ras to activate the MAP kinase pathway to its full potential in nonadherent cells.
Table 1.
Stable Transformation Assay
| Vectors | HygR | Foci in 10% CS | Foci in 0.5% CS | Soft agar colonies |
|---|---|---|---|---|
| Control | 1,190 ± 137 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ras | 1,217 ± 93 | 1,598 ± 56 | 1,472 ± 83 | 1,481 ± 29 |
| IL2Rβ1 | 1,053 ± 64 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ras+IL2Rβ1 | 1,095 ± 80 | 1,417 ± 15 | 1,338 ± 55 | 1,325 ± 40 |
| CD2FAK | 1,137 ± 129 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Ras+CD2FAK | 1,213 ± 110 | 1,080 ± 246 | 1,078 ± 314 | 1,030 ± 320 |
NIH 3T3 cells were cotransfected with RSVHyg and combinations of the plasmids for Ras G12V, IL2Rβ1, CD2FAK, or empty control vector. Total DNA was kept constant. Cells were trypsinized 2 d after transfection and replated in media containing 10% CS with 200 μg/ml hygromycin B, 10% CS, 0.5% CS, or soft agar to measure foci and colony formation. Values are means ± SD from three separate experiments representing the total number of foci or colonies calculated for the entire transfection.
Table 2.
Soft Agar and Minimal Medium Colony Size
| Vectors | Soft agar | Foci in 0.5% CS | ||
|---|---|---|---|---|
| Colony volume* | Relative | Foci size‡ | Relative | |
| Ras | 0.29 ± 0.08 | 1.00 | 93 ± 6 | 1.00 |
| Ras+IL2Rβ1 | 1.84 ± 0.01 | 6.23 | 87 ± 7 | 0.94 |
| Ras+CD2FAK | 1.49 ± 0.03 | 5.07 | 95 ± 7 | 1.02 |
Transfections from Table were further analyzed by measuring the soft agar colony diameter and calculating (4/3πr3) the volume (× 10−2 mM3)*. Values represent the average from two independent experiments in which 15 colonies were measured for each transfection combination. ‡ Minimal medium foci size was determined by trypsinization followed by replating in soft agar to determine the number of transformed cells per minimal media foci. Values represent the average of two experiments in which at least 40 foci from each transfection were trypsinized.
FAK in Transformation by v-src
FAK was originally identified as a protein whose tyrosine phosphorylation increased in v-src transformed cells (Kanner et al. 1990). Furthermore, oncogenic src was shown to stimulate FAK activity in both adherent and suspended cells (Guan and Shalloway 1992). Transforming variants of src also induce activation of endogenous ras (Gibbs et al. 1990). These results suggest the interesting prediction that by activating both FAK and Ras, v-src might efficiently promote activation of MAP kinase in suspended cells. Thus, to further test the role of FAK in transformation, the activity of MAP kinase in cells transfected with activated src was assayed. These experiments showed that MAP kinase activity in suspended src-expressing cells was 96 ± 4% of that in adherent cells, compared with 16 ± 2% for suspended ras-transfected cells (n = 4). Thus, anchorage-independent activation of FAK correlates with anchorage-independent activation of MAP kinase.
To determine if FAK activity was required for the adhesion-independent activation of MAP kinase by activated src, we cotransfected src and HA-Erk2 along with either a dominant negative FAK (FRNK) or an empty control vector. Coexpression of dominant negative FAK inhibited Erk2 activation in the src-transfected cells by 60–70% in either suspended or adherent cells (Fig. 6). Consistent with our results in which FRNK inhibited the serum activation of MAP kinase in adherent cells, coexpression of FRNK also strongly inhibited ras activation of MAP kinase in adherent cells, but had only a weak effect in suspended ras cells. We also tested whether colony formation was inhibited when src was cotransfection with the FRNK construct. However, no decrease in soft agar growth was observed (data not shown). This result may demonstrate that FAK is not essential to transformation by activated src, or may only indicate that inhibitory levels of FRNK are difficult to achieve in stable cotransfectants.
Figure 6.
v-src activation of MAP kinase is dependent upon FAK. (a) NIH 3T3 cells were cotransfected with HA-Erk2 and either active src (pSrcII) or ras G12V in combination with FRNK or the empty control vector. MAP kinase activity was measured in both adherent and suspended cells in low serum. (b) Bar graphs depict the means ± range from two experiments. Kinase activity is normalized to Erk2 protein levels.
Discussion
First, our data demonstrate that the integrin signal that mediates the serum activation of the MAP kinase pathway emanates from the β cytoplasmic domain of the integrin receptor. This conclusion is based on the result that an activated IL2Rβ1 chimera containing only the cytoplasmic domain from the integrin was sufficient to restore MAP kinase in suspended cells. However, this construct did not induce serum-independent MAP kinase activity. Thus, the contributions of integrins and growth factor receptors to the MAP kinase pathway can be separated, and the IL2Rβ1 activates only the integrin component of the pathway.
Second, we found that this integrin signal is mediated by FAK. Expression of the IL2Rβ1 chimera, which was previously shown to induce FAK phosphorylation (Akiyama et al. 1994; Lukashev et al. 1994), restored activation of MAP kinase in suspended cells. Consistent with this result, we found that expression of a constitutively active FAK was also sufficient to restore MAP kinase induction in suspension. Conversely, dominant negative FAK constructs inhibited the activation of MAP kinase induced by serum in adherent cells or the rescue by IL2Rβ1 in suspended cells. These conclusions were confirmed in FAK−/− MEF cells, which showed a complete loss of the effect of cell adhesion. Expression of FAK restored the integrin regulation of MAP kinase induction by serum in FAK−/− cells, indicating that the differences in FAK−/− cells were not due to secondary genetic alterations. These results are all the more surprising in light of recent findings that FAK is not essential for the transient activation of MAP kinase that occurs when suspended cells are replated on FN or other ECM proteins (Wary et al. 1996; Lin, 1997a; Sieg et al. 1998). Therefore, these results suggest that FAK may be more crucial for the synergism with growth factor induction of MAP kinase than it is for the direct integrin induction of MAP kinase activity. However, it should be noted that the experimental conditions employed for these published studies of direct integrin activation of Erk differ from ours in some respects. FAK is activated by a variety of ECM proteins that bind different integrins, suggesting that the effect we describe here should relatively widespread, so that many different matrices can promote growth factor activation of MAP kinase. However, we have not compared different ECM proteins for their potency in this regard.
A number of signaling molecules known to associate with FAK in focal adhesions such as paxillin (Richardson and Parsons 1996) and Cas (Polte and Hanks 1995) represent potential downstream targets for FAK. However, further work will be required to elucidate this pathway. A critical role for FAK in growth factor induction of MAP kinase activity is likely to explain the loss of cell cycle progression when FAK is inhibited (Gilmore and Romer 1996), and may be important in the embryonic lethality of FAK−/− mouse embryos. MAP kinase has been demonstrated to regulate cell migration through the enhancement of myosin light chain phosphorylation (Klemke et al. 1997). Thus, the requirement for FAK activity in the efficient activation of MAP kinase could also be related to effects of FAK on cell motility, as demonstrated in a number of studies (Ilic et al. 1995; Cary et al. 1996; Gilmore and Romer 1996).
Previous studies have shown that FAK and src family kinase function in a highly cooperative manner (M.H. Cobb et al. 1994; Schlaepfer and Hunter, 1994, Schlaepfer and Hunter 1997). Thus, the Y397F mutant of FAK that does not bind c-src functions as a strong dominant negative for FAK, whereas the K454R kinase defective mutant that still binds c-src does not (Cary et al. 1996; Schlaepfer and Hunter 1997). Our result that the Y397F but not the K454R mutant blocks FAK signaling in adherent cells is, therefore, consistent with previous studies, and most likely reflects the ability of the K454R mutant to be phosphorylated by other kinases (or endogenous FAK), enabling it to bind c-src. Therefore, it is likely that src family kinases make a critical contribution to the effects described here, either by activating FAK or by directly phosphorylating key downstream substrates.
Third, our data implicate FAK in oncogenic transformation by v-src. Constitutive FAK activation is not sufficient to transform cells but substantially potentiates anchorage-independent growth induced by v-ras without altering growth of adherent ras cells. It is important to note in this regard that while ras-transformed cells form colonies in soft agar, their growth is markedly slower in suspension than when adherent. v-src, which induces constitutive activation of FAK (Kanner et al. 1990; Guan and Shalloway 1992), as well as activation of ras stimulates MAP kinase in an adhesion-independent manner. MAP kinase activation by v-src was inhibited by dominant negative FAK in both adherent and suspended cells, indicating that FAK contributes to v-src induction of MAP kinase under both conditions.
Tumorigenesis is thought to be a multistep process. Deregulation of FAK could greatly enhance the tumorigenicity of transformed cells in vivo via effects on both growth and motility. Anchorage-independent growth is the in vitro characteristic that correlates most closely with in vivo tumorigenicity (Freedman and Shin 1974). Motility plays a critical role in invasion and metastasis. FAK expression correlates with motility in human melanoma cell lines (Akasaka et al. 1995), and its activity and expression increase in metastatic tumors (Weiner et al. 1993; Owens et al. 1995). Elevated FAK expression also correlated with the invasive potential of tumors (Owens et al. 1995). These data support the notion that elevation of FAK levels or activity can contribute to progression of human tumors.
In mammalian cells, cellular functions such as growth, gene expression, and migration are controlled by multiple external stimuli, and understanding how these inputs are integrated is an important goal in cell biology. Our results identify a point of intersection between integrin and growth factor pathways, and identify FAK as a key component of the integrin but not the growth factor arm of this pathway. These data also represent the first example where oncogene function can be understood in terms of specific activation of an integrin-mediated event. Therefore, this work contributes to our understanding of both normal cell regulation and the subversion of these pathways in cancer.
Acknowledgments
The FAK+/+ and FAK−/− MEFs were a gift from Dr. Dusko Ilic (University of California, San Francisco, San Francisco, CA). The CD2FAK constructs were a gift from Dr. A. Aruffo at Bristol-Myers-Squibb (Seattle, WA). The FRNK and wt FAK constructs were the gift of Dr. David D. Schlaepfer at Scripps Research Institute (La Jolla, CA).
This work was supported by the National Institutes of Health, grant numbers P01 HL57900 (MAS) and F32 GM18298 (MWR).
Footnotes
1.used in this paper: ECM, extracellular matrix; Erk, extracellular regulated kinase; FAK, focal adhesion kinase; FN, fibronectin; HA, hemagglutinin; MAP, mitogen-activated protein
References
- Akasaka T., van Leeuwen I.G., Yoshinaga I.G., Mihm M.C., Byers H.R. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J. Investig. Dermatol. 1995;105:104–108. doi: 10.1111/1523-1747.ep12313396. [DOI] [PubMed] [Google Scholar]
- Akiyama S.K., Yamada S.S., Yamada K.M., LaFlamme S.E. Transmembrane signal transduction by integrin cytoplasmic domain expressed in single-subunit chimeras. J. Biol. Chem. 1994;269:15961–15964. [PubMed] [Google Scholar]
- Blumer K.J., Johnson G.L. Diversity in function and regulation of MAP kinase pathways. TIBS (Trends Biochem. Sci.) 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Cary L.A., Chang J.F., Guan J.L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with src and fyn. J. Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- Chan P.-Y., Kanner S.B., Whitney G., Aruffo A. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK . J. Biol. Chem. 1994;269:20567–20574. [PubMed] [Google Scholar]
- Chen H.C., Guan J.L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Kinch M.S., Lin T.H., Burridge K., Juliano R.L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chen Q., Lin T.S., Der C.J., Juliano R.L. Integrin-mediated activation of mitogen-activated protein (MAP) or extracellular signal related kinase kinase (MEK) and kinase is independent of Ras. J. Biol. Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- Cobb B.S., Schaller M.D., Leu T.H., Parsons J.T. Stable association of pp60src and pp50fyn with the focal adhesion associated protein tyrosine kinase pp125FAK. Mol. Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M.H., Xu S., Hepler J.E., Hutchinson M., Frost J., Robbins D.J. Regulation of the MAP kinase cascade. Cell Mol. Biol. Res. 1994;40:253–256. [PubMed] [Google Scholar]
- Crews C.M., Erikson R.L. Extracellular signals and reversible protein phosphorylationwhat to MEK of it all. Cell. 1993;74:215–217. doi: 10.1016/0092-8674(93)90411-i. [DOI] [PubMed] [Google Scholar]
- Cybulsky A.V., McTavish A.J., Cyr M.D. Extracellular matrix modulates epidermal growth factor receptor activation in rat glomerular epithelial cells. J. Clin. Invest. 1994;94:68–78. doi: 10.1172/JCI117350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky A.V., McTavish A.J. Extracellular matrix is required for MAP kinase activation and proliferation of rat glomerular epithelial cells. Biochem. Biophys. Res. Commun. 1997;231:160–166. doi: 10.1006/bbrc.1997.6064. [DOI] [PubMed] [Google Scholar]
- Deibler G.E., Martenson R.E., Kies M.W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep. Biochem. 1972;2:139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Freedman V.H., Shin S. Cellular tumorigenicity in nude micecorrelation with cell growth in semisolid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Frisch S.M., Vuori K., Ruoslahti E., Chan P.Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Ilic D., Kanazawa S., Yamamoto T., Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- George E., Georges-Labouesse E., Patel-King R., Rayburn H., Hynes R. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gibbs J.B., Marshall M.S., Scolnick E.M., Dixon R.A.F., U.S. Vogel Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and GTPase activating protein (GAP) J. Biol. Chem. 1990;265:20437–20442. [PubMed] [Google Scholar]
- Gilmore A.P., Romer L.H. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J.-L., Shalloway D. Regulation of pp125FAK both by cellular adhesion and by oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Hipskind R.A., Baccarini M., Nordheim A. Transient activation of Raf- 1, MEK, and Erk2 coincides kinetically with ternary complex factor phosphorylation and immediate early gene promoter activity in vivo. Mol. Cell Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerford J.E., Compton M.T., Matter M.L., Hoffstrom B.G., Otey C.A. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J. Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T., Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Inoue H., Yamashita A., Hahura A. Adhesion-dependency of serum-induced p42/p44 MAP kinase activation is released by retroviral oncogenes. Virology. 1996;225:223–226. doi: 10.1006/viro.1996.0591. [DOI] [PubMed] [Google Scholar]
- Johnson G.L., Vaillancourt R.R. Sequential protein kinase reactions controlling cell growth and differentiation. Curr. Opin. Cell Biol. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Kamashita I., Fujisawa H. A sensitive method for detection of calmodulin dependent protein kinase II activity in sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kanner S.B., Reynolds A.B., Vines R.R., Parsons J.T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc. Natl. Acad. Sci. USA. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R., Solski P.A., Clark G.J., Kinch M.S., Der C.J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke R.L., Cai S., Giannini A.L., Gallagher P.J., de Lanerolle P., Cheresh D.A. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L., Earp H.S., Parsons J.T., Schaller M., Juliano R.L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J. Biol. Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- LaFlamme S.E., Thomas L.A., Yamada S.S., Yamada K.M. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J. Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.H., Alpin A.E., Shen Y., Chen Q., Schaller M., Romer L., Aukhil I., Juliano R.L. Integrin-mediated activation of MAP kinase is independent of FAKevidence for dual integrin signaling pathways in fibroblasts J. Cell Biol. 136 1997. 1385 1395a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.H., Chen Q., Howe A., Juliano R.L. Cell anchorage permits efficient signal transduction between ras and its downstream kinases J. Biol. Chem. 272 1997. 8849 8852b [PubMed] [Google Scholar]
- Lipfert L., Haimovitch B., Schaller M.D., Cobb B.S., Parsons J.T., Brugge J.S. Integrin dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J. Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev M.E., Sheppard S., Pytela R. Disruption of integrin function and induction of tyrosine phosphorylation by the autonomously expressed β1 integrin cytoplasmic domain. J. Biol. Chem. 1994;269:18311–18314. [PubMed] [Google Scholar]
- Marshall C.J. Specificity of receptor tyrosine kinase signalingtransient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Matsumoto K., Nakamura T., Kramer R.H. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J. Biol. Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- Miyamoto S., Teramoto H., Gutkind J.S., Yamada K.M. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activationroles of integrin aggregation and occupancy of receptors. J. Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino N., Mimura T., Hamasaki K., Tobe K., Ueki K., Kikuchi K., Takehara K., Kadowaki T., Yazaki Y., Nojima Y. Matrix/integrin interaction activates the mitogen activated protein kinase p44erk-1 and p42erk-2. J. Biol. Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Owens L.V., Hu L., Craven R.J., Dent G.A., Weiner T.M., Kornberg L., Liu E.T., Cance W.G. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- Polte T.R., Hanks S.K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130cas . Proc. Natl. Acad. Sci. USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S., Hooshmand-Rad R., Claussen-Welch L., Rozengurt E. Requirement for phosphatidylinositol 3-kinase activity in platelet derived growth factor-stimulated tyrosine phosphorylation of p125 focal adhesion kinase and paxillin. J. Biol. Chem. 1996;271:7829–7834. doi: 10.1074/jbc.271.13.7829. [DOI] [PubMed] [Google Scholar]
- Renshaw M.W., Toksoz D., Schwartz M.A. Involvement of the small GTPase Rho in integrin-mediated activation of MAP kinase. J. Biol. Chem. 1996;271:21691–21694. doi: 10.1074/jbc.271.36.21691. [DOI] [PubMed] [Google Scholar]
- Renshaw M.W., Ren X.-D., Schwartz M.A. Growth factor activation of MAP kinase requires cell adhesion. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A., Parsons J.T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK . Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- Richardson A., Malik R.K., Hildebrand J.D., Parsons J.T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAKa role for paxillin tyrosine phosphorylation. Mol. Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D., Borgman C.A., Cobb B.S., Vines R.R., Reynolds A.B., Parsons J.T. PP125FAK, a structurally unique protein kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hanks S.K., Hunter T., vanderGeer P. Integrin-mediated signal transduction linked to ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hunter T. FAK overexpression enhances Ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- Sieg D.J., Ilic D., Jones K.C., Damsky C.H., Hunter T., Schlaepfer D.D. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.E., Miller J.T. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domainsidentification of a vinculin and pp125FAK binding region. J. Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- Wary K.K., Maneiro F., Isakoff S.J., Marcantonio E.E., Giancotti F.G. The adapter protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Weiner T.M., Liu E.T., Craven R.J., Cance W.G. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342:1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- White M.A., Nicolette C., Minden A., Polverini A., Aelst L.V., Karin M., Wigler M.H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. J. Biol. Chem. 1992;267:19031–19034. [PubMed] [Google Scholar]
- Zhang Q., Checovich W.J., Peters D.M., Albrecht R.M., Mosher D.F. Modulation of cell surface fibronectin assembly by lysophosphatidic acid. J. Cell Biol. 1994;127:1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Assoian R.K. Integrin-dependent activation of MAP kinasea link to shape-dependent cell proliferation. Mol. Biol. Cell. 1995;6:273–283. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]