Summary
Transfer of autologous tumor-specific tumor infiltrating lymphocytes (TILs) in adoptive immunotherapy can mediate the regression of tumor in patients with metastatic melanoma. In this procedure, TILs from resected tumors are expanded in vitro, then administered to patients and further stimulated to proliferate in vivo by the administration of high dose IL-2. After in vitro expansion, TILs are often dominated by a few specific clonotypes, and recently it was reported that the persistence in vivo of one or more of these clonotypes correlated with positive therapeutic response. We and others have previously shown that repeated in vitro stimulation and clonal expansion of normal human T lymphocytes results in progressive decrease in telomerase activity and shortening of telomeres, ultimately resulting in replicative senescence. In the studies reported here, we therefore compared telomerase activity and telomere length in persistent and nonpersistent TIL clonotypes before transfer in vivo, and found a correlation between telomere length and clonal persistence. We also observed that TILs proliferate extensively in vivo in the days after transfer, but fail to induce substantial telomerase activity, and undergo rapid decreases in telomere length within days after transfer. Thus, in vivo loss of telomeres by clonotypes that have the shortest telomeres at the time of administration may drive these clones to replicative senescence, whereas cells with longer telomeres are able to persist and mediate antitumor effects. These findings are relevant both to predicting effectiveness of adoptive immunotherapy and in deriving strategies for improving effectiveness by sustaining telomere length.
Keywords: cancer, immunotherapy, telomere, telomerase
Adoptive immunotherapy using autologous tumor infiltrating lymphocytes (TILs) can mediate the regression of tumors in patients with metastatic melanoma.1,2 TILs from resected tumor metastases are cultured extensively in vitro to produce a highly activated and tumor-specific oligoclonal population comprised of a few clonotypes as determined by their T cell receptor (TCR) Vβ expression.1–3 After lymphodepleting chemotherapy, adoptive transfer of autologous in vitro expanded TILs into melanoma patients results in positive clinical responses in approximately 50% of treated patients.1,2 Although the factors that contribute to positive response are unclear, it was observed that positive clinical response correlated with the ability of at least one clonotype to persist at a significant percentage for a prolonged duration after transfer.4 Because telomere length has been shown to be a critical determinant of T cell survival and replicative capacity,5–7 we have investigated telomere length as a determinant of persistence and clinical response elicited by TILs.
In human cells telomeres are present at the ends of all chromosomes and consist of 5 to 15 kb of tandemly repeated (TTAGGG)n sequence and associated proteins.8 Owing to incomplete terminal replication during DNA synthesis, telomeres shorten with cell division.9,10 Telomerase, a unique RNA-dependent DNA polymerase, is capable of synthesizing terminal telomeric sequences and compensating for this telomere shortening.11 In normal human T lymphocytes, extensive proliferation ultimately leads to a reduction in telomerase activity and to the shortening of telomeres, eventually reaching a critically short length at which telomere function becomes compromised.12 These events trigger the cell to enter into a state of senescence which is characterized by altered function and notably the inability to further proliferate. 13,14
Because of the extensive in vitro proliferation and clonal expansion involved in the generation of TIL cultures for use in adoptive immunotherapy, we hypothesized that the ability to persist after transfer in vivo may depend on telomere length of administered TILs. After in vitro expansion, some TILs may have experienced significant telomere erosion such that further loss through stimulation and expansion in vivo cause them to reach critical shortening. In contrast, TILs with sufficient telomere length or ability to maintain telomeres after in vitro expansion may avoid senescence and retain the ability to persist and to mediate antitumor effector function in vivo.
To test this hypothesis, we measured the telomere length of persistent and nonpersistent TIL clonotypes before adoptive transfer, and at various times after administration in vivo. We have previously shown that clonotypes which persist in vivo after transfer to recipient patients start with longer telomeres at the time of TIL administration.15 We have now confirmed these results with an enhanced flow cytometric technique. In addition, we have extended these observations in demonstrating that TILs undergo significant proliferation accompanied by telomere shortening within days after transfer in vivo. We further show that TILs fail to express significant telomerase activity when induced to proliferate in vivo or in vitro. Our work suggests that in the absence of the capacity to express substantial telomerase activity, telomere length may be a critical factor that limits the ability of TILs to proliferate and persist in vivo.
MATERIALS AND METHODS
Patient Populations
All patients in this study signed an Institutional Review Board approved consent and were treated with autologous TILs after lymphodepleting chemotherapy as described previously.1,2 The patients studied here represent a population extended from and partially overlapping with those which we have previously characterized.15 Clinical responses in patients were defined according to Response Evaluation Criteria in Solid Tumor criteria, and described previously.1,2 The techniques of TIL culture have been described in detail.3 Peripheral blood samples were obtained from patients at 1 week, again at 1 month, and at later times after adoptive transfer of autologous TILs, and peripheral blood mononuclear cells (PBMC) were separated from peripheral blood samples using Lymphocyte Separation Medium (MP Biomedicals, Irvine, CA). TILs and PBMC were cryopreserved and thawed into prewarmed human AB serum for analyses. CD8T cells in PBMC were enriched by passing samples over a MACS CD8 negative selection kit (Miltenyi Biotec, Auburn, CA).
Analysis of In Vivo T cell persistence
The analysis of in vivo persistence of TIL clonotypes using 5′ rapid amplification of complementary DNA ends sequencing of TCR Vβ expression in patients after adoptive transfer of autologous TILs was previously described.16 Persistence is defined as the presence of a TIL clonotypic population at ≥ 5% of the total peripheral CD8 population 1 month after transfer.
Telomere Length Measurement Using 2-color Flow-fluorescence In Situ Hybridization
Telomere length was measured using a 2-color modification of the flow-fluorescence in situ hybridization (FISH) protocol.10,17 Cells were first surface stained with TCR Vβ-specific antibodies labeled with either fluorescein isothiocyanate (FITC) or biotin. Biotinylated antibodies were subsequently detected with Alexa-488-streptavidin (Molecular Probes, Eugene, OR). All antibodies were obtained from Beckman-Coulter, Fullterton, CA. After extensive washing to remove unbound antibodies, cells were resuspended in 100 μL phosphate-buffered saline (PBS), and TCR Vβ-specific antibodies were then cross-linked to the cell surface with 100 μL of a 1.14 mg/mL solution of BS3 (Pierce, Rockford, IL) in PBS for 30 minutes at 4°C. The cross-linking reaction was then quenched with 1mL 50mM Tris in PBS for 20 minutes at room temperature. To detect telomeres in these cells, they were then washed and incubated in a prewash buffer (PBS + 1% bovine serum albumin) for 30 minutes at room temperature. Cells were then incubated with 300 μL of a hybridization solution containing 0.75 μg/mL Cy5-labeled (CCCTAA)3 PNA telomere probe (Applied Biosystems, Foster City, CA) for 15 minutes, and hybridized at 82°C for 15 minutes. Cell bodies were then gently washed to remove unbound telomere probe, and resuspended in prewash buffer. Intensity of telomere binding was measured using a FACSCalibur (BD Biosciences, San Jose, CA), gating on single cell bodies by scatter and specific clonotypes by FITC- or Alexa488-positive cells. A standard curve to convert fluorescence units to physical length was generated by comparison of a set of standard samples analyzed in parallel by flow-FISH and by gel electrophoresis as described.17 Telomere signals were normalized to a control preparation of PBMC run with each experiment.17–19
Ki-67 Staining
Cryopreserved TILs and PBMC were thawed into prewarmed human AB serum (Gemini Bio-Products, Woodland, CA), washed and resuspended in PBS with 2% fetal bovine serum (Gemini Bio-Products, Woodland, CA) at 5 × 106 cells/mL and blocked with 10% normal mouse Ig (Caltag Laboratories, Burlingame, CA) for 10 minutes on ice. Cells (5 × 105) in 100 μL were stained with antibodies specific for the β chain variable region of the T-cell receptor (Immunotech, Marseille, France) and antihuman CD8 antibody (BD Biosciences, San Jose, CA) at 4°C for 40 minutes in the dark. Cells were washed twice and permeabilized and fixed using the Cytofix/Cytoplug Plus with GolgiPlug kit (BD PharMingen, San Diego, CA) according to manufacturer's instructions. Cells were then stained with FITC conjugated Ki-67 antibody, washed twice and subsequently analyzed using a FACSCalibur instrument (BD Biosciences, San Jose, CA).
Stimulation of Cells for Telomerase Activity
One to 2 × 106 TILs or PMBC were stimulated with medium containing 0.3 μg/mL OKT3 (Ortho-Biotech, Bridgewater, NJ), 50:1 irradiated allogeneic feeder cells, and 6000 IU/mL IL-2 for 4 days. Live cells were then separated with Lymphocyte Separation Medium (MP Biomedicals, Irving, CA), and specific clonotypes were isolated with either phycoerythrin (PE)-labeled MART-1 tetramer (Beckman-Coulter, Fullerton, CA) (for persistent clonotypes with MART-1 reactivity2) or PE-labeled Vβ antibody (Beckman-Coulter, Fullerton, CA) and positively selected with a MACS anti-PE kit (Miltenyi Biotec). CD8 cells within stimulated PBMC cultures were selected using a MACS CD8 negative selection kit (Miltenyi Biotec). Purity of separation was generally greater than 85%.
Telomerase Activity Assay
Telomerase activity was quantified with a polymerase chain reaction-based telomerase repeat amplification protocol, using the TRAPeze telomerase detection kit (Chemicon, Temecula, CA) and following the manufacturer's instructions. Briefly, lysates of cells were made at concentration of 107 cells/mL in 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate detergent. Serial dilutions of cell lysates were tested for telomerase activity, and products were separated by gel electrophoresis and visualized by SYBR Green (Molecular Probes, Carlsbad, CA). Telomerase activity was quantified as the total intensity of telomerase product bands divided by the intensity of a competitive internal standard included in each polymerase chain reaction. Telomerase activity in each experiment was normalized to that of EL4, a telomerase positive mouse tumor cell line that was analyzed in each reaction.
Statistical Analysis
Significance of differences in telomere length between groups was evaluated using 2-tailed Student t test.
RESULTS
Telomere Length of Persistent Versus Nonpersistent TIL Clonotypes
In previous studies, we showed that the persistence of transferred TILs in the peripheral blood of patients correlates with clinical response.4,15 In that work, we also reported that persistent clonotypes had longer telomeres after in vitro expansion than did nonpersistent clonotypes. Persistent clonotypes were identified by 5′ rapid amplification of complementary DNA ends as those specific TCR Vβ populations that each remained at ≥ 5% of total CD8 population 1 month after transfer. In the present study, we expanded this analysis using 2 parameter flow cytometry to assess telomere length in persistent and nonpersistent clonotypes within the same TIL population. In addition, we have gone on to assess telomere length changes and expression of telomerase activity in TIL populations subsequent to in vivo adoptive transfer.
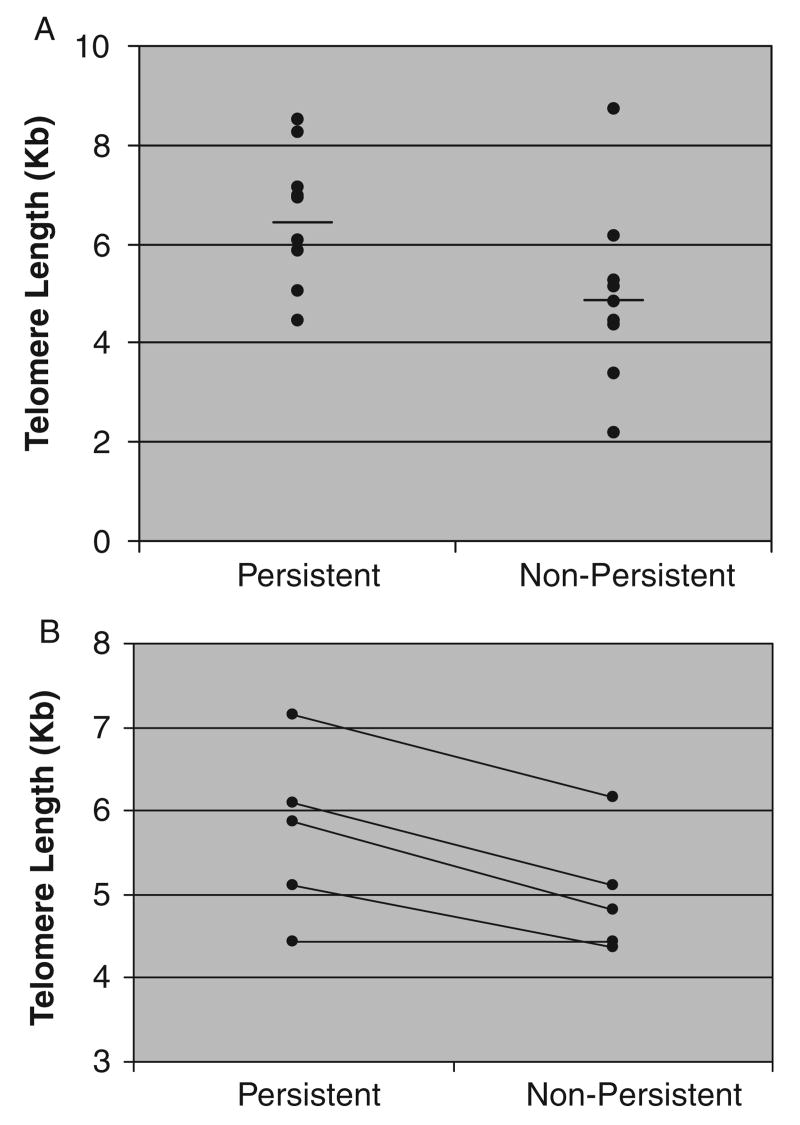
Clonotypes within a total TIL population were labeled with Vβ-specific antibodies and telomere length measured using 2 color flow-FISH. The 2 color flow-FISH technique allows reliable measurements of telomere length of multiple clones within a population and avoids the need for cell separation to isolate individual clonotypes. It also provides an internal control for staining by concurrent measurement of clonotype-positive and clonotype-negative subpopulations within the whole TIL population. Assessing telomere lengths in TIL populations from 14 patients, it was found that clonotypes which persisted after in vivo transfer had longer telomeres than those which did not persist P < 0.04 (Fig. 1A). One notable exception to the correlation between telomere length and clonal persistence was a patient whose TIL were dominated (96%) by a single clonotype that did not persist but had the longest telomeres of any we measured. Inclusion of this patient reduced what was otherwise an even stronger correlation between telomere length and persistence.
FIGURE 1.
Telomere length of persistent and nonpersistent clonotypes. Each dot represents the average (when more than 1 was assayed) of persistent or nonpersistent clonotypes from 1 patient. A, Telomere length of persistent and nonpersistent clonotypes across individual patients. Mean length of persistent clonotypes was 6.6 kb and nonpersistent was 5.0 kb (P<0.04). For this calculation, because some patients had more than 1 persistent or nonpersistent clonotype, the average telomere length of persisters or nonpersisters in that patient was calculated and used in statistical analysis. When telomere lengths for each individual clonotype were used for analysis, mean length of persistent clonotypes was 6.4 kb and mean of nonpersistent clonotypes was 5.0 kb (P<0.02). A total of 27 clonotypes from 14 patients were measured. B, Pair-wise comparison of persistent and nonpersistent clonotypes from the same patient (P<0.02). Mean lengths of persistent or nonpersistent clonotypes for an individual patient were used in this pair-wise comparison. This represents 14 clonotypes from 5 patients.
To assess the relationship between telomere length and persistence or nonpersistence of multiple TIL clones in the same host environment, we analyzed 5 patients who received TILs that contained both persistent and nonpersistent clonotypes. In 4 of 5 patients, the transferred clonotypes that persisted in vivo were longer than those clonotypes from the same individual that did not persist. In the remaining patient, persistent and nonpersistent clonotypes had similar length telomeres (Fig. 1B). This difference across all patients studied was statistically significant by paired t test (P < 0.02), and suggests that telomere length contributed to the ability of TILs to persist in vivo in the same patient, and therefore independent of differences between individual patients.
Telomere Length Changes in Persistent Clonotypes After Transfer In Vivo
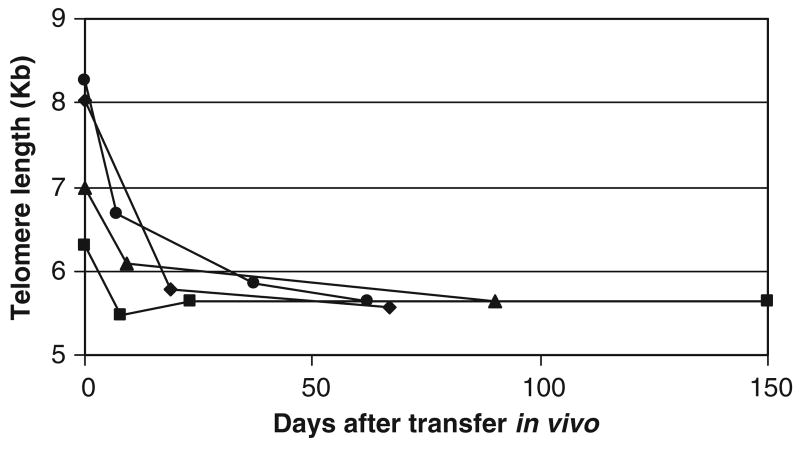
To further investigate how telomere length may influence persistence, we followed the telomere length of TILs after transfer in vivo. Telomere length of persisting clonotypes in the peripheral blood from 4 patients was measured by 2 color flow-FISH. In each of 4 patients, the persistent clonotype had an initial decrease of 10% to 30% in telomere length within days after transfer. After this initial decrease, telomere length remained unchanged for months (Fig. 2). This decrease in telomere length suggested that TILs proliferated to a significant degree immediately after transfer, and became quiescent with longer time in vivo.
FIGURE 2.
Telomere length of persistent clonotype after transfer in vivo. Telomere lengths of the persistent clonotype in 4 patients were measured by 2 color flow-FISH. Each showed an initial decline in length, followed by maintenance.
Proliferation of Transferred TILs In Vivo
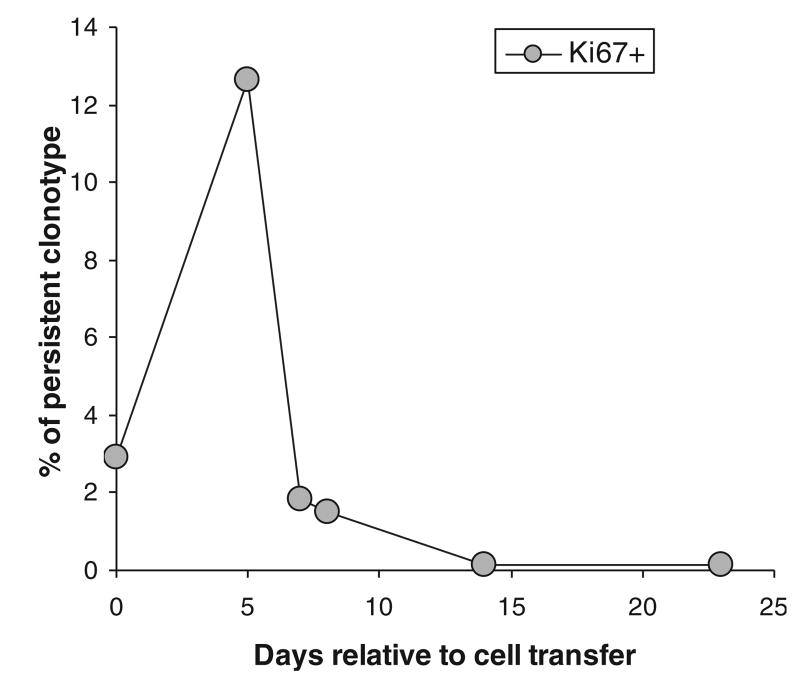
To directly assess the proliferation of TILs in vivo, we stained specific persistent clonotypes derived from the peripheral blood of patients after transfer for the expression of Ki-67 as a marker of cycling cells. As shown in a representative experiment, the transferred TILs significantly up-regulated Ki-67 by 5 days in vivo, and this expression fell to undetectable levels at later time points (Fig. 3). In 4 patients tested, high levels (13% to 44%) of Ki67 + TIL were present in the peripheral blood, whereas peripheral blood from normal donors had low Ki67 expression (0.5% to 0.7%). BrdU incorporation in these cells followed similar patterns with significant uptake at 1 week, which disappeared at later time points (data not shown). Taken together these 2 experimental approaches indicate that TILs undergo substantial proliferation that is restricted to the first 5 to 7 days after in vivo transfer. Thus, the observed shortening of telomeres that occurs immediately after adoptive transfer occurs during a period of substantial cell division, and suggests that compensatory mechanisms such as telomerase are not sufficient to compensate for telomere loss during cell division and chromosomal replication.
FIGURE 3.
Ki-67 expression of TILs after transfer in vivo. This representative experiment presents the percentages of cells with a persistent TIL clonotype that express Ki-67 increase in the few days after transfer, indicating an early and transient peak of proliferative activity.
Telomerase Activity of TILs
In normal T lymphocytes, stimulation and subsequent proliferation are often accompanied by activation of the enzyme telomerase.5,20,21 High telomerase activity can initially compensate for the loss of telomeres that occurs with cell division.21,22 However, with repeated stimulation in vitro or in vivo, the ability to activate telomerase activity decreases and telomere length shortens.5,7,23 To determine the extent to which TILs are able to activate telomerase as a mechanism for maintaining telomere length in vivo, we measured the telomerase activity of TILs and persistent clonotypes after transfer.
In the process of generating TILs for in vivo transfer, cells undergo substantial in vitro expansion. Cells harvested from in vitro culture and just before in vivo administration were found to have very low to undetectable telomerase activity (Fig. 4). Likewise, at 1 week after transfer, a time point when TILs seem to be proliferating in vivo, telomerase activity was very low. To further probe the ability of TIL to express telomerase in response to stimulation, we attempted to mimic conditions of in vivo antigen encounter and activation by restimulating pretransfer TILs in vitro with anti-CD3 mAb, high-dose IL-2, and irradiated allogeneic feeder cells. These TILs responded by proliferating to an extent similar to that observed in freshly isolated normal peripheral blood CD8T cells stimulated in the same manner (data not shown). However, TILs did not increase expression of telomerase above marginally detectable levels, whereas significant up-regulation of telomerase was observed in activated normal peripheral blood CD8 T cells (Fig. 4). Thus, TILs seem to have a limited ability to activate telomerase and may therefore be unable to compensate for telomere loss that occurs during the proliferative response that is observed after in vivo transfer.
FIGURE 4.
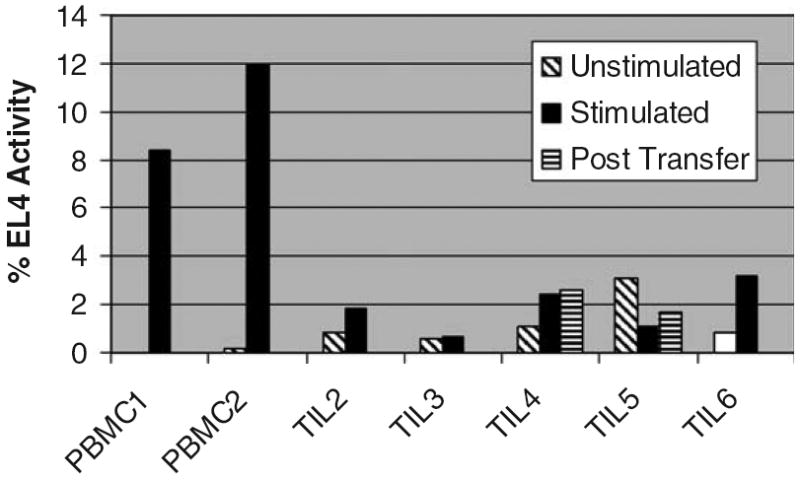
Telomerase activity in TILs. TILs after expansion had very low telomerase activity, in vitro and in vivo, and did not up-regulate telomerase upon restimulation. TILs or freshly isolated peripheral blood leukocytes were stimulated with anti-CD3 and APC as described in Materials and Methods. CD8-enriched populations of peripheral blood leukocyte were isolated after stimulation; and clonotypic TIL populations were isolated by tetramer binding or TCR Vβ expression. Lysates from these populations were assayed for telomerase activity as described, and activity is expressed in relative units on the basis of a standard included in each assay. Telomerase posttransfer in TIL2 and TIL6 were not examined due to unavailability of samples.
DISCUSSION
Adoptive cell transfer immunotherapy can be used to treat patients with metastatic melanoma. In response to the treatment protocol employed in the present study, about half of patients achieve a positive response, defined by Response Evaluation Criteria in Solid Tumor criteria. 1,2 Initial comparisons between patients who responded and those who did not, yielded few differences in either host factors or the TILs used to treat them (data not shown). The notable exception is the observation that persistence, defined as the ability of at least 1 clonotype to remain in the peripheral blood 1 month after transfer at 5% or greater of the total CD8T cell population, correlates very strongly with clinical response.4 Here, we investigate a potential mechanism of why some TILs fail to persist, and suggest potential strategies to improve the success of this therapy.
Expansion of TILs for treatment requires extensive proliferation in vitro before transfer.1–3 In normal T lymphocytes, extensive in vitro proliferation5,12 and clonal expansion in vivo24,25 result in the progressive shortening of telomeres. When telomeres reach a critically short length, the cell enters into a state of senescence characterized by functional changes, including replicative arrest.12–14 Experiments have shown that peripheral human CD4 and CD8T cells may be cultured in vitro for 20 to 30 population doublings before they reach senescence.7,26–28 By comparison, TIL cultures start from a small tumor mass and undergo a ∼ 104 to 105-fold in vitro expansion before transfer.3 This corresponds to ∼ 13 to 17 population doublings, and is likely to represent much greater clonal expansion, depending how many (or few) TILs in the initial tumor mass were precursors of the expanded populations, and how much cell death occurred in culture. This degree of expansion places TILs in a range where telomere shortening could affect their function.
Telomeres were on average longer in persisting clones than in nonpersisters in the present study. However, when analyzed across populations of TILs generated from multiple patients, we could not identify a single telomere length that differentiated TILs destined to persist from those that do not. To eliminate the effect of interindividual differences on the effect of telomere shortening, we were able to compare the telomere length of persistent versus nonpersistent clonotypes within in the same individual. By this internally controlled paired analysis, persistent clonotypes were significantly longer than nonpersistent clonotypes, suggesting that telomere length is a major determinant of persistence within individuals.
In the studies reported here, we were able to extend the analysis of TIL populations used in adoptive immunotherapy by studying their proliferation and telomere dynamics after transfer in vivo, as well as their ability to express telomerase. Generally, in in vivo situations both homeostatic29 and antigen-driven proliferation are accompanied by high telomerase activity and maintenance of telomere length.21,30 Transferred TILs behaved in a unique manner in that extensive proliferation occurred in vivo, but was accompanied by little if any detectable increase in telomerase activity. In the clinical protocol studied here, TILs are transferred into an environment where they potentially receive strong signals to proliferate, from tumor antigen stimulation, high-dose IL-2 treatment, and homeostatic forces to repopulate the lymphodepleted host. Despite apparent proliferation as indicated by Ki-67 expression and BrdU incorporation, we detected little compensating telomerase activity, in either in vivo or in vitro stimulation conditions. Thus, persistent TILs proliferate after transfer but fail to express telomerase activity, potentially leading to the observed rapid decrease in telomere length of persistent clonotypes within days after transfer. Nonpersistent clonotypes, with shorter telomeres at the time of administration, may be unable to tolerate further telomere loss, and may rapidly undergo senescence and/or apoptotic death as a consequence of critical telomere shortening.
Although we could not directly assay the telomere length of nonpersistent clonotypes after transfer, persistent and nonpersistent TILs share a remarkable degree of similarity in expression of a wide array of activation markers (CD69, CD25, and CD40L), homing molecules (CCR7, CXCR4), and differentiation markers (CD45RO, CD27).15 CD28 expression was low in both persistent and nonpersistent clones, but tended to be lower in nonpersisters. Thus, at our present stage of understanding, telomere length of TILs is the phenotypic variable that best predicts clonotypic survival and concomitant probability of clinical response. Given the correlation to persistence, telomere length might also be a determinant of clinical response, which is itself correlated with TIL clonal persistence.4 Our previous study of telomere length of TILs indeed reported a significant correlation between average telomere length of the overall pretransfer TIL culture and clinical response.15 We also observed this trend in the present study; however, it did not reach statistical significant (data not shown) due in part to the inclusion of 3 recently studied nonresponding patients who had persistent clonotypes with long telomere length. These cases suggest that although telomere length and persistence may be necessary conditions to positive clinical outcome, other factors may also influence clinical response.
Identifying telomere shortening and consequent senescence and/or cell death as a possible mechanism mediating failure of clonotype survival suggests strategies to improve effectiveness of adoptive immunotherapy. Most immediately, measuring relative telomere length of the multiple TIL cultures routinely generated from distinct tumor sites in each patient might provide a means for selecting the clones that are most likely to persist and to be effective. Additionally, strategies such as transduction of hTERT, the catalytic component of telomerase, can increase proliferative lifespan of human cells, including T cells, without apparent transformation or loss of function,31,32 and may improve the immune function of transduced lymphocytes.33 Manipulation of telomere length by expression of hTERT in TILs may thus represent a useful approach to increasing effectiveness in adoptive immunotherapy.
Footnotes
The authors have declared there are no financial conflicts of interest related to this work.
References
- 1.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley M, Mark E, Wunderlich JR, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weng NP, Palmer LD, Levine BL, et al. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 6.Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20:682. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 7.Weng N, Levine B, June C, et al. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. PNAS. 1995;92:11091. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackburn E. Structure and function of telomeres. Nature. 1991;350:569. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 9.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 10.Rufer N, Dragowska W, Thornbury G, et al. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Hodes R, Richard J, Hathcock KS, et al. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 13.Effros RB, Dagarag M, Spaulding C, et al. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Shen X, Hodes RJ, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Dudley ME, Rosenberg SA, et al. Selective growth, in vitro and in vivo, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J Immunol. 2004;173:7622. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hathcock KS, Hodes RJ, Weng Np. Analysis of telomere length and telomerase activity. In: Coligan JE, Kruisbeek AM, Margulies DH, et al., editors. Current Protocols in Immunology. 30. Vol. 10. New York: Greene Publishing Associates and Wiley-Interscience; 2004. pp. 1–27. [DOI] [PubMed] [Google Scholar]
- 18.Plunkett FJ, Soares MV, Annels N, et al. The flow cytometric analysis of telomere length in antigen-specific CD8+ T cells during acute Epstein-Barr virus infection. Blood. 2001;97:700. doi: 10.1182/blood.v97.3.700. [DOI] [PubMed] [Google Scholar]
- 19.Baerlocher GM, Lansdorp PM. Telomere length measurements using fluorescence in situ hybridization and flow cytometry. Methods Cell Biol. 2004;75:719. doi: 10.1016/s0091-679x(04)75031-1. [DOI] [PubMed] [Google Scholar]
- 20.Weng N, Levine B, June C, et al. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maini MK, Soares MVD, Zilch CF, et al. Virus-induced CD8+ T cell clonal expansion is associated with telomerase up-regulation and telomere length preservation: a mechanism for rescue from replicative senescence. J Immunol. 1999;162:4521. [PubMed] [Google Scholar]
- 22.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43:405. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 23.Roth A, Yssel H, Pene J, et al. Telomerase levels control the lifespan of human T lymphocytes. Blood. 2003;102:849. doi: 10.1182/blood-2002-07-2015. [DOI] [PubMed] [Google Scholar]
- 24.Reed JR, Vukmanovic-Stejic M, Fletcher JM, et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 2004;199:1433. doi: 10.1084/jem.20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner UG, Koetz K, Weyand CM, et al. Perturbation of the T cell repertoire in rheumatoid arthritis. PNAS. 1998;95:14447. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migliaccio M, Amacker M, Just T, et al. Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8+ T lymphocyte immortalization. J Immunol. 2000;165:4978. doi: 10.4049/jimmunol.165.9.4978. [DOI] [PubMed] [Google Scholar]
- 27.Perillo NL, Naeim F, Walford RL, et al. In vitro cellular aging in T-lymphocyte cultures: analysis of DNA content and cell size. Exp Cell Res. 1993;207:131. doi: 10.1006/excr.1993.1171. [DOI] [PubMed] [Google Scholar]
- 28.Perillo NL, Naeim F, Walford RL, et al. In vitro cellular aging in T-lymphocyte cultures: analysis of DNA content and cell size. Exp Cell Res. 1993;207:131. doi: 10.1006/excr.1993.1171. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zhi W, Wareski P, et al. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol. 2005;174:4019. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
- 30.Soares MVD, Plunkett FJ, Verbeke CS, et al. Integration of apoptosis and telomere erosion in virus-specific CD8+ T cells from blood and tonsils during primary infection. Blood. 2004;103:162. doi: 10.1182/blood-2003-06-1791. [DOI] [PubMed] [Google Scholar]
- 31.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 32.Rufer N, Migliaccio M, Antonchuk J, et al. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98:597. doi: 10.1182/blood.v98.3.597. [DOI] [PubMed] [Google Scholar]
- 33.Dagarag M, Evazyan T, Rao N, et al. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]