Abstract
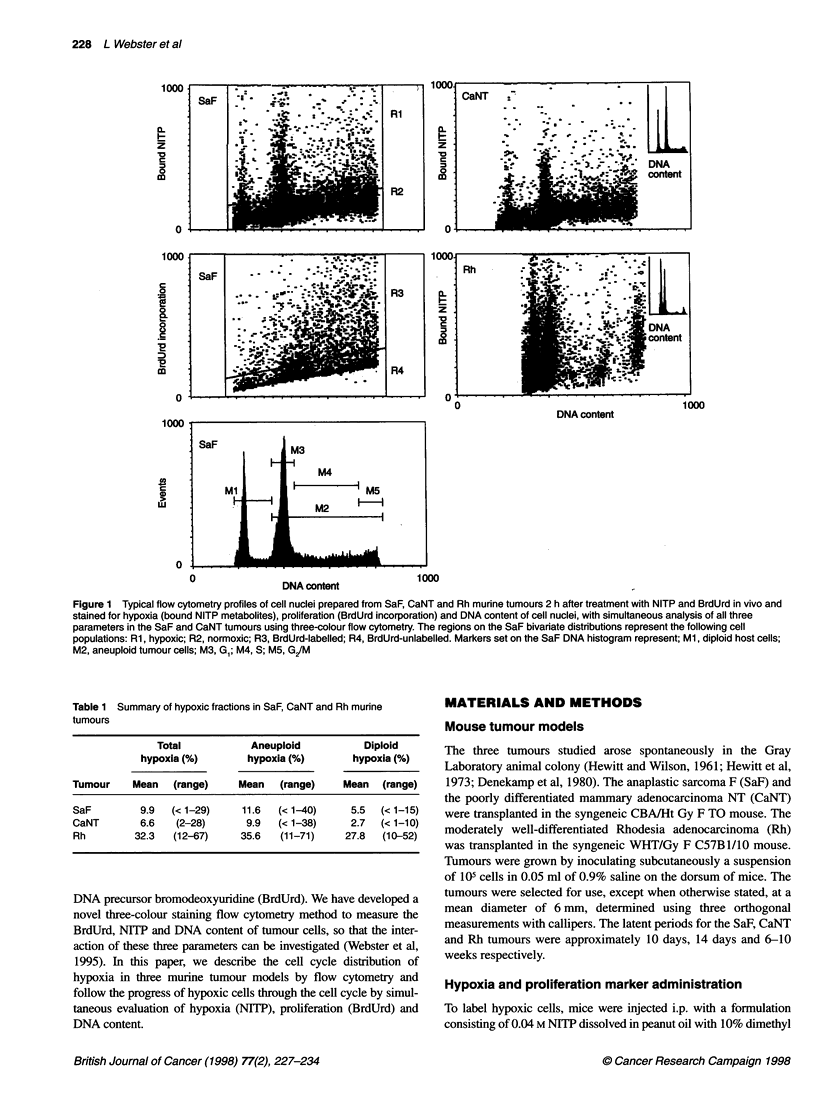
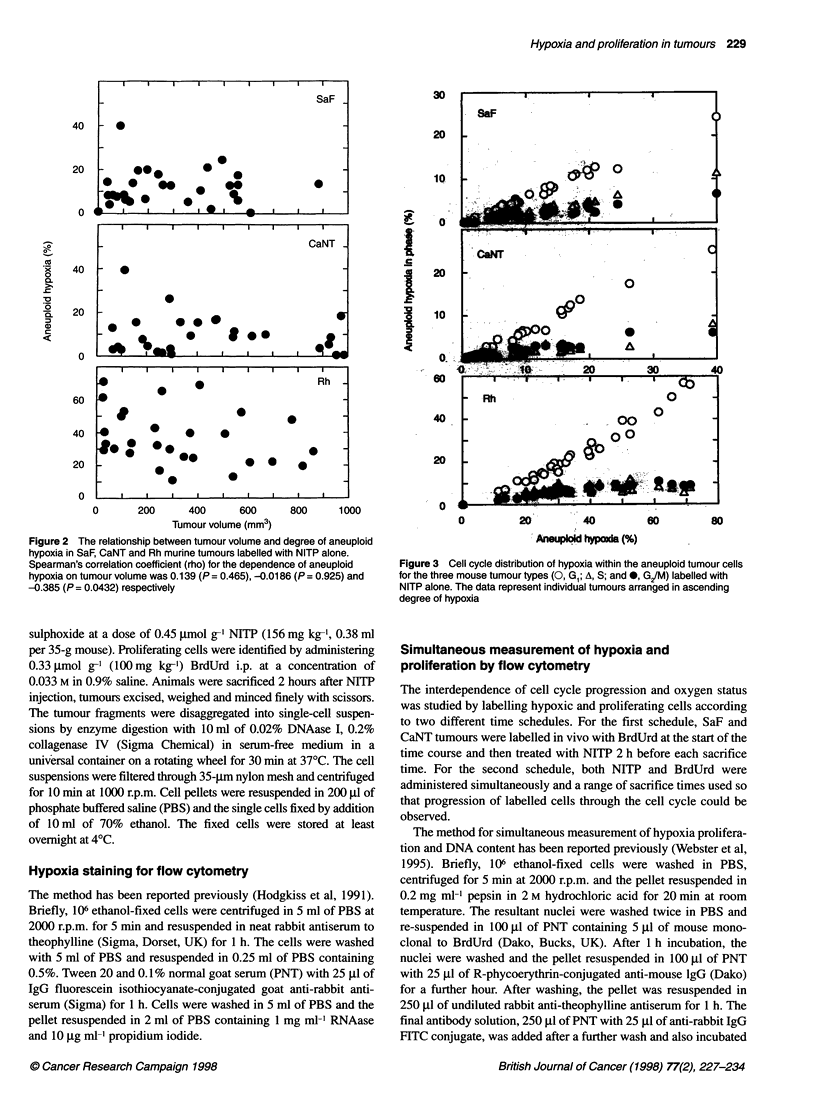
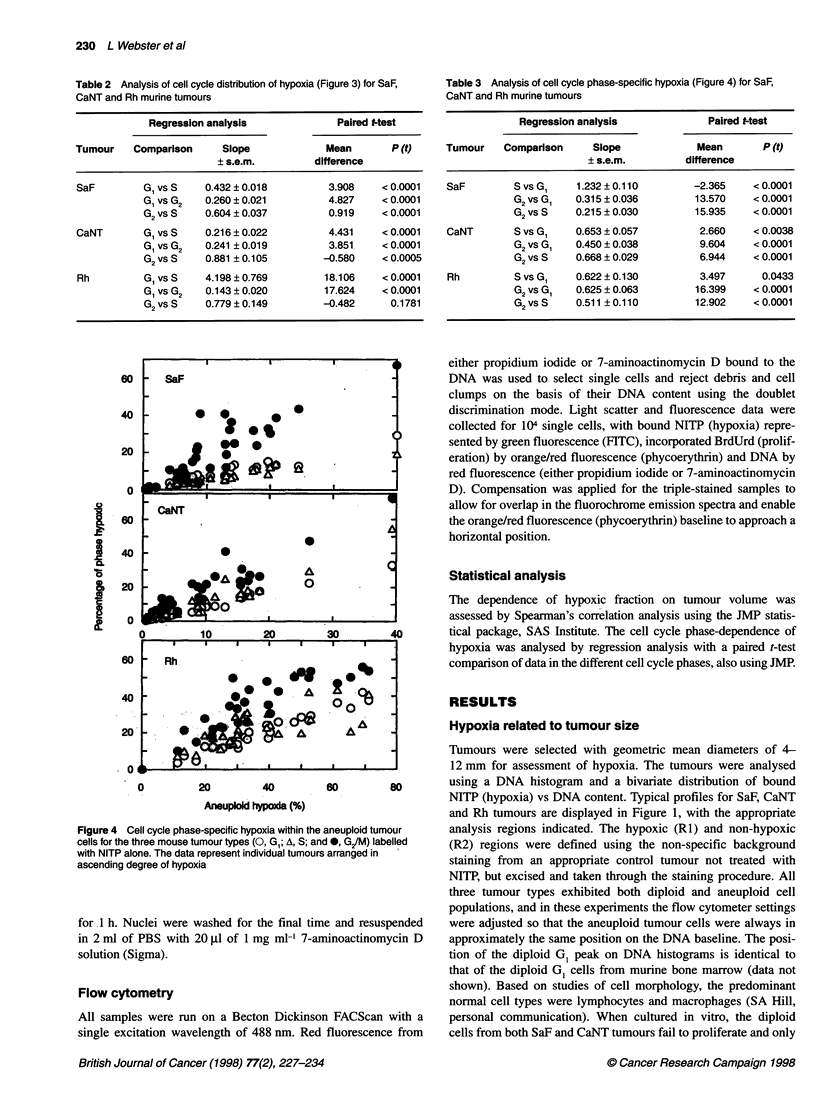
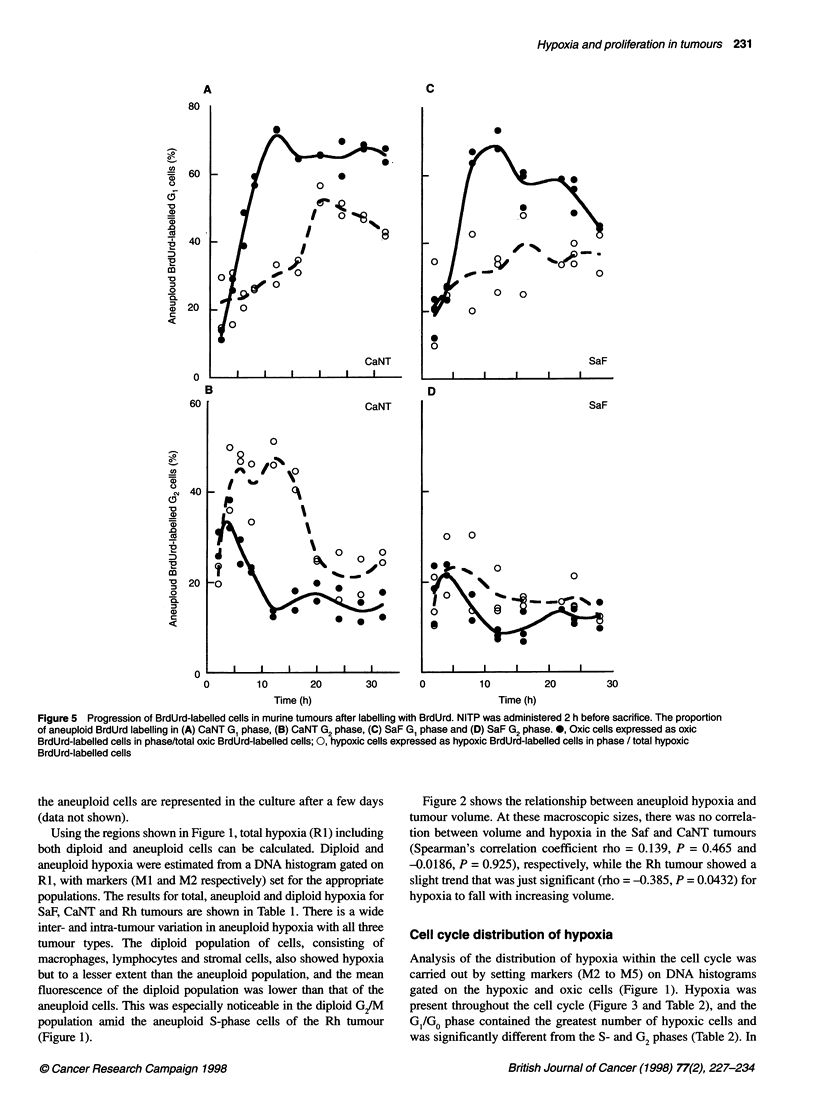
Hypoxia was assessed in three murine tumour models in vivo by measuring the incorporation of 7-(4'-(2-nitroimidazole-1-yl)-butyl)-theophylline (NITP), an immunologically identifiable hypoxia marker that binds bioreductively to cells under low-oxygen conditions. Proliferating cells were labelled in the same tumours by administering the thymidine analogue bromodeoxyuridine (BrdUrd). The relative hypoxia in each cell cycle phase of cells isolated from tumours was assessed by addition of propidium iodide with analysis by flow cytometry. There was no relationship between tumour volume and hypoxia in either the anaplastic sarcoma SaF or the poorly differentiated carcinoma CaNT and only a slight negative correlation in moderately well-differentiated carcinoma Rh. The G1/G0 phase contained the greatest number of aneuploid hypoxic cells (aneuploid hypoxia ranging from less than 1% up to 40%, 38% and 71% in SaF, CaNT and Rh respectively), although there were significant amounts of hypoxia present in S- and G2/M phases for all three tumours examined. However, the highest proportion of hypoxia occurred in the G2/M phase, in which up to 60% of the cells were hypoxic. Simultaneous measurement of hypoxia, proliferation and DNA content using a novel triple-staining flow cytometry method showed that hypoxic cells could actively participate in the cell cycle. In addition, the cell cycle distribution of NITP and BrdUrd labelling showed that hypoxic cells could progress through the cell cycle, although their rate of progression was slower than that of better oxygenated cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amellem O., Pettersen E. O. Cell inactivation and cell cycle inhibition as induced by extreme hypoxia: the possible role of cell cycle arrest as a protection against hypoxia-induced lethal damage. Cell Prolif. 1991 Mar;24(2):127–141. doi: 10.1111/j.1365-2184.1991.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Begg A. C., McNally N. J., Shrieve D. C., Kärcher H. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry. 1985 Nov;6(6):620–626. doi: 10.1002/cyto.990060618. [DOI] [PubMed] [Google Scholar]
- Brizel D. M., Scully S. P., Harrelson J. M., Layfield L. J., Bean J. M., Prosnitz L. R., Dewhirst M. W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996 Mar 1;56(5):941–943. [PubMed] [Google Scholar]
- Corvò R., Giaretti W., Sanguineti G., Geido E., Orecchia R., Guenzi M., Margarino G., Bacigalupo A., Garaventa G., Barbieri M. In vivo cell kinetics in head and neck squamous cell carcinomas predicts local control and helps guide radiotherapy regimen. J Clin Oncol. 1995 Aug;13(8):1843–1850. doi: 10.1200/JCO.1995.13.8.1843. [DOI] [PubMed] [Google Scholar]
- Cox L. S., Lane D. P. Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays. 1995 Jun;17(6):501–508. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Hirst D. G., Stewart F. A., Terry N. H. Is tumour radiosensitization by misonidazole a general phenomenon? Br J Cancer. 1980 Jan;41(1):1–9. doi: 10.1038/bjc.1980.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. K., Wendland M. F., Iyer S. B., Lam K. N., Engeseth H., James T. L. Correlations between in vivo 31P NMR spectroscopy measurements, tumor size, hypoxic fraction and cell survival after radiotherapy. Int J Radiat Oncol Biol Phys. 1990 Jun;18(6):1341–1350. doi: 10.1016/0360-3016(90)90307-6. [DOI] [PubMed] [Google Scholar]
- GRAY L. H., CONGER A. D., EBERT M., HORNSEY S., SCOTT O. C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953 Dec;26(312):638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Gelfant S. A new concept of tissue and tumor cell proliferation. Cancer Res. 1977 Nov;37(11):3845–3862. [PubMed] [Google Scholar]
- Graeber T. G., Osmanian C., Jacks T., Housman D. E., Koch C. J., Lowe S. W., Giaccia A. J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996 Jan 4;379(6560):88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Graeber T. G., Peterson J. F., Tsai M., Monica K., Fornace A. J., Jr, Giaccia A. J. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994 Sep;14(9):6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEWITT H. B., WILSON C. W. Survival curves for tumor cells irradiated in vivo. Ann N Y Acad Sci. 1961 Nov 13;95:818–827. doi: 10.1111/j.1749-6632.1961.tb50078.x. [DOI] [PubMed] [Google Scholar]
- Hewitt H. B., Blake E., Proter E. H. The effect of lethally irradiated cells on the transplantability of murine tumours. Br J Cancer. 1973 Aug;28(2):123–135. doi: 10.1038/bjc.1973.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst D. G., Denekamp J. Tumour cell proliferation in relation to the vasculature. Cell Tissue Kinet. 1979 Jan;12(1):31–42. doi: 10.1111/j.1365-2184.1979.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Hodgkiss R. J., Jones G., Long A., Parrick J., Smith K. A., Stratford M. R., Wilson G. D. Flow cytometric evaluation of hypoxic cells in solid experimental tumours using fluorescence immunodetection. Br J Cancer. 1991 Jan;63(1):119–125. doi: 10.1038/bjc.1991.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkiss R. J., Stratford M. R., Dennis M. F., Hill S. A. Pharmacokinetics and binding of the bioreductive probe for hypoxia, NITP: effect of route of administration. Br J Cancer. 1995 Dec;72(6):1462–1468. doi: 10.1038/bjc.1995.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Nordsmark M., Høyer M., Keller J., Nielsen O. S., Jensen O. M., Overgaard J. The relationship between tumor oxygenation and cell proliferation in human soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1996 Jul 1;35(4):701–708. doi: 10.1016/0360-3016(96)00132-0. [DOI] [PubMed] [Google Scholar]
- Okunieff P. G., Koutcher J. A., Gerweck L., McFarland E., Hitzig B., Urano M., Brady T., Neuringer L., Suit H. D. Tumor size dependent changes in a murine fibrosarcoma: use of in vivo 31P NMR for non-invasive evaluation of tumor metabolic status. Int J Radiat Oncol Biol Phys. 1986 May;12(5):793–799. doi: 10.1016/0360-3016(86)90038-6. [DOI] [PubMed] [Google Scholar]
- Pallavicini M. G., Lalande M. E., Miller R. G., Hill R. P. Cell cycle distribution of chronically hypoxic cells and determination of the clonogenic potential of cells accumulated in G2 + M phases after irradiation of a solid tumor in vivo. Cancer Res. 1979 Jun;39(6 Pt 1):1891–1897. [PubMed] [Google Scholar]
- Pettersen E. O., Lindmo T. Inhibition of cell-cycle progression by acute treatment with various degrees of hypoxia: modifications induced by low concentrations of misonidazole present during hypoxia. Br J Cancer. 1983 Dec;48(6):809–817. doi: 10.1038/bjc.1983.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A., Fisher D. E. p53 in life and death. Clin Cancer Res. 1996 Mar;2(3):435–440. [PubMed] [Google Scholar]
- Shrieve D. C., Begg A. C. Cell cycle kinetics of aerated, hypoxic and re-aerated cells in vitro using flow cytometric determination of cellular DNA and incorporated bromodeoxyuridine. Cell Tissue Kinet. 1985 Nov;18(6):641–651. doi: 10.1111/j.1365-2184.1985.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Siemann D. W., Keng P. C. Characterization of radiation resistant hypoxic cell subpopulations in KHT sarcomas. (II). Cell sorting. Br J Cancer. 1988 Sep;58(3):296–300. doi: 10.1038/bjc.1988.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann D. W. Tumour size: a factor influencing the isoeffect analysis of tumour response to combined modalities. Br J Cancer Suppl. 1980 Apr;4:294–298. [PMC free article] [PubMed] [Google Scholar]
- Spiro I. J., Rice G. C., Durand R. E., Stickler R., Ling C. C. Cell killing, radiosensitization and cell cycle redistribution induced by chronic hypoxia. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1275–1280. doi: 10.1016/0360-3016(84)90332-8. [DOI] [PubMed] [Google Scholar]
- Stanley J. A., Shipley W. U., Steel G. G. Influence of tumour size on hypoxic fraction and therapeutic sensitivity of Lewis lung tumour. Br J Cancer. 1977 Jul;36(1):105–113. doi: 10.1038/bjc.1977.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart N., Hicks G. G., Paraskevas F., Mowat M. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 1995 Jan 5;10(1):109–115. [PubMed] [Google Scholar]
- Stone H. B., Brown J. M., Phillips T. L., Sutherland R. M. Oxygen in human tumors: correlations between methods of measurement and response to therapy. Summary of a workshop held November 19-20, 1992, at the National Cancer Institute, Bethesda, Maryland. Radiat Res. 1993 Dec;136(3):422–434. [PubMed] [Google Scholar]
- Sutherland R. M., Sordat B., Bamat J., Gabbert H., Bourrat B., Mueller-Klieser W. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986 Oct;46(10):5320–5329. [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen C. A., Michaelson S. M., Wheeler K. T. Evidence for an unconventional radiosensitivity of rat 9L subcutaneous tumors. Radiat Res. 1980 Dec;84(3):529–541. [PubMed] [Google Scholar]
- Webster L., Hodgkiss R. J., Wilson G. D. Simultaneous triple staining for hypoxia, proliferation, and DNA content in murine tumours. Cytometry. 1995 Dec 1;21(4):344–351. doi: 10.1002/cyto.990210406. [DOI] [PubMed] [Google Scholar]
- Wilson G. D., Martindale C. A., Soranson J. A., Bourhis J., Carl U. M., McNally N. J. Radiation-induced cell cycle delay measured in two mouse tumors in vivo using bromodeoxyuridine. Radiat Res. 1994 Feb;137(2):177–185. [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dische S., Saunders M. I., Des Rochers C., Lewis A. A., Bennett M. H. Measurement of cell kinetics in human tumours in vivo using bromodeoxyuridine incorporation and flow cytometry. Br J Cancer. 1988 Oct;58(4):423–431. doi: 10.1038/bjc.1988.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dunphy E., Kärcher H., Pfragner R. The labelling index of human and mouse tumours assessed by bromodeoxyuridine staining in vitro and in vivo and flow cytometry. Cytometry. 1985 Nov;6(6):641–647. doi: 10.1002/cyto.990060621. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Calkins D. P., Cline J. M., Thrall D. E., Raleigh J. A. The relationship between proliferative and oxygenation status in spontaneous canine tumors. Int J Radiat Oncol Biol Phys. 1993 Nov 15;27(4):891–898. doi: 10.1016/0360-3016(93)90465-8. [DOI] [PubMed] [Google Scholar]


