Abstract
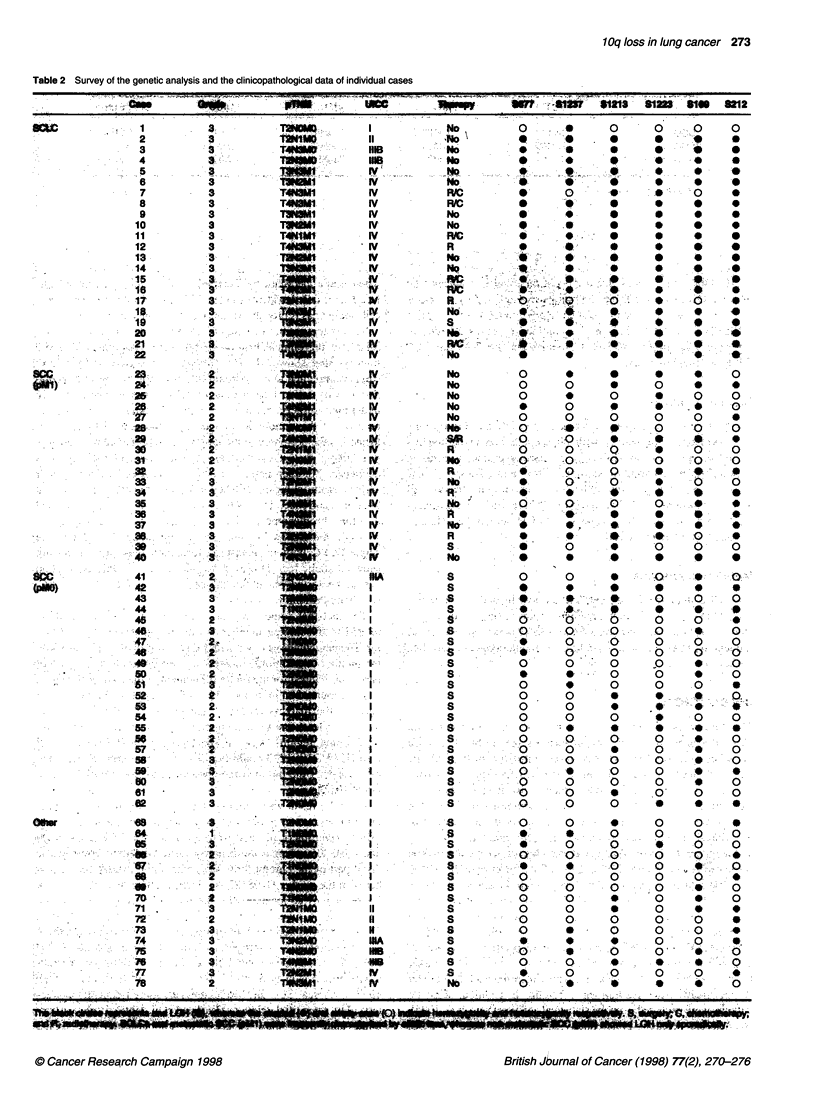
We analysed 78 carcinomas of the lung for allelic losses on chromosome 10q. The tumours were of different stage and grade and comprised 22 small-cell lung carcinomas (SCLC), 40 squamous cell carcinomas (SCC), 11 adenocarcinomas, four large-cell carcinomas and one carcinoid. They were investigated by six polymorphic markers located between 10q21 and 10qter. We observed a high incidence of loss of heterozygosity (LOH) in SCLC (91%) and metastatic SCC (56%). Non-metastatic SCC showed deletions in three cases (14%) and no LOH was found in the other types of non-small-cell lung cancer. The statistical analysis indicated that the presence of LOH correlated significantly with advanced tumour stages in the entire collective and in particular within the SCLC and SCC subgroups. For SCC, a positive association was found between LOH and metastases formation, while in SCLC the number of non-metastatic tumours was too small for a final conclusion. Whereas SCLC was frequently characterized by multiple allelic losses, suggesting the deletion of the entire chromosomal arm, SCC showed interstitial imbalances. A high incidence of allelic loss was observed between the markers D10S677 and D10S1223. The analysis of five informative cases suggested the presence of two non-overlapping regions between the loci D10S677/D10S1237 and D10S1213/D10S1223. In SCLC, we did not find mutations in the putative tumour-suppressor gene MXI1. The data indicate that LOH on chromosome 10q is associated with tumour progression in SCC and SCLC. Thus it may become a useful genetic marker in the assessment of the malignant potential of these tumour types.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockmühl U., Schwendel A., Dietel M., Petersen I. Distinct patterns of chromosomal alterations in high- and low-grade head and neck squamous cell carcinomas. Cancer Res. 1996 Dec 1;56(23):5325–5329. [PubMed] [Google Scholar]
- Califano J., van der Riet P., Westra W., Nawroz H., Clayman G., Piantadosi S., Corio R., Lee D., Greenberg B., Koch W. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996 Jun 1;56(11):2488–2492. [PubMed] [Google Scholar]
- Churg A., Johnston W. H., Stulbarg M. Small cell squamous and mixed small cell squamous--small cell anaplastic carcinomas of the lung. Am J Surg Pathol. 1980 Jun;4(3):255–263. doi: 10.1097/00000478-198006000-00006. [DOI] [PubMed] [Google Scholar]
- Dubovsky J., Sheffield V. C., Duyk G. M., Weber J. L. Sets of short tandem repeat polymorphisms for efficient linkage screening of the human genome. Hum Mol Genet. 1995 Mar;4(3):449–452. doi: 10.1093/hmg/4.3.449. [DOI] [PubMed] [Google Scholar]
- Eagle L. R., Yin X., Brothman A. R., Williams B. J., Atkin N. B., Prochownik E. V. Mutation of the MXI1 gene in prostate cancer. Nat Genet. 1995 Mar;9(3):249–255. doi: 10.1038/ng0395-249. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Bader S., Hung J., Kishimoto Y., Sekido Y., Sugio K., Virmani A., Fleming J., Carbone D. P., Minna J. D. Molecular genetic changes found in human lung cancer and its precursor lesions. Cold Spring Harb Symp Quant Biol. 1994;59:565–572. doi: 10.1101/sqb.1994.059.01.063. [DOI] [PubMed] [Google Scholar]
- Gray I. C., Phillips S. M., Lee S. J., Neoptolemos J. P., Weissenbach J., Spurr N. K. Loss of the chromosomal region 10q23-25 in prostate cancer. Cancer Res. 1995 Nov 1;55(21):4800–4803. [PubMed] [Google Scholar]
- Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996 Aug 9;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hirsch F. R., Ottesen G., Pødenphant J., Olsen J. Tumor heterogeneity in lung cancer based on light microscopic features. A retrospective study of a consecutive series of 200 patients, treated surgically. Virchows Arch A Pathol Anat Histopathol. 1983;402(2):147–153. doi: 10.1007/BF00695056. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Soylemezoglu F., Schäuble B., Scheithauer B. W., Burger P. C. Histopathology, classification, and grading of gliomas. Glia. 1995 Nov;15(3):211–221. doi: 10.1002/glia.440150303. [DOI] [PubMed] [Google Scholar]
- Levin N. A., Brzoska P., Gupta N., Minna J. D., Gray J. W., Christman M. F. Identification of frequent novel genetic alterations in small cell lung carcinoma. Cancer Res. 1994 Oct 1;54(19):5086–5091. [PubMed] [Google Scholar]
- Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997 Mar 28;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Mountain C. F., Greenberg S. D., Fraire A. E. Tumor stage in non-small cell carcinoma of the lung. Chest. 1991 May;99(5):1258–1260. doi: 10.1378/chest.99.5.1258. [DOI] [PubMed] [Google Scholar]
- Müller K. M., Fisseler-Eckhoff A. What's new in lung tumor heterogeneity? Pathol Res Pract. 1988 Dec;184(1):108–115. doi: 10.1016/S0344-0338(88)80201-2. [DOI] [PubMed] [Google Scholar]
- Nesbitt J. C., Putnam J. B., Jr, Walsh G. L., Roth J. A., Mountain C. F. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995 Aug;60(2):466–472. doi: 10.1016/0003-4975(95)00169-l. [DOI] [PubMed] [Google Scholar]
- Petersen I., Bujard M., Petersen S., Wolf G., Goeze A., Schwendel A., Langreck H., Gellert K., Reichel M., Just K. Patterns of chromosomal imbalances in adenocarcinoma and squamous cell carcinoma of the lung. Cancer Res. 1997 Jun 15;57(12):2331–2335. [PubMed] [Google Scholar]
- Petersen I., Langreck H., Wolf G., Schwendel A., Psille R., Vogt P., Reichel M. B., Ried T., Dietel M. Small-cell lung cancer is characterized by a high incidence of deletions on chromosomes 3p, 4q, 5q, 10q, 13q and 17p. Br J Cancer. 1997;75(1):79–86. doi: 10.1038/bjc.1997.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I., Reichel M. B., Dietel M. Use of non-radioactive detection in SSCP, direct DNA sequencing and LOH analysis. Clin Mol Pathol. 1996 Apr;49(2):M118–M121. doi: 10.1136/mp.49.2.m118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani P., Parkin D. M., Ferlay J. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993 Dec 2;55(6):891–903. doi: 10.1002/ijc.2910550604. [DOI] [PubMed] [Google Scholar]
- Rasheed B. K., McLendon R. E., Friedman H. S., Friedman A. H., Fuchs H. E., Bigner D. D., Bigner S. H. Chromosome 10 deletion mapping in human gliomas: a common deletion region in 10q25. Oncogene. 1995 Jun 1;10(11):2243–2246. [PubMed] [Google Scholar]
- Rempel S. A., Schwechheimer K., Davis R. L., Cavenee W. K., Rosenblum M. L. Loss of heterozygosity for loci on chromosome 10 is associated with morphologically malignant meningioma progression. Cancer Res. 1993 May 15;53(10 Suppl):2386–2392. [PubMed] [Google Scholar]
- Ried T., Petersen I., Holtgreve-Grez H., Speicher M. R., Schröck E., du Manoir S., Cremer T. Mapping of multiple DNA gains and losses in primary small cell lung carcinomas by comparative genomic hybridization. Cancer Res. 1994 Apr 1;54(7):1801–1806. [PubMed] [Google Scholar]
- Sato S., Nakamura Y., Tsuchiya E. Difference of allelotype between squamous cell carcinoma and adenocarcinoma of the lung. Cancer Res. 1994 Nov 1;54(21):5652–5655. [PubMed] [Google Scholar]
- Schwendel A., Langreck H., Reichel M., Schröck E., Ried T., Dietel M., Petersen I. Primary small-cell lung carcinomas and their metastases are characterized by a recurrent pattern of genetic alterations. Int J Cancer. 1997 Feb 20;74(1):86–93. doi: 10.1002/(sici)1097-0215(19970220)74:1<86::aid-ijc15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Steck P. A., Pershouse M. A., Jasser S. A., Yung W. K., Lin H., Ligon A. H., Langford L. A., Baumgard M. L., Hattier T., Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997 Apr;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Thiberville L., Payne P., Vielkinds J., LeRiche J., Horsman D., Nouvet G., Palcic B., Lam S. Evidence of cumulative gene losses with progression of premalignant epithelial lesions to carcinoma of the bronchus. Cancer Res. 1995 Nov 15;55(22):5133–5139. [PubMed] [Google Scholar]
- Tsuchiya E., Nakamura Y., Weng S. Y., Nakagawa K., Tsuchiya S., Sugano H., Kitagawa T. Allelotype of non-small cell lung carcinoma--comparison between loss of heterozygosity in squamous cell carcinoma and adenocarcinoma. Cancer Res. 1992 May 1;52(9):2478–2481. [PubMed] [Google Scholar]
- von Deimling A., Louis D. N., von Ammon K., Petersen I., Hoell T., Chung R. Y., Martuza R. L., Schoenfeld D. A., Yaşargil M. G., Wiestler O. D. Association of epidermal growth factor receptor gene amplification with loss of chromosome 10 in human glioblastoma multiforme. J Neurosurg. 1992 Aug;77(2):295–301. doi: 10.3171/jns.1992.77.2.0295. [DOI] [PubMed] [Google Scholar]



