Abstract
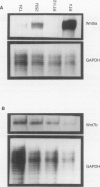
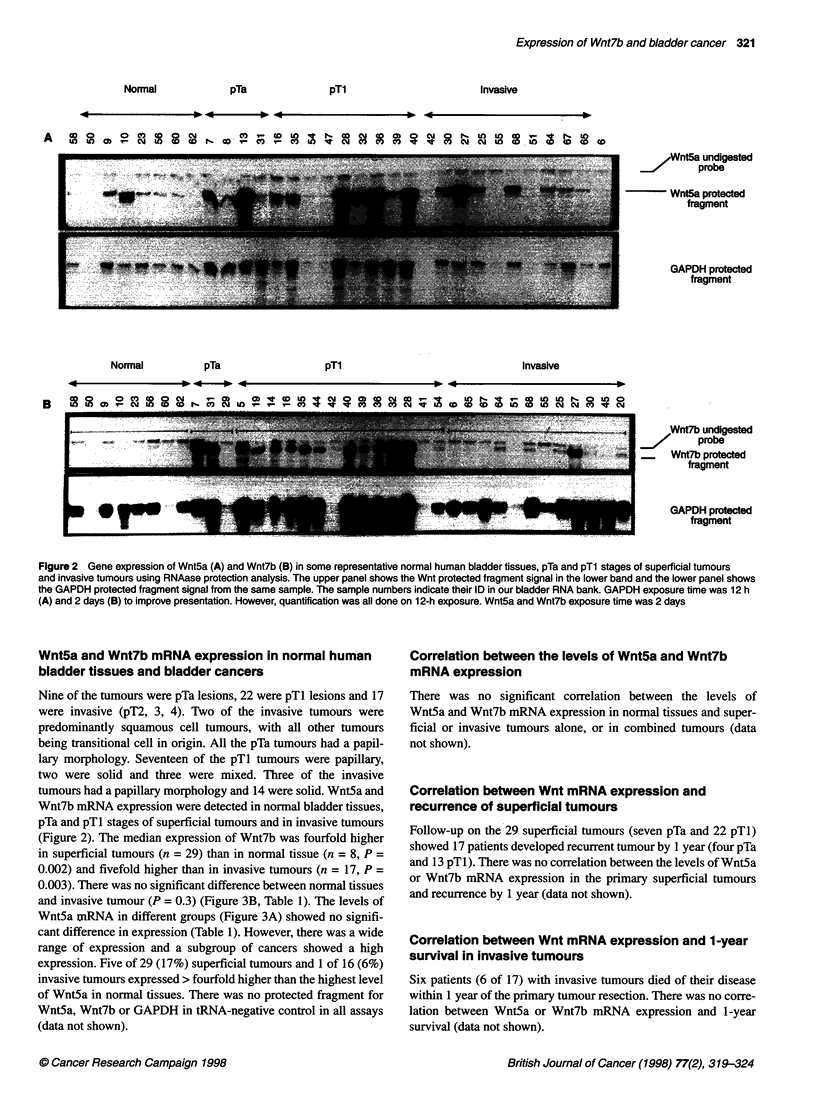
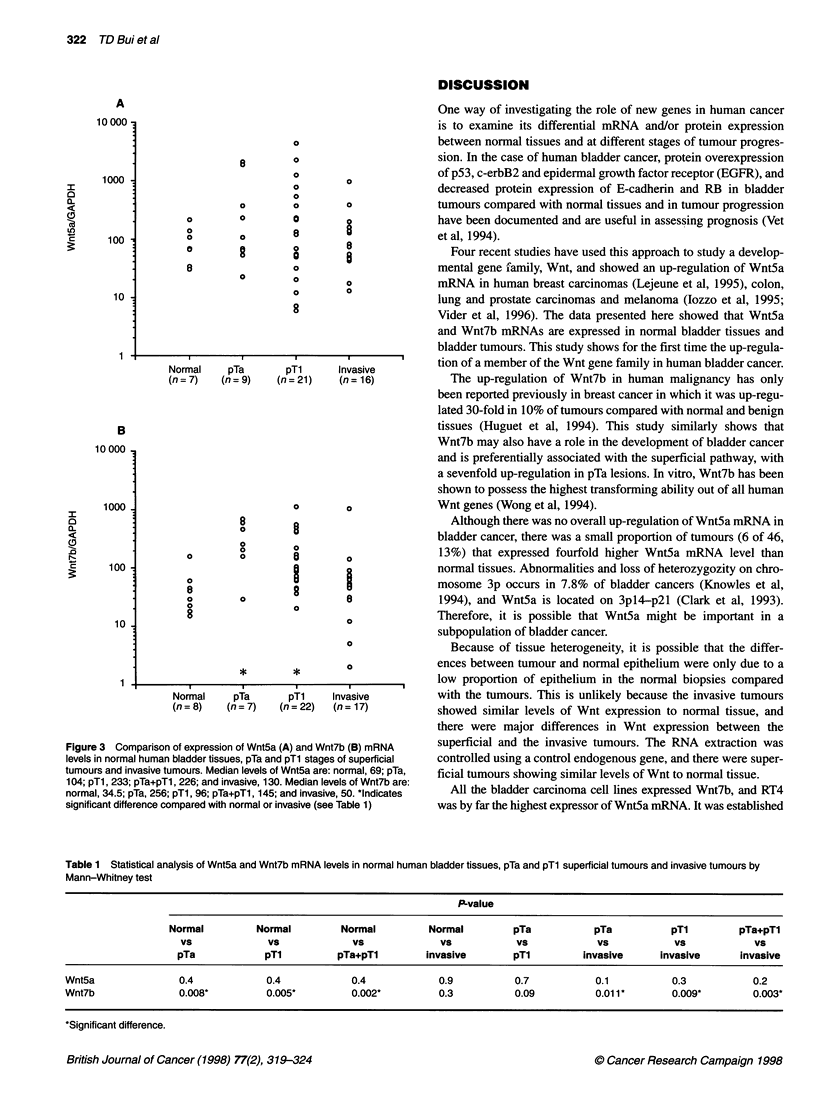
Aberrant Wnt gene expression is involved in the development of breast cancer, but its role in other tumours is unknown. Wnts regulate cadherin function, previously shown to be more commonly deregulated in invasive bladder cancer. This study investigated whether factors upstream of cadherins were aberrantly expressed in superficial bladder cancer. The expression of one transforming (Wnt7b) and one non-transforming (Wnt5a) Wnt gene in four human bladder carcinoma cell lines, and in normal human bladder tissues (n = 8) and bladder cancers (n = 48) were analysed by ribonuclease protection analysis. All cell lines expressed an approximately equal level of Wnt7b mRNA. Wnt5a and Wnt7b mRNAs were both expressed in normal bladder tissues and bladder tumours. The median expression of Wnt7b was fourfold higher in superficial tumours (n = 29) than in normal tissues (n = 8, P = 0.002) and five fold higher than in invasive tumours (n = 17, P = 0.003). There was no significant difference between normal tissues and invasive tumours (P = 0.3). The expression of Wnt5a did not vary significantly between normal tissues and superficial tumours (P = 0.4), normal tissues and invasive tumours (P = 0.3) or superficial tumours and invasive tumours (P = 0.2). The differential expression of Wnt7b suggests a role in the early events of superficial bladder tumorigenesis involving cell adhesion and provides further evidence of different pathways of evolution of superficial and invasive cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley R. S., Brown A. M. A soluble form of Wnt-1 protein with mitogenic activity on mammary epithelial cells. Mol Cell Biol. 1995 Aug;15(8):4616–4622. doi: 10.1128/mcb.15.8.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R. S., Brown A. M. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990 May;9(5):1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R. S., Cowin P., Brown A. M. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993 Dec;123(6 Pt 2):1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringuier P. P., Umbas R., Schaafsma H. E., Karthaus H. F., Debruyne F. M., Schalken J. A. Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res. 1993 Jul 15;53(14):3241–3245. [PubMed] [Google Scholar]
- Brown A. M., Papkoff J., Fung Y. K., Shackleford G. M., Varmus H. E. Identification of protein products encoded by the proto-oncogene int-1. Mol Cell Biol. 1987 Nov;7(11):3971–3977. doi: 10.1128/mcb.7.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrus L. W., McMahon A. P. Biochemical analysis of murine Wnt proteins reveals both shared and distinct properties. Exp Cell Res. 1995 Oct;220(2):363–373. doi: 10.1006/excr.1995.1327. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Cohen I., Eichstetter I., Cannizzaro L. A., McPherson J. D., Wasmuth J. J., Iozzo R. V. Molecular cloning of the human proto-oncogene Wnt-5A and mapping of the gene (WNT5A) to chromosome 3p14-p21. Genomics. 1993 Nov;18(2):249–260. doi: 10.1006/geno.1993.1463. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Gumbiner B. M. Cell contact-dependent signaling. Dev Biol. 1996 Dec 15;180(2):445–454. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- Griffiths T. R., Brotherick I., Bishop R. I., White M. D., McKenna D. M., Horne C. H., Shenton B. K., Neal D. E., Mellon J. K. Cell adhesion molecules in bladder cancer: soluble serum E-cadherin correlates with predictors of recurrence. Br J Cancer. 1996 Aug;74(4):579–584. doi: 10.1038/bjc.1996.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L., Nelson W. J., Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994 Mar;124(5):729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet E. L., McMahon J. A., McMahon A. P., Bicknell R., Harris A. L. Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res. 1994 May 15;54(10):2615–2621. [PubMed] [Google Scholar]
- Huguet E. L., Smith K., Bicknell R., Harris A. L. Regulation of Wnt5a mRNA expression in human mammary epithelial cells by cell shape, confluence, and hepatocyte growth factor. J Biol Chem. 1995 May 26;270(21):12851–12856. doi: 10.1074/jbc.270.21.12851. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Eichstetter I., Danielson K. G. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995 Aug 15;55(16):3495–3499. [PubMed] [Google Scholar]
- Kitajewski J., Mason J. O., Varmus H. E. Interaction of Wnt-1 proteins with the binding protein BiP. Mol Cell Biol. 1992 Feb;12(2):784–790. doi: 10.1128/mcb.12.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. A., Elder P. A., Williamson M., Cairns J. P., Shaw M. E., Law M. G. Allelotype of human bladder cancer. Cancer Res. 1994 Jan 15;54(2):531–538. [PubMed] [Google Scholar]
- Kühl M., Wedlich D. Wnt signalling goes nuclear. Bioessays. 1997 Feb;19(2):101–104. doi: 10.1002/bies.950190204. [DOI] [PubMed] [Google Scholar]
- Lejeune S., Huguet E. L., Hamby A., Poulsom R., Harris A. L. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res. 1995 Feb;1(2):215–222. [PubMed] [Google Scholar]
- Mason J. O., Kitajewski J., Varmus H. E. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Mol Biol Cell. 1992 May;3(5):521–533. doi: 10.1091/mbc.3.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S. A., Bicknell R. Responses of pertussis toxin-treated microvascular endothelial cells to transforming growth factor beta 1. No evidence for pertussis-sensitive G-protein involvement in TGF-beta signal transduction. J Biol Chem. 1992 Oct 25;267(30):21617–21622. [PubMed] [Google Scholar]
- Moon R. T., Campbell R. M., Christian J. L., McGrew L. L., Shih J., Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993 Sep;119(1):97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Neal D. E., Sharples L., Smith K., Fennelly J., Hall R. R., Harris A. L. The epidermal growth factor receptor and the prognosis of bladder cancer. Cancer. 1990 Apr 1;65(7):1619–1625. doi: 10.1002/1097-0142(19900401)65:7<1619::aid-cncr2820650728>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Wnt genes. Cell. 1992 Jun 26;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- O'Brien T., Cranston D., Fuggle S., Bicknell R., Harris A. L. Different angiogenic pathways characterize superficial and invasive bladder cancer. Cancer Res. 1995 Feb 1;55(3):510–513. [PubMed] [Google Scholar]
- Papkoff J., Brown A. M., Varmus H. E. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol. 1987 Nov;7(11):3978–3984. doi: 10.1128/mcb.7.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J. Inducible overexpression and secretion of int-1 protein. Mol Cell Biol. 1989 Aug;9(8):3377–3384. doi: 10.1128/mcb.9.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990 Jun;10(6):2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr B. A., McMahon A. P. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995 Mar 23;374(6520):350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Presti J. C., Jr, Reuter V. E., Galan T., Fair W. R., Cordon-Cardo C. Molecular genetic alterations in superficial and locally advanced human bladder cancer. Cancer Res. 1991 Oct 1;51(19):5405–5409. [PubMed] [Google Scholar]
- Roelink H., Wang J., Black D. M., Solomon E., Nusse R. Molecular cloning and chromosomal localization to 17q21 of the human WNT3 gene. Genomics. 1993 Sep;17(3):790–792. doi: 10.1006/geno.1993.1412. [DOI] [PubMed] [Google Scholar]
- Shimazui T., Schalken J. A., Giroldi L. A., Jansen C. F., Akaza H., Koiso K., Debruyne F. M., Bringuier P. P. Prognostic value of cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120cas) in bladder tumors. Cancer Res. 1996 Sep 15;56(18):4154–4158. [PubMed] [Google Scholar]
- Stark K., Vainio S., Vassileva G., McMahon A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994 Dec 15;372(6507):679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Syrigos K. N., Krausz T., Waxman J., Pandha H., Rowlinson-Busza G., Verne J., Epenetos A. A., Pignatelli M. E-cadherin expression in bladder cancer using formalin-fixed, paraffin-embedded tissues: correlation with histopathological grade, tumour stage and survival. Int J Cancer. 1995 Dec 20;64(6):367–370. doi: 10.1002/ijc.2910640603. [DOI] [PubMed] [Google Scholar]
- Vet J. A., Debruyne F. M., Schalken J. A. Molecular prognostic factors in bladder cancer. World J Urol. 1994;12(2):84–88. doi: 10.1007/BF00184242. [DOI] [PubMed] [Google Scholar]
- Vider B. Z., Zimber A., Chastre E., Prevot S., Gespach C., Estlein D., Wolloch Y., Tronick S. R., Gazit A., Yaniv A. Evidence for the involvement of the Wnt 2 gene in human colorectal cancer. Oncogene. 1996 Jan 4;12(1):153–158. [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Stanier P., Watson E. K., Bell G., Wicking C., Estivill X., Courtney M., Boue A., Pedersen P. S. Isolation of a human gene with protein sequence similarity to human and murine int-1 and the Drosophila segment polarity mutant wingless. EMBO J. 1988 Jun;7(6):1743–1748. doi: 10.1002/j.1460-2075.1988.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki S., Watanabe R., Saito K., Saito T., Katagiri A., Sato S., Tomita Y. Loss of human E-cadherin (ECD) correlated with invasiveness of transitional cell cancer in the renal pelvis, ureter and urinary bladder. Cancer Lett. 1996 May 15;103(1):11–17. doi: 10.1016/0304-3835(96)04194-8. [DOI] [PubMed] [Google Scholar]
- Whitehead I., Kirk H., Kay R. Expression cloning of oncogenes by retroviral transfer of cDNA libraries. Mol Cell Biol. 1995 Feb;15(2):704–710. doi: 10.1128/mcb.15.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witjes J. A., Umbas R., Debruyne F. M., Schalken J. A. Expression of markers for transitional cell carcinoma in normal bladder mucosa of patients with bladder cancer. J Urol. 1995 Dec;154(6):2185–2189. [PubMed] [Google Scholar]
- Wong G. T., Gavin B. J., McMahon A. P. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994 Sep;14(9):6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., Kwee V., Nusse R. The nucleotide sequence of the human int-1 mammary oncogene; evolutionary conservation of coding and non-coding sequences. EMBO J. 1985 Nov;4(11):2905–2909. doi: 10.1002/j.1460-2075.1985.tb04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]