Abstract
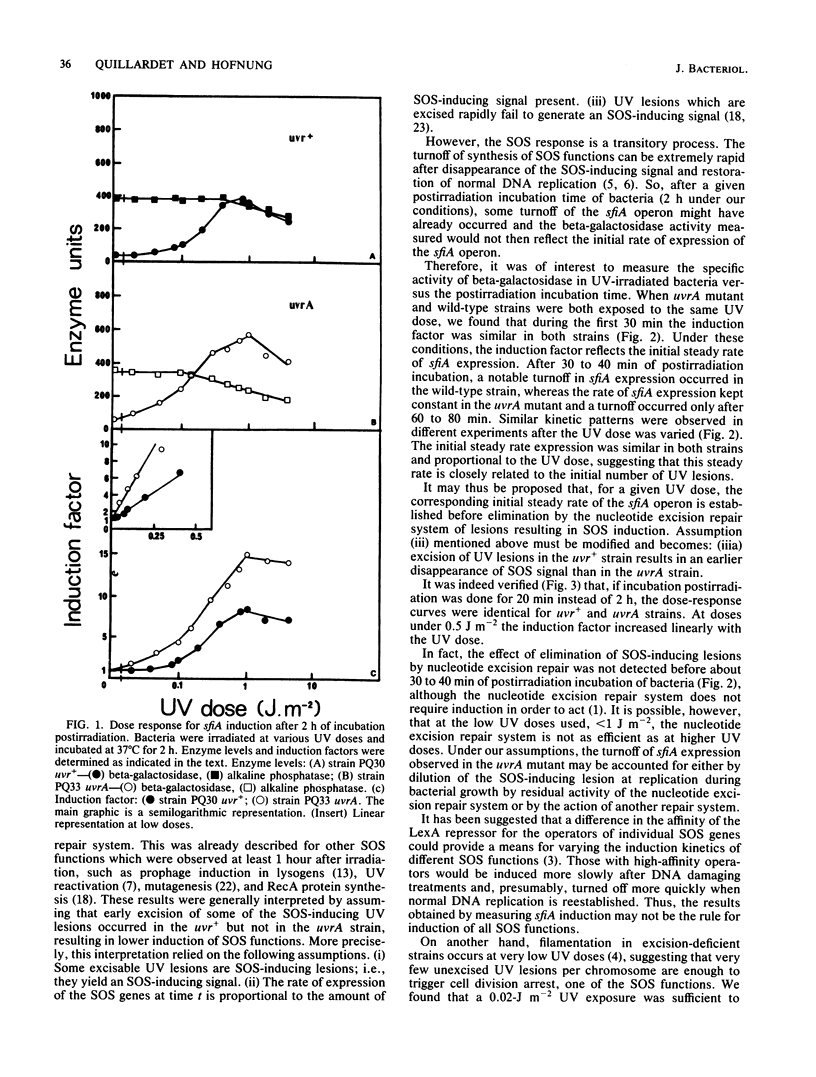
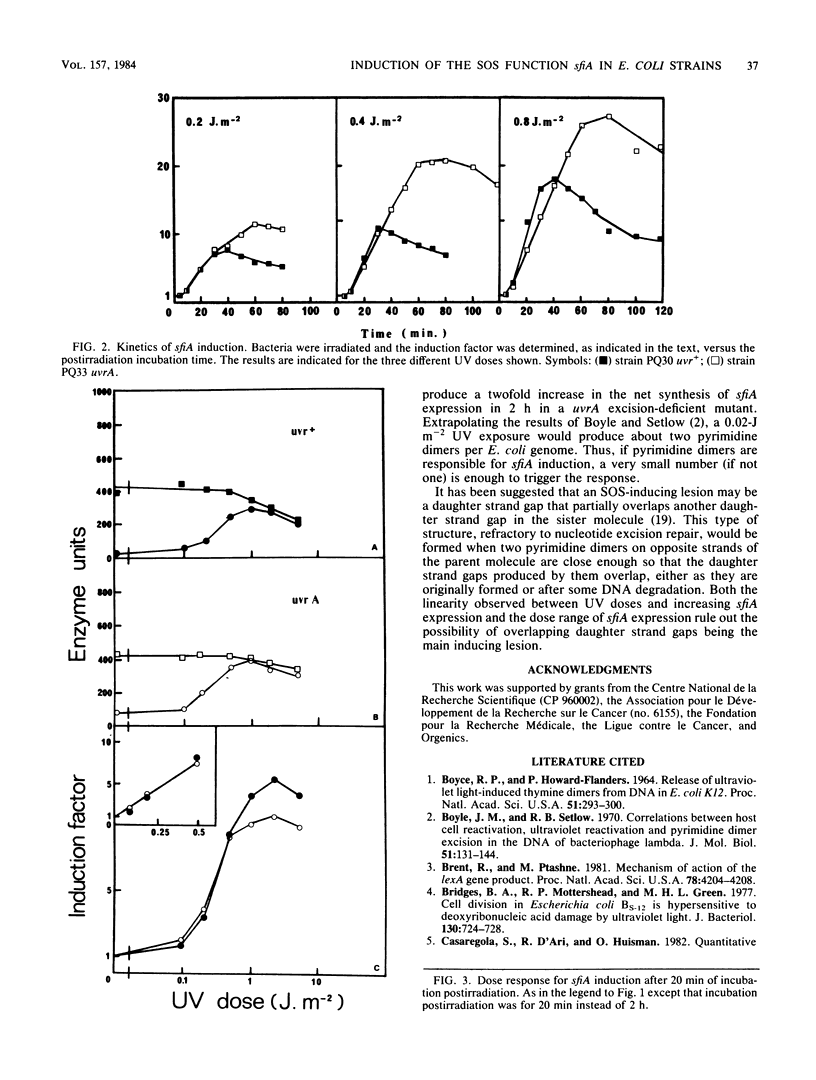
The influence of the nucleotide excision repair system on the induction by UV irradiation of the SOS function sfiA has been investigated. The level of sfiA expression was monitored by means of a sfiA::lacZ operon fusion in both the wild-type strain and a uvrA mutant. We found that the initial steady rate of sfiA expression was proportional to the UV dose and was identical in uvr+ and uvrA backgrounds. This suggests that the initial steady rate of sfiA expression is determined by the initial number of lesions and before any effect of excision repair. We confirmed that after 2 h of expression the net synthesis of sfiA product is, for the same UV dose, about five times lower in uvr+ than in uvrA strains. We show that this is due to earlier repression of the SOS system in uvr+ than in uvrA strains and not to different initial rates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Setlow R. B. Correlations between host-cell reactivation, ultraviolet reactivation and pyrimidine dimer excision in the DNA of bacteriophage lambda. J Mol Biol. 1970 Jul 14;51(1):131–144. doi: 10.1016/0022-2836(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P., Green M. H. Cell division in Escherichia coli BS-12 is hypersensitive to deoxyribonucleic acid damage by ultraviolet light. J Bacteriol. 1977 May;130(2):724–728. doi: 10.1128/jb.130.2.724-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaregola S., D'Ari R., Huisman O. Role of DNA replication in the induction and turn-off of the SOS response in Escherichia coli. Mol Gen Genet. 1982;185(3):440–444. doi: 10.1007/BF00334136. [DOI] [PubMed] [Google Scholar]
- Defais M., Fauquet P., Radman M., Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of lambda in different genetic systems. Virology. 1971 Feb;43(2):495–503. doi: 10.1016/0042-6822(71)90321-7. [DOI] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Monk M., Peacey M., Gross J. D. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. doi: 10.1016/0022-2836(71)90376-7. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Huisman O., D'Ari R., Hofnung M. SOS chromotest, a direct assay of induction of an SOS function in Escherichia coli K-12 to measure genotoxicity. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5971–5975. doi: 10.1073/pnas.79.19.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert C. S. Enzymatic photoreactivation: overview. Basic Life Sci. 1975;5A:73–87. doi: 10.1007/978-1-4684-2895-7_11. [DOI] [PubMed] [Google Scholar]
- Salles B., Paoletti C. Control of UV induction of recA protein. Proc Natl Acad Sci U S A. 1983 Jan;80(1):65–69. doi: 10.1073/pnas.80.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G. Genetic and kinetic evidence for different types of postreplication repair in Escherichia coli B. J Bacteriol. 1975 Jul;123(1):154–161. doi: 10.1128/jb.123.1.154-161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Nissen-Meyer J., Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976 Oct 7;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Persistence and decay of thermoinducible error-prone repair activity in nonfilamentous derivatives of tif-1, Escherichia coli B/r: the timing of some critical events in ultraviolet mutagenesis. Mol Gen Genet. 1975 Dec 29;142(2):87–103. doi: 10.1007/BF00266092. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


