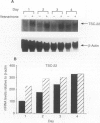
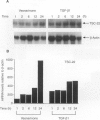
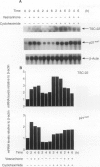
Abstract
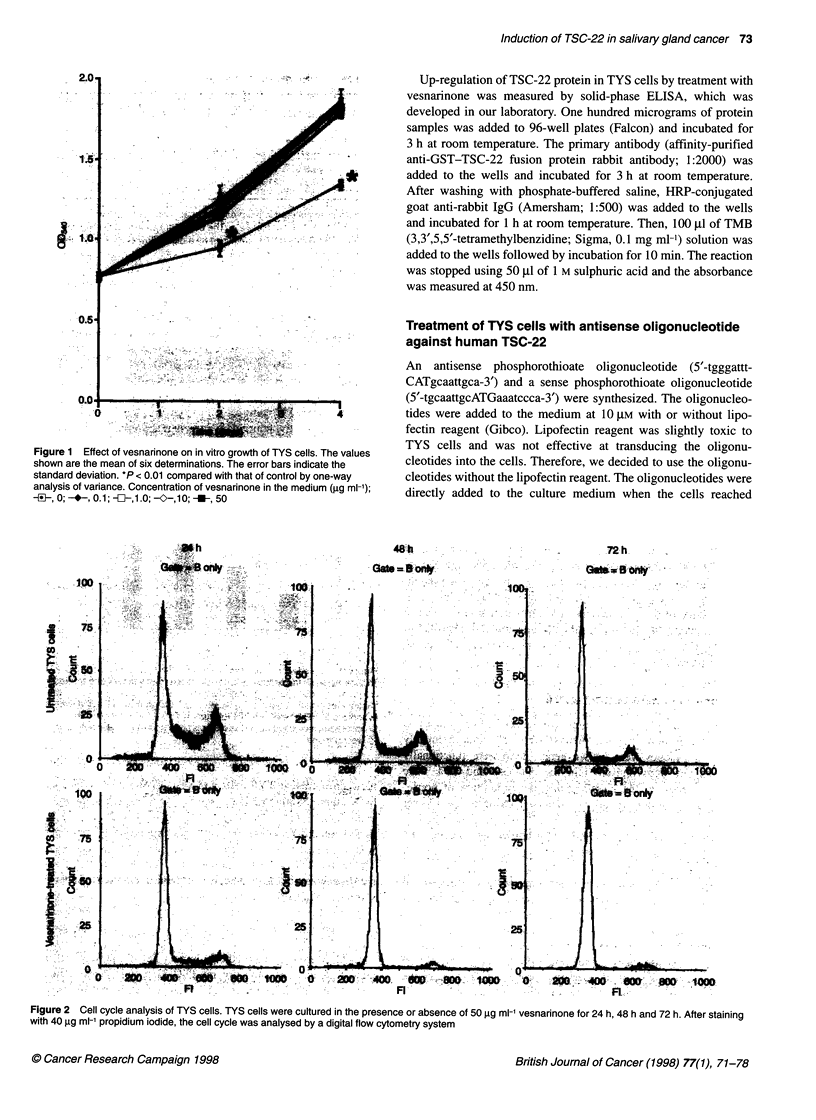
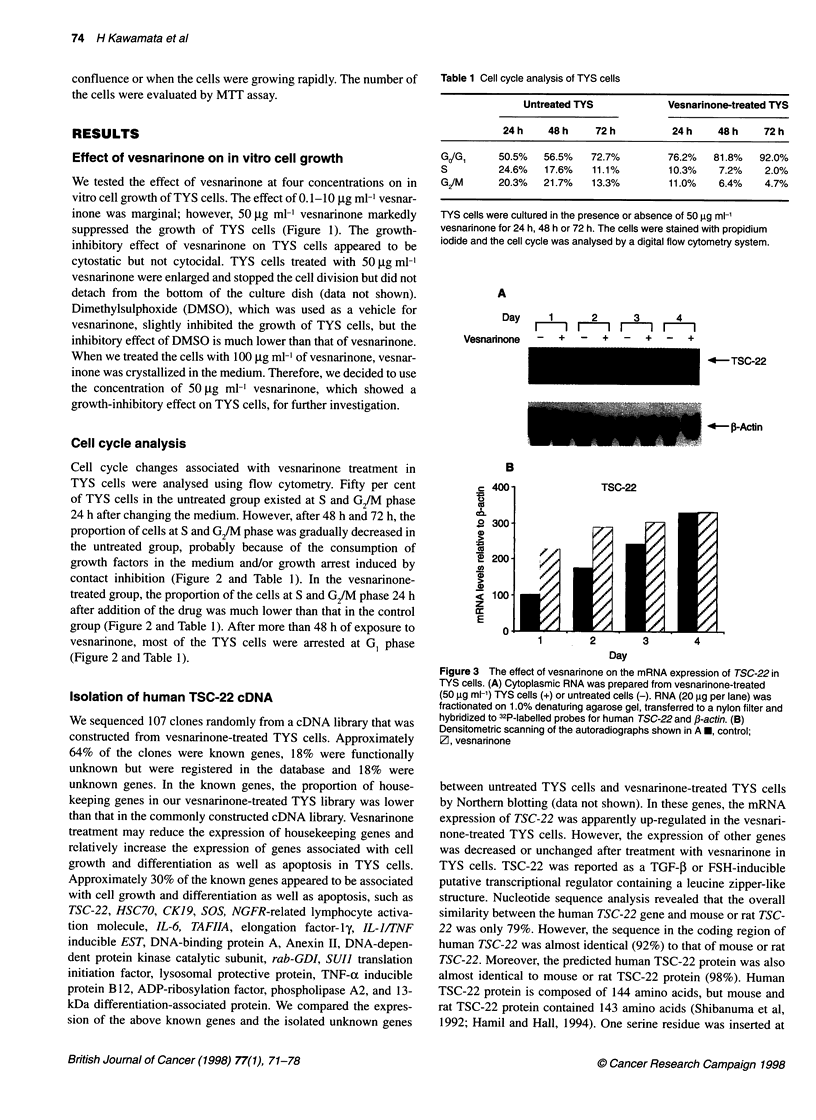
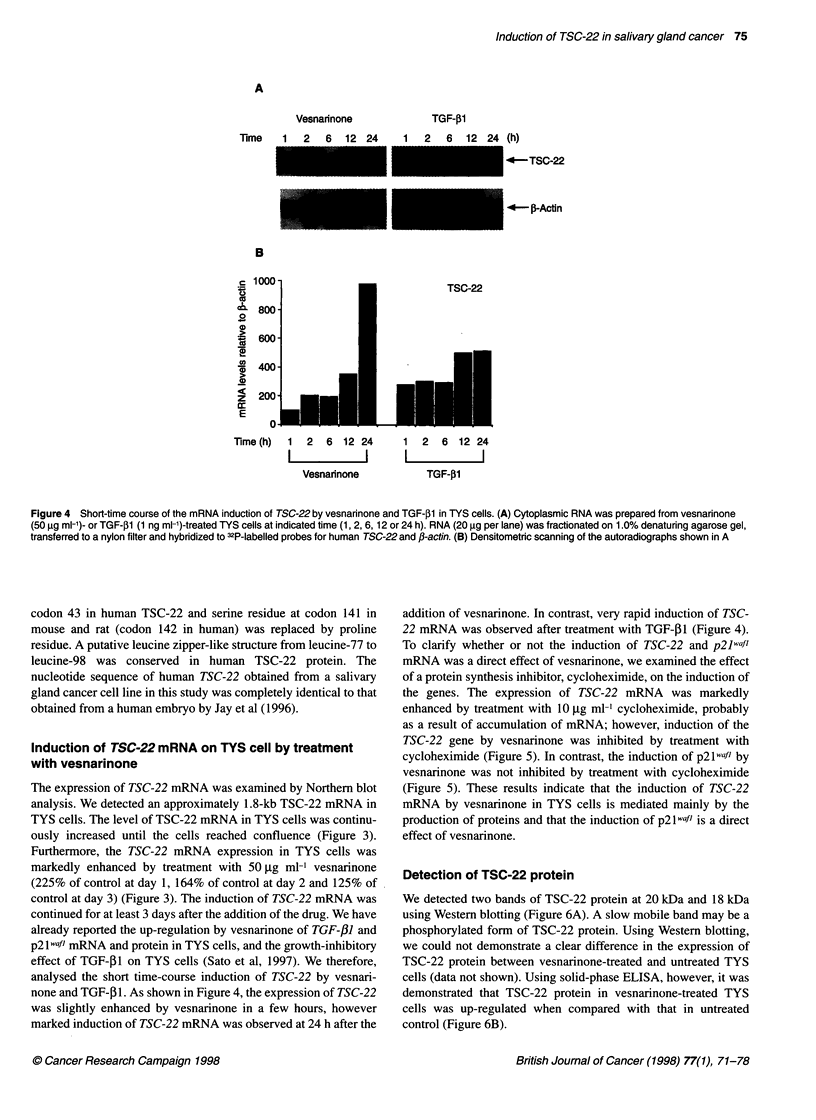
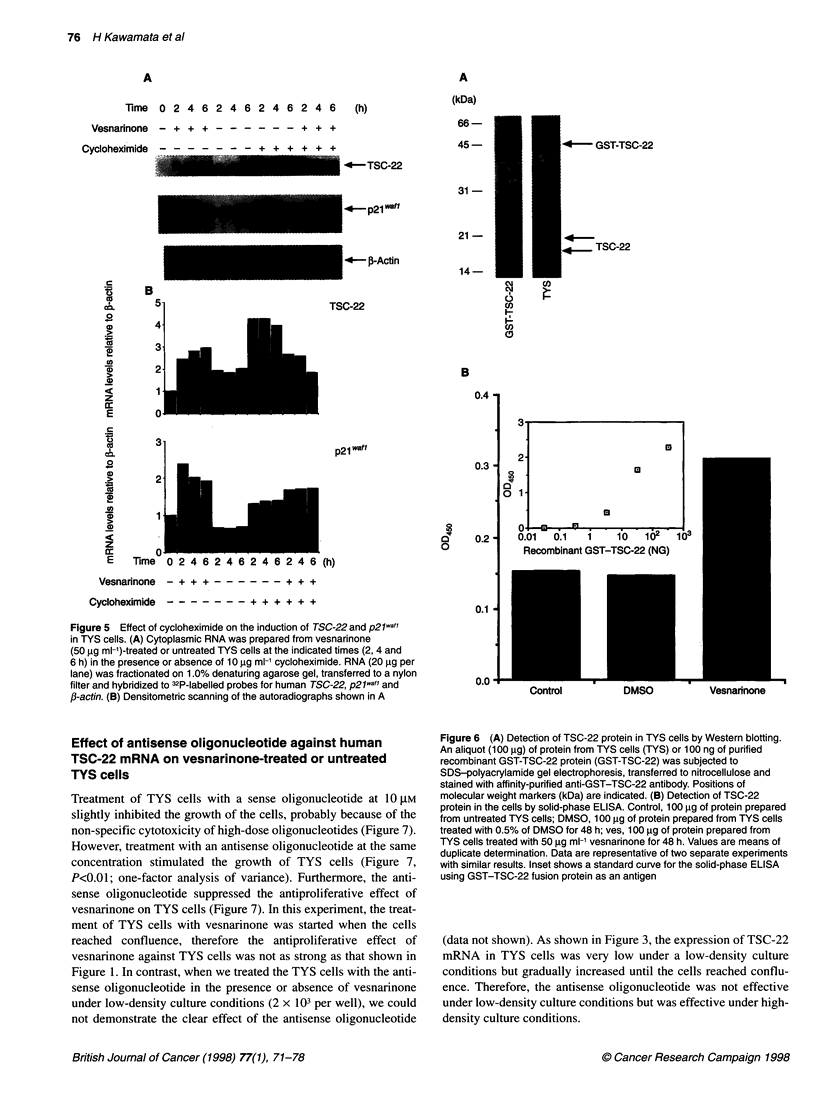
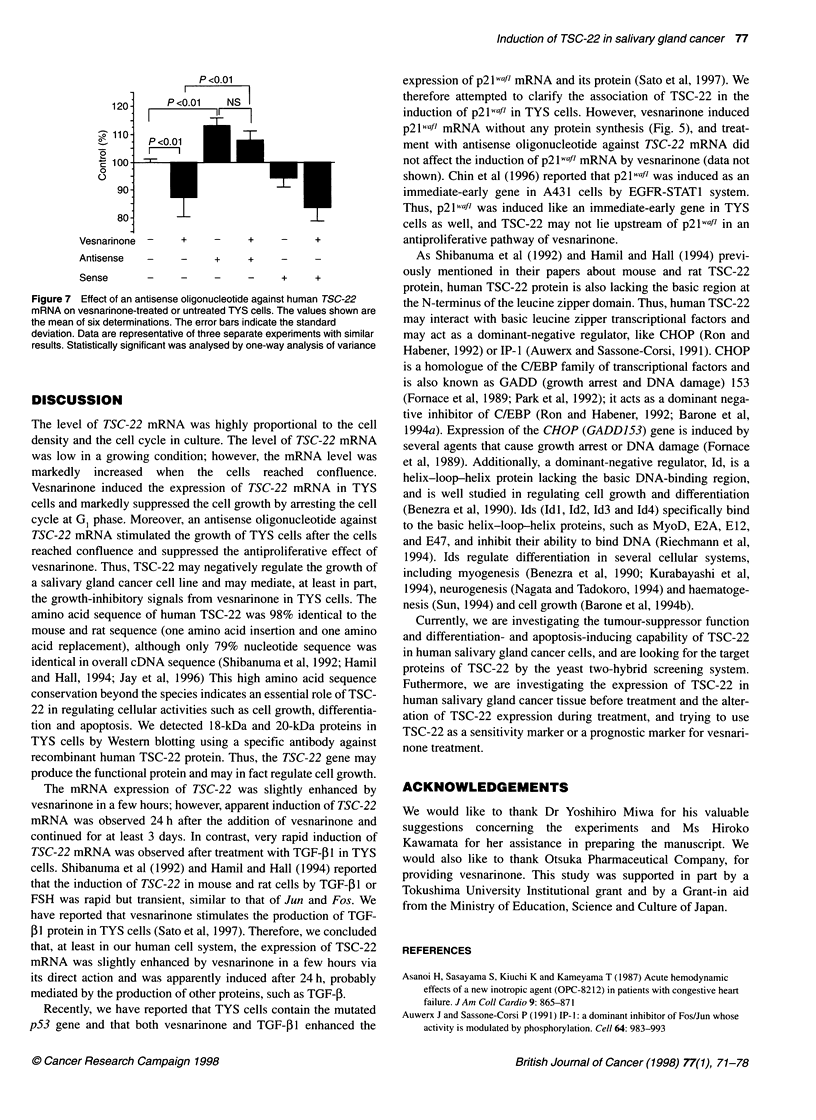
We undertook the present study to clarify the molecular mechanism of the effect of a new anti-cancer drug, vesnarinone, on a human salivary gland cancer cell line, TYS. We isolated TSC-22cDNA as avesnarinone-inducible gene from a cDNA library constructed from vesnarinone-treated TYS cells. TSC-22 was originally reported as a transforming growth factor (TGF)-beta-inducible gene. The expression of TSC-22 was up-regulated within a few hours after treatment with vesnarinone and was continued for 3 days. The level of TSC-22 mRNA in TYS cells was continuously increased until the cells reached confluency. Furthermore, the induction of TSC-22 by vesnarinone was inhibited by treatment with cycloheximide. When we treated the cells with an antisense oligonucleotide against TSC-22 mRNA under quiescent conditions, the antisense oligonucleotide stimulated the growth of TYS cells; however, under growing conditions the antisense oligonucleotide did not affect cell growth. Furthermore, the antisense oligonucleotide suppressed the antiproliferative effect of vesnarinone. These results suggest that TSC-22 may be a negative growth regulator and may play an important role in the antiproliferative effect of vesnarinone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asanoi H., Sasayama S., Iuchi K., Kameyama T. Acute hemodynamic effects of a new inotropic agent (OPC-8212) in patients with congestive heart failure. J Am Coll Cardiol. 1987 Apr;9(4):865–871. doi: 10.1016/s0735-1097(87)80243-7. [DOI] [PubMed] [Google Scholar]
- Auwerx J., Sassone-Corsi P. IP-1: a dominant inhibitor of Fos/Jun whose activity is modulated by phosphorylation. Cell. 1991 Mar 8;64(5):983–993. doi: 10.1016/0092-8674(91)90322-p. [DOI] [PubMed] [Google Scholar]
- Barone M. V., Crozat A., Tabaee A., Philipson L., Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994 Feb 15;8(4):453–464. doi: 10.1101/gad.8.4.453. [DOI] [PubMed] [Google Scholar]
- Barone M. V., Pepperkok R., Peverali F. A., Philipson L. Id proteins control growth induction in mammalian cells. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4985–4988. doi: 10.1073/pnas.91.11.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Chin Y. E., Kitagawa M., Su W. C., You Z. H., Iwamoto Y., Fu X. Y. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996 May 3;272(5262):719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamil K. G., Hall S. H. Cloning of rat Sertoli cell follicle-stimulating hormone primary response complementary deoxyribonucleic acid: regulation of TSC-22 gene expression. Endocrinology. 1994 Mar;134(3):1205–1212. doi: 10.1210/endo.134.3.8161377. [DOI] [PubMed] [Google Scholar]
- Jay P., Ji J. W., Marsollier C., Taviaux S., Bergé-Lefranc J. L., Berta P. Cloning of the human homologue of the TGF beta-stimulated clone 22 gene. Biochem Biophys Res Commun. 1996 May 24;222(3):821–826. doi: 10.1006/bbrc.1996.0825. [DOI] [PubMed] [Google Scholar]
- Kurabayashi M., Jeyaseelan R., Kedes L. Doxorubicin represses the function of the myogenic helix-loop-helix transcription factor MyoD. Involvement of Id gene induction. J Biol Chem. 1994 Feb 25;269(8):6031–6039. [PubMed] [Google Scholar]
- Nagata Y., Todokoro K. Activation of helix-loop-helix proteins Id1, Id2 and Id3 during neural differentiation. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1355–1362. doi: 10.1006/bbrc.1994.1380. [DOI] [PubMed] [Google Scholar]
- Park J. S., Luethy J. D., Wang M. G., Fargnoli J., Fornace A. J., Jr, McBride O. W., Holbrook N. J. Isolation, characterization and chromosomal localization of the human GADD153 gene. Gene. 1992 Jul 15;116(2):259–267. doi: 10.1016/0378-1119(92)90523-r. [DOI] [PubMed] [Google Scholar]
- Riechmann V., van Crüchten I., Sablitzky F. The expression pattern of Id4, a novel dominant negative helix-loop-helix protein, is distinct from Id1, Id2 and Id3. Nucleic Acids Res. 1994 Mar 11;22(5):749–755. doi: 10.1093/nar/22.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Habener J. F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992 Mar;6(3):439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- Sato M., Harada K., Bando T., Shirakami T., Nakashiro K., Yoshida H., Nakai S., Kawai K., Adachi M. Characteristics of antitumor activity of 3,4-dihydro-6-[4-(3,4-dimethoxybenzoyl)-1-piperazinyl]- 2(1H)-quinolinone (vesnarinone) against a human adenoid squamous carcinoma-forming cell line grown in athymic nude mice. Cancer Lett. 1995 May 4;91(1):1–9. doi: 10.1016/0304-3835(95)03713-7. [DOI] [PubMed] [Google Scholar]
- Sato M., Kawamata H., Harada K., Nakashiro K., Ikeda Y., Gohda H., Yoshida H., Nishida T., Ono K., Kinoshita M. Induction of cyclin-dependent kinase inhibitor, p21WAF1, by treatment with 3,4-dihydro-6-[4-(3,4)-dimethoxybenzoyl)-1-piperazinyl]-2(1H)-quinoline (vesnarinone) in a human salivary cancer cell line with mutant p53 gene. Cancer Lett. 1997 Jan 30;112(2):181–189. doi: 10.1016/s0304-3835(96)04581-8. [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Kuroki T., Nose K. Isolation of a gene encoding a putative leucine zipper structure that is induced by transforming growth factor beta 1 and other growth factors. J Biol Chem. 1992 May 25;267(15):10219–10224. [PubMed] [Google Scholar]
- Sun X. H. Constitutive expression of the Id1 gene impairs mouse B cell development. Cell. 1994 Dec 2;79(5):893–900. doi: 10.1016/0092-8674(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Yanagawa T., Hayashi Y., Yoshida H., Yura Y., Nagamine S., Bando T., Sato M. An adenoid squamous carcinoma-forming cell line established from an oral keratinizing squamous cell carcinoma expressing carcinoembryonic antigen. Am J Pathol. 1986 Sep;124(3):496–509. [PMC free article] [PubMed] [Google Scholar]