Abstract
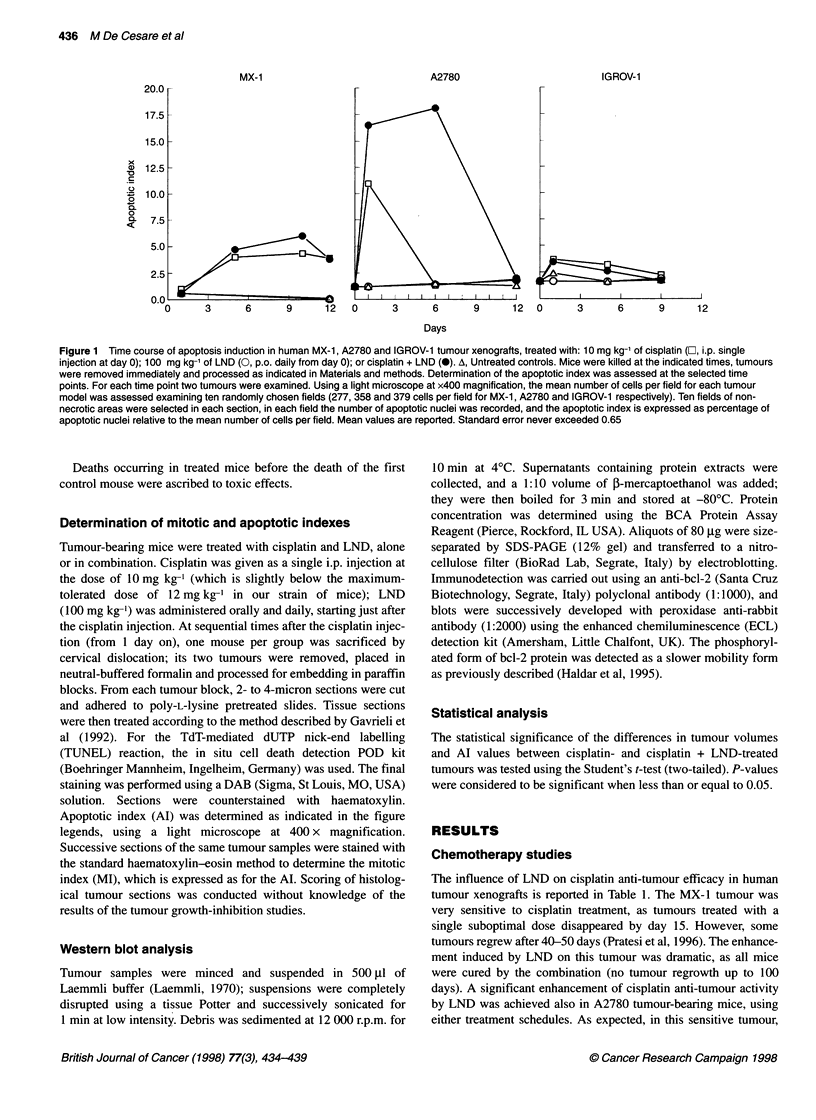
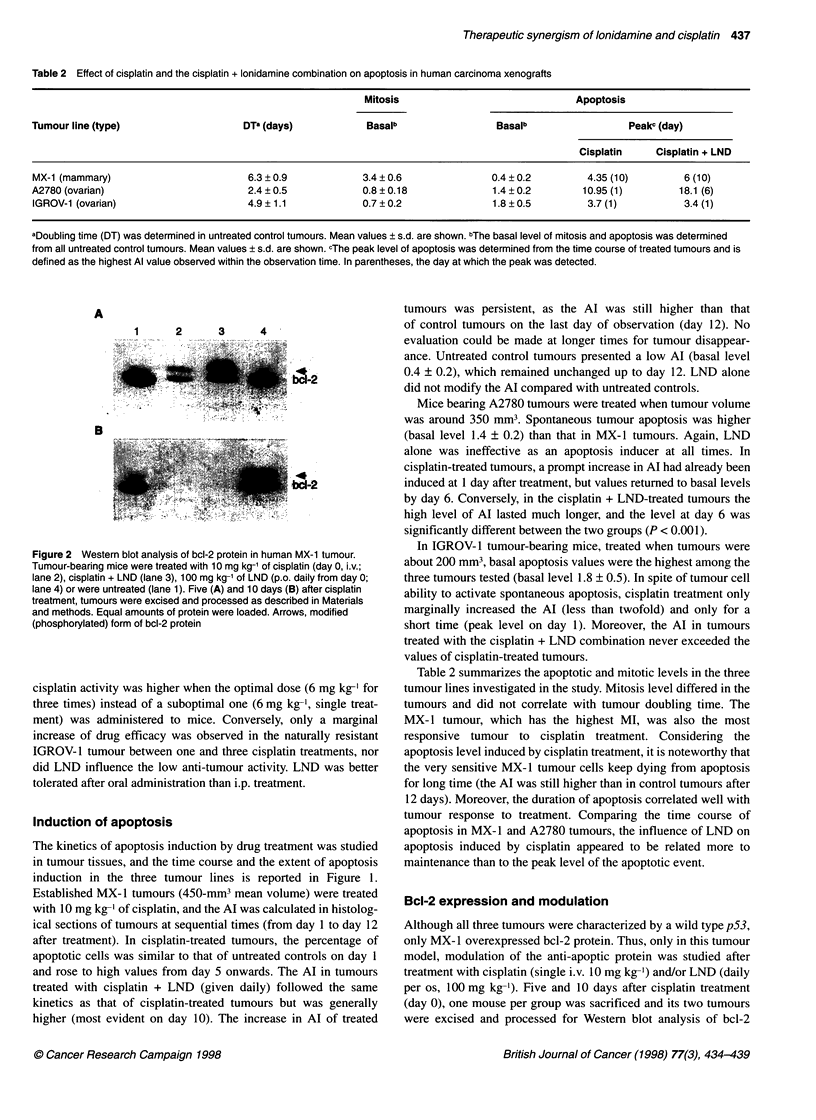
The pharmacological interest in lonidamine is related to its ability to enhance the cytotoxic effects of several DNA-damaging anti-tumour agents. This study was undertaken to better understand the in vivo interaction between lonidamine and cisplatin in the treatment of human tumour xenografts, including three carcinoma models characterized by a different responsiveness to cisplatin, in spite of the presence of a wild-type p53 gene in all tumours. The drug combination was more effective in tumour growth inhibition than cisplatin alone against MX-1 breast carcinoma and A2780 ovarian carcinoma, both highly responsive to cisplatin, whereas no influence of ionidamine was observed on anti-tumour activity of cisplatin in the treatment of the relatively resistant IGROV-1 ovarian carcinoma. As cisplatin activity is related to induction of apoptosis, the modulation of drug-induced apoptosis by lonidamine was investigated. Under conditions in which lonidamine itself had negligible effects on tumour growth and apoptosis, the modulating agent stimulated the apoptotic response induced by cisplatin in the responsive but not in the resistant tumours. Tumour response was dependent not only on the drug activation of apoptosis, but mainly on the persistence over time of the event. In the breast carcinoma MX-1, hypersensitive to cisplatin and to the lonidamine+cisplatin combination, the efficacy of drug treatment was associated with phosphorylation of bcl-2 followed by down-regulation of the protein. Lonidamine itself caused a delayed phosphorylation of bcl-2. These results are consistent with the interpretation that lonidamine is effective in modulating biochemical factors involved in regulation of apoptosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcamone F., Animati F., Berettoni M., Bigioni M., Capranico G., Casazza A. M., Caserini C., Cipollone A., De Cesare M., Franciotti M. Doxorubicin disaccharide analogue: apoptosis-related improvement of efficacy in vivo. J Natl Cancer Inst. 1997 Aug 20;89(16):1217–1223. doi: 10.1093/jnci/89.16.1217. [DOI] [PubMed] [Google Scholar]
- Caserini C., Pratesi G., Tortoreto M., Bedogné B., Carenini N., Supino R., Perego P., Righetti S. C., Zunino F. Apoptosis as a determinant of tumor sensitivity to topotecan in human ovarian tumors: preclinical in vitro/in vivo studies. Clin Cancer Res. 1997 Jun;3(6):955–961. [PubMed] [Google Scholar]
- Chitnis M., Adwankar M. Potentiation of adriamycin cytotoxicity in P388 murine leukemia sensitive and resistant to adriamycin by use of lonidamine and hyperthermia. Tumori. 1986 Oct 31;72(5):469–473. doi: 10.1177/030089168607200503. [DOI] [PubMed] [Google Scholar]
- Del Bufalo D., Biroccio A., Soddu S., Laudonio N., D'Angelo C., Sacchi A., Zupi G. Lonidamine induces apoptosis in drug-resistant cells independently of the p53 gene. J Clin Invest. 1996 Sep 1;98(5):1165–1173. doi: 10.1172/JCI118900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floridi A., Paggi M. G., D'Atri S., De Martino C., Marcante M. L., Silvestrini B., Caputo A. Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer Res. 1981 Nov;41(11 Pt 1):4661–4666. [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G. M., van Kersen I., Silvestrini B. Inhibition of the recovery from potentially lethal damage by lonidamine. Br J Cancer. 1984 Nov;50(5):657–660. doi: 10.1038/bjc.1984.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S., Basu A., Croce C. M. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997 Jan 15;57(2):229–233. [PubMed] [Google Scholar]
- Haldar S., Jena N., Croce C. M. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Weidemann M. J. Role and regulation of glucose metabolism in proliferating cells. J Natl Cancer Inst. 1979 Jan;62(1):3–8. [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997 Jun;3(6):614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Perego P., Giarola M., Righetti S. C., Supino R., Caserini C., Delia D., Pierotti M. A., Miyashita T., Reed J. C., Zunino F. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 1996 Feb 1;56(3):556–562. [PubMed] [Google Scholar]
- Pratesi G., Dal Bo L., Paolicchi A., Tonarelli P., Tongiani R., Zunino F. The role of the glutathione-dependent system in tumor sensitivity to cisplatin: a study of human tumor xenografts. Ann Oncol. 1995 Mar;6(3):283–289. doi: 10.1093/oxfordjournals.annonc.a059159. [DOI] [PubMed] [Google Scholar]
- Pratesi G., De Cesare M., Zunino F. Efficacy of lonidamine combined with different DNA-damaging agents in the treatment of the MX-1 tumor xenograft. Cancer Chemother Pharmacol. 1996;38(2):123–128. doi: 10.1007/s002800050459. [DOI] [PubMed] [Google Scholar]
- Raaphorst G. P., Feeley M. M., Heller D. P., Danjoux C. E., Martin L., Maroun J. A., De Sanctis A. J. Lonidamine can enhance the cytotoxic effect of cisplatin in human tumour cells and rodent cells. Anticancer Res. 1990 Jul-Aug;10(4):923–927. [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti S. C., Della Torre G., Pilotti S., Ménard S., Ottone F., Colnaghi M. I., Pierotti M. A., Lavarino C., Cornarotti M., Oriana S. A comparative study of p53 gene mutations, protein accumulation, and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Res. 1996 Feb 15;56(4):689–693. [PubMed] [Google Scholar]
- Rusch V., Klimstra D., Venkatraman E., Oliver J., Martini N., Gralla R., Kris M., Dmitrovsky E. Aberrant p53 expression predicts clinical resistance to cisplatin-based chemotherapy in locally advanced non-small cell lung cancer. Cancer Res. 1995 Nov 1;55(21):5038–5042. [PubMed] [Google Scholar]
- Sarti P., Antonini G., Arancia G., Blanck T. J., Citro G., Meloni A., Molinari A., Malatesta F. Lonidamine-mediated respiratory changes in rat heart myocytes: a re-examination of the functional response of mitochondrial cytochrome c oxidase. Biochem Pharmacol. 1994 Jun 15;47(12):2221–2225. doi: 10.1016/0006-2952(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Silvestrini B. Lonidamine: an overview. Semin Oncol. 1991 Apr;18(2 Suppl 4):2–6. [PubMed] [Google Scholar]
- UKCCCR guidelines for the welfare of animals in experimental neoplasia. Br J Cancer. 1988 Jul;58(1):109–113. doi: 10.1038/bjc.1988.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. S., El-Deiry W. S. Apoptotic death of tumor cells correlates with chemosensitivity, independent of p53 or bcl-2. Clin Cancer Res. 1996 Apr;2(4):623–633. [PubMed] [Google Scholar]
- Zupi G., Greco C., Laudonio N., Benassi M., Silvestrini B., Caputo A. In vitro and in vivo potentiation by lonidamine of the antitumor effect of adriamycin. Anticancer Res. 1986 Sep-Oct;6(5):1245–1249. [PubMed] [Google Scholar]



