Abstract
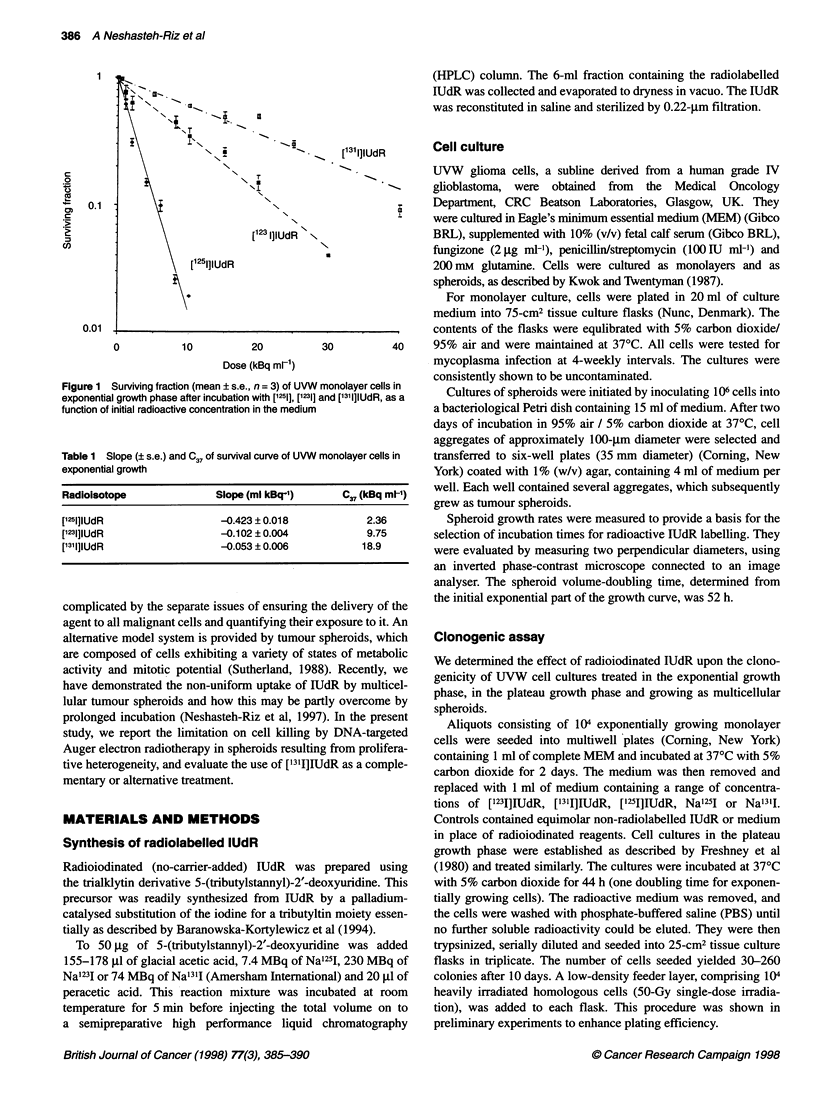
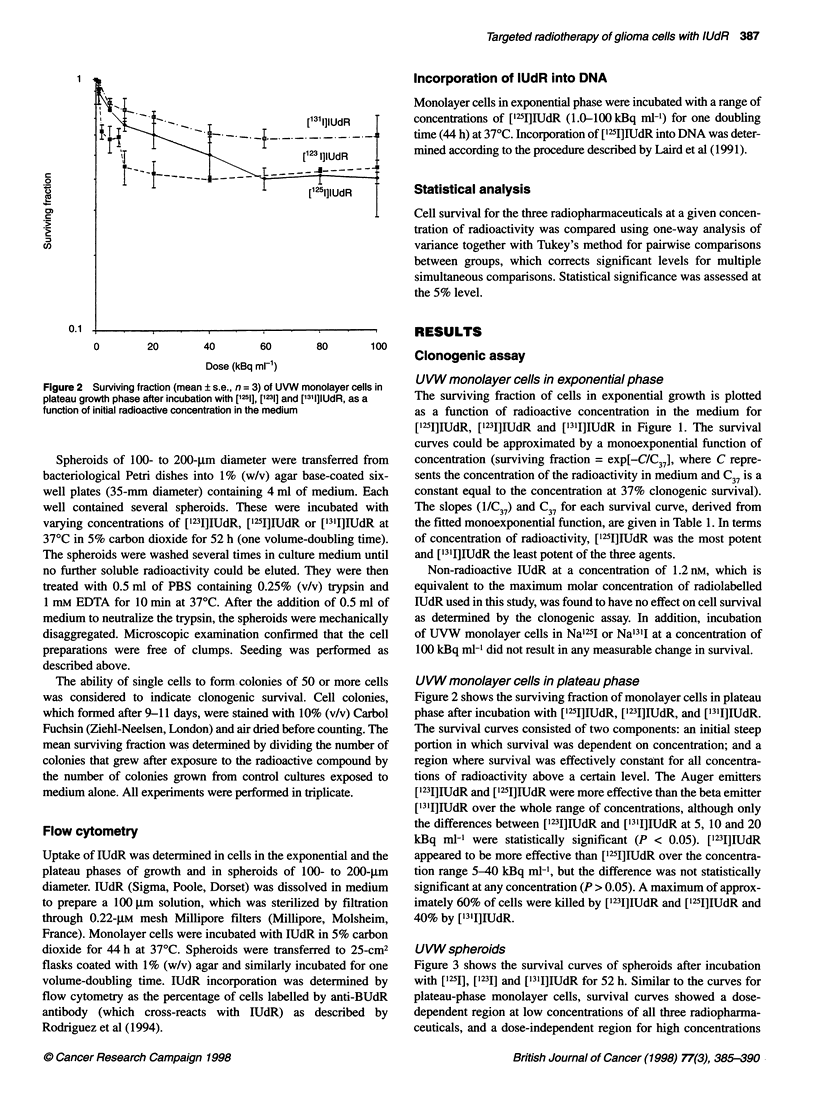
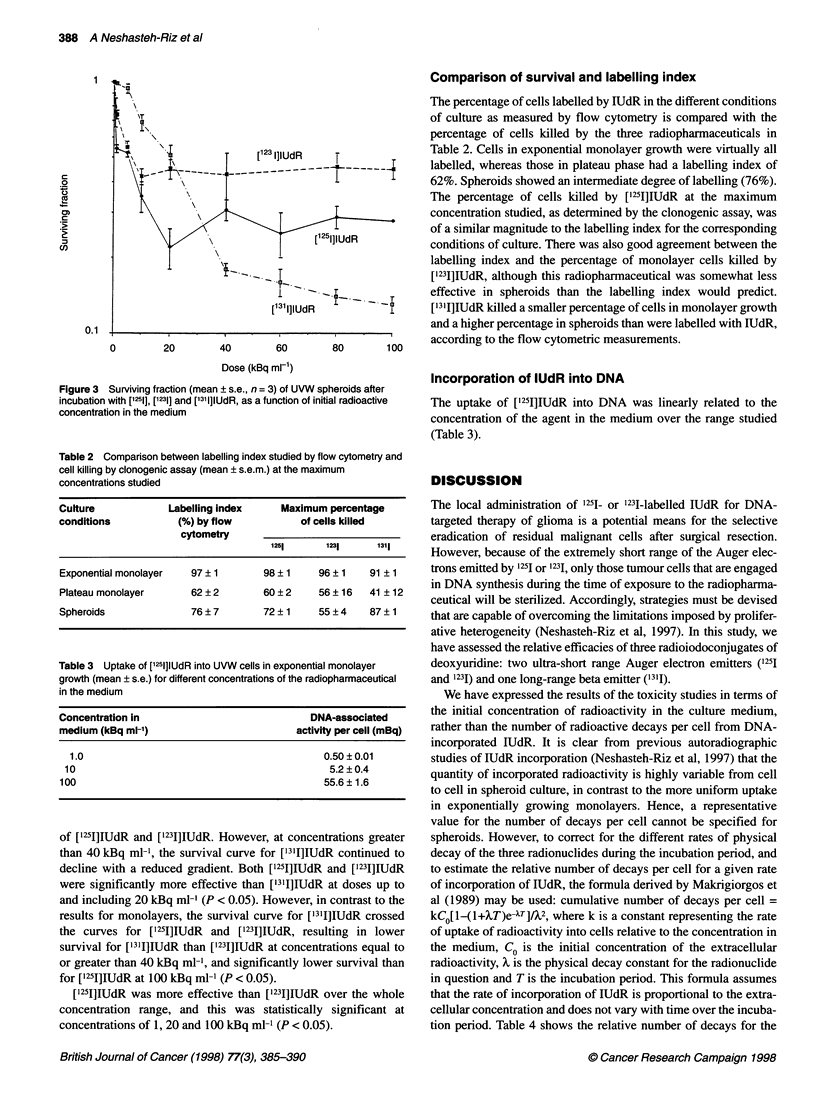
Radioiodinated iododeoxyuridine (IUdR) is a novel, cycle-specific agent that has potential for the treatment of residual malignant glioma after surgery. As only cells in S-phase incorporate IUdR into DNA, a major limitation to this therapy is likely to be proliferative heterogeneity of the tumour cell population. Using a clonogenic end point, we have compared the toxicities of three radioiodoanalogues of IUdR--[123I]IUdR, [125I]IUdR and [131I]IUdR--to the human glioma cell line UVW, cultured as monolayers in the exponential and the plateau phase of growth and as multicellular spheroids. Monolayers treated in the exponential growth phase were most efficiently sterilized by [125I]IUdR (concentration resulting in 37% survival (C37) = 2.36 kBq ml(-1)), while [123I]IUdR and [131I]IUdR were less effective eradicators of clonogens (C37 = 9.75 and 18.9 kBq ml(-1) respectively). Plateau-phase monolayer cultures were marginally more susceptible to treatment with [123I]IUdR and [125I]IUdR (40% clonogenic survival) than [131I]IUdR (60% clonogenic survival). In cells derived from glioma spheroids, both [125I]IUdR and [123I]IUdR were again more effective than [131I]IUdR at concentrations up to and including 20 kBq ml(-1). However, the survival curve for [131I]IUdR crossed the curves for the other agents, resulting in lower survival for [131I]IUdR than [123I]IUdR and [125I]IUdR at concentrations of 40 kBq ml(-1) and higher, the clonogenic survival values at 100 kBq ml(-1) were 13%, 45% and 28% respectively. It was concluded that IUdR incorporating the Auger electron emitters 123I and 125I killed only cells that were in S-phase during the period of incubation with the radiopharmaceutical, whereas the superior toxicity to clonogenic cells in spheroids of [131I]IUdR at higher concentration was due to cross-fire beta-irradiation. These findings suggest that [131I]IUdR or combinations of [131I]IUdR and [123I]IUdR or [125I]IUdR may be more effective than Auger electron emitters alone for the treatment of residual glioma, if proliferative heterogeneity exists.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranowska-Kortylewicz J., Makrigiorgos G. M., Van den Abbeele A. D., Berman R. M., Adelstein S. J., Kassis A. I. 5-[123I]iodo-2'-deoxyuridine in the radiotherapy of an early ascites tumor model. Int J Radiat Oncol Biol Phys. 1991 Nov;21(6):1541–1551. doi: 10.1016/0360-3016(91)90331-w. [DOI] [PubMed] [Google Scholar]
- Chan P. C., Lisco E., Lisco H., Adelstein S. J. The radiotoxicity of iodine-125 in mammalian cells II. A comparative study on cell survival and cytogenetic responses to 125IUdR, 131TUdR, and 3HTdR. Radiat Res. 1976 Aug;67(2):332–343. [PubMed] [Google Scholar]
- Daghighian F., Humm J. L., Macapinlac H. A., Zhang J., Izzo J., Finn R., Kemeny N., Larson S. M. Pharmacokinetics and dosimetry of iodine-125-IUdR in the treatment of colorectal cancer metastatic to liver. J Nucl Med. 1996 Apr;37(4 Suppl):29S–32S. [PubMed] [Google Scholar]
- Freshney R. I., Sherry A., Hassanzadah M., Freshney M., Crilly P., Morgan D. Control of cell proliferation in human glioma by glucocorticoids. Br J Cancer. 1980 Jun;41(6):857–866. doi: 10.1038/bjc.1980.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer K. G., Harris C. R., Smith J. M. Rdiotoxicity of intracellular 67Ga, 125I and 3H. Nuclear versus cytoplasmic radiation effects in murine L1210 leukaemia. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Sep;28(3):225–241. doi: 10.1080/09553007514550991. [DOI] [PubMed] [Google Scholar]
- Hofer K. G., Hughes W. L. Radiotoxicity of intranuclear tritium, 125 iodine and 131 iodine. Radiat Res. 1971 Jul;47(1):94–101. [PubMed] [Google Scholar]
- Humm J. L. Dosimetric aspects of radiolabeled antibodies for tumor therapy. J Nucl Med. 1986 Sep;27(9):1490–1497. [PubMed] [Google Scholar]
- Kassis A. I., Fayad F., Kinsey B. M., Sastry K. S., Taube R. A., Adelstein S. J. Radiotoxicity of 125I in mammalian cells. Radiat Res. 1987 Aug;111(2):305–318. [PubMed] [Google Scholar]
- Kassis A. I., Jones P. L., Matalka K. Z., Adelstein S. J. Antibody-dependent signal amplification in tumor xenografts after pretreatment with biotinylated monoclonal antibody and avidin or streptavidin. J Nucl Med. 1996 Feb;37(2):343–352. [PubMed] [Google Scholar]
- Kassis A. I., Sastry K. S., Adelstein S. J. Kinetics of uptake, retention, and radiotoxicity of 125IUdR in mammalian cells: implications of localized energy deposition by Auger processes. Radiat Res. 1987 Jan;109(1):78–89. [PubMed] [Google Scholar]
- Kassis A. I., Tumeh S. S., Wen P. Y., Baranowska-Kortylewicz J., Van den Abbeele A. D., Zimmerman R. E., Carvalho P. A., Garada B. M., DeSisto W. C., Bailey N. O. Intratumoral administration of 5-[123I]iodo-2'-deoxyuridine in a patient with a brain tumor. J Nucl Med. 1996 Apr;37(4 Suppl):19S–22S. [PubMed] [Google Scholar]
- Kassis A. I., Van den Abbeele A. D., Wen P. Y., Baranowska-Kortylewicz J., Aaronson R. A., DeSisto W. C., Lampson L. A., Black P. M., Adelstein S. J. Specific uptake of the auger electron-emitting thymidine analogue 5-[123I/125I]iodo-2'-deoxyuridine in rat brain tumors: diagnostic and therapeutic implications in humans. Cancer Res. 1990 Aug 15;50(16):5199–5203. [PubMed] [Google Scholar]
- Kwok T. T., Twentyman P. R. Use of a tritiated thymidine suicide technique in the study of the cytotoxic drug response of cells located at different depths within multicellular spheroids. Br J Cancer. 1987 Apr;55(4):367–374. doi: 10.1038/bjc.1987.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macapinlac H. A., Kemeny N., Daghighian F., Finn R., Zhang J., Humm J., Squire O., Larson S. M. Pilot clinical trial of 5-[125I]iodo-2'-deoxyuridine in the treatment of colorectal cancer metastatic to the liver. J Nucl Med. 1996 Apr;37(4 Suppl):25S–29S. [PubMed] [Google Scholar]
- Makrigiorgos G. M., Kassis A. I., Baranowska-Kortylewicz J., McElvany K. D., Welch M. J., Sastry K. S., Adelstein S. J. Radiotoxicity of 5-[123I]iodo-2'-deoxyuridine in V79 cells: a comparison with 5-[125I]iodo-2'-deoxyuridine. Radiat Res. 1989 Jun;118(3):532–544. [PubMed] [Google Scholar]
- Mariani G., Collecchi P., Baldassarri S., Di Luca L., Buralli S., Fontanini G., Baranowska-Kortylewicz J., Adelstein S. J., Kassis A. I. Tumor uptake and mitotic activity pattern of 5-[125I]iodo-2'- deoxyuridine after intravesical infusion in patients with bladder cancer. J Nucl Med. 1996 Apr;37(4 Suppl):16S–19S. [PubMed] [Google Scholar]
- Mariani G., Di Sacco S., Volterrani D., Di Luca L., Buralli S., Di Stefano R., Baranowska-Kortylewicz J., Bonora D., Matteucci F., Ricci S. Tumor targeting by intra-arterial infusion of 5-[123I]iodo-2'-deoxyuridine in patients with liver metastases from colorectal cancer. J Nucl Med. 1996 Apr;37(4 Suppl):22S–25S. [PubMed] [Google Scholar]
- Martin R. F., Haseltine W. A. Range of radiochemical damage to DNA with decay of iodine-125. Science. 1981 Aug 21;213(4510):896–898. doi: 10.1126/science.7256283. [DOI] [PubMed] [Google Scholar]
- Neshasteh-Riz A., Angerson W. J., Reeves J. R., Smith G., Rampling R., Mairs R. J. Incorporation of iododeoxyuridine in multicellular glioma spheroids: implications for DNA-targeted radiotherapy using Auger electron emitters. Br J Cancer. 1997;75(4):493–499. doi: 10.1038/bjc.1997.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue J. A., Bardiès M., Wheldon T. E. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995 Oct;36(10):1902–1909. [PubMed] [Google Scholar]
- Raaphorst G. P., Feeley M. M., Da Silva V. F., Danjoux C. E., Gerig L. H. A comparison of heat and radiation sensitivity of three human glioma cell lines. Int J Radiat Oncol Biol Phys. 1989 Sep;17(3):615–622. doi: 10.1016/0360-3016(89)90114-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Ritter M. A., Fowler J. F., Kinsella T. J. Kinetics of cell labeling and thymidine replacement after continuous infusion of halogenated pyrimidines in vivo. Int J Radiat Oncol Biol Phys. 1994 Apr 30;29(1):105–113. doi: 10.1016/0360-3016(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Schneiderman M. H., Schneiderman G. S. Radioiododeoxyuridine in cancer therapy: an in vitro approach to developing in vivo strategies. J Nucl Med. 1996 Apr;37(4 Suppl):6S–9S. [PubMed] [Google Scholar]
- Schwartz J. L., Mustafi R., Hughes A., DeSombre E. R. DNA and chromosome breaks induced by iodine-123-labeled estrogen in Chinese hamster ovary cells. Radiat Res. 1996 Aug;146(2):151–158. [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- Taghian A., Suit H., Pardo F., Gioioso D., Tomkinson K., DuBois W., Gerweck L. In vitro intrinsic radiation sensitivity of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1992;23(1):55–62. doi: 10.1016/0360-3016(92)90543-q. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan G., Larsen R. H., Zalutsky M. R. 5-[211 At]astato-2'-deoxyuridine, an alpha particle-emitting endoradiotherapeutic agent undergoing DNA incorporation. Cancer Res. 1996 Mar 15;56(6):1204–1209. [PubMed] [Google Scholar]
- Wheldon T. E., O'Donoghue J. A. The radiobiology of targeted radiotherapy. Int J Radiat Biol. 1990 Jul;58(1):1–21. doi: 10.1080/09553009014551401. [DOI] [PubMed] [Google Scholar]


