Abstract
As the overall prognosis for patients with ovarian cancer is poor, the management of this condition should be restricted to expert multi-disciplinary teams in gynaecological oncology. Apparent early stage ovarian cancer requires accurate and complete staging so that potential sites for metastases are not missed. Omitting adequate staging may have significant consequences including a negative impact on survival rates in young patients. The challenge with advanced ovarian cancer is to obtain a detailed appreciation of the extent of disease. This information allows treatment with primary chemotherapy if the cancer is considered to be inoperable and/or the general condition of the patient renders her unfit for appropriate surgery. Available data would suggest that a 5-year survival rate of 50% is only possible for those patients who have had complete cytoreduction of all tumour. Therefore, the best surgical option for patients with advanced ovarian cancer is a ‘complete’ primary surgical procedure that achieves complete clearance of the abdominal cavity rather than ‘optimal’ surgery that leaves tumour nodules up to 1 cm in diameter in situ in the patient.
Keywords: Ovarian cancer, cytoreductive surgery
Introduction
In the UK, there are over 6600 new cases of ovarian cancer per year resulting in more than 4000 deaths (http://www.cancerresearchuk.org). The condition presents at an early stage in approximately 25% of patients. Unfortunately ovarian cancers are usually discovered at an advanced stage (FIGO stages III and IV) resulting in the poor overall prognosis of this condition. The 5-year survival rate of advanced ovarian cancer is 20–30% in advanced stage disease and the treatment involves surgery and platinum-based chemotherapy. Medical treatment alone rarely results in cure and optimising surgery continues to be the best way to improve survival.
Surgery for early stage ovarian cancer
There is no doubt that complete surgical staging is the standard of care for apparent early stage ovarian cancer. However, evidence from the literature shows that surgery is inadequate in 75% of cases when the procedure is performed by inexperienced surgeons. These data report a 50–100% difference in 5-year overall survival. In fact, 30% of early stage ovarian cancers are upstaged by a formal restaging procedure1–4. Staging is mandatory as it may alter both prognosis and treatment. It has been shown that the prognosis for accurately staged disease confined to the ovaries is excellent even without chemotherapy.
Although adequate surgical staging can obviate the requirement for chemotherapy, chemotherapy does not make up for the effects of inadequate surgery. The EORTC-ACTION trial has demonstrated that patients with adequate staging procedures had a better prognosis than those without5.
What is an appropriate staging procedure?
Exploration of the abdominal cavity is critical and must be systematic. The right and left paracolic gutters, and the small and large bowel including the appendix, the diaphragm anterior and posterior to the liver must be examined. This usually requires mobilization of the liver. Experience shows that peritoneal carcinosis can spread directly to the peri-hepatic areas, skipping the pelvis. This is a cardinal reason for underestimating the extent of disease. The gallbladder, porta hepatis, lesser sac, spleen, anterior and posterior aspects of the stomach and the pancreas are systematically visualised and/or palpated.
Then, the pelvis is assessed: the operative notes must include a description of both ovaries and Fallopian tubes documenting diameter, vegetations, adherence to other structures and also the findings on careful examination of the pouch of Douglas and the recto-sigmoid colon.
In early stage disease, an average of five random peritoneal biopsies should be performed as 7% of diaphragmatic peritoneal biopsies, 5% of omentectomy specimens and also 10% of biopsies taken from all other peritoneal areas are positive. Twenty percent of restaging procedures have positive peritoneal washings6.
Total abdominal hysterectomy and bilateral salpingoophorectomy are standard. Total omentectomy is also important as the supracolic omentum is the site of subclinical metastases in 10–30% of cases5. However, infra-colic omentectomy remains an option for the management of early stage disease but is only acceptable if a thorough assessment of the supracolic area between the stomach and transverse colon is performed. Appendicectomy is also advisable. Metastases to the appendix are seen in 23% of cases, usually with grade 2/3 tumours and those of mucinous type7.
In apparent early stage disease, nodal metastases are of great relevance as this affects both prognosis and treatment. An apparent early stage cancer is upstaged to FIGO stage IIlc if nodal disease is confirmed. Occult involvement of retroperitoneal lymph nodes may occur in 5–25% of cases where disease is initially considered to be limited to the ovaries. Nodal metastases are more common with serous (28%) and clear cell histology (14.5%) and less so with mucinous (2.6%) and endometrioid cancers (4.1%)8. It is known that in 50% of cases, nodes are affected in both the pelvic and aorto-lumbar regions. In 25% of cases the nodes affected are in the aorto-lumbar area only and in a further 25% the metastatic nodes are confined to the pelvis9. However, in apparent early stage ovarian cancer the aortic area seems to be most commonly involved9. These figures indicate that lymphadenectomy must be complete and must include both pelvic and para-aortic areas. Thus, the finding of an invasive epithelial tumour apparently limited to the ovary justifies systematic bilateral pelvic lymphadenectomy and complete para-aortic lymphadenectomy extending to the left renal vein cephalad and to the gonadal vessels laterally. A prospective study of early stage cases reported that the 5-year progression-free survival was 71.3% and 78.3% in the no lymphadenectomy group and the lymphadenectomy group respectively but the difference was not statistically different10.
Surgery for advanced ovarian cancer
The challenge in surgery for advanced ovarian cancer lies with the approach to upper abdominal and retroperitoneal disease. Surgery for advanced ovarian cancer may include bowel resection, cholecystectomy, splenectomy and extensive peritonectomy with diaphragmatic resection, pleural drainage and excision of any enlarged lymph nodes. The classical surgical approach was first described by Hudson where a retroperitoneal en bloc resection of the recto-sigmoid colon with the pelvic tumour is required to achieve complete extirpation of pelvic disease11. This extent of surgery is required in up to 50% of cases in advanced disease12. This type of surgery requires a colo-rectal anastomosis. Clearly peri-operative and post-operative morbidity can be increased significantly when extensive visceral resections are carried out. In this situation, careful intraoperative assessment and decision making are essential. This assessment includes disease-related factors, associated surgical procedures, the blood loss, the patient's age and nutritional status and most importantly the experience of the surgical team. A prolonged period in the intensive care unit and/or a complicated post-op course increases the delay before initiating chemotherapy. However, two major studies have reported that the interval before starting chemotherapy is not a prognostic indicator13,14.
Rationale for maximal surgical effort
Bristow has published a meta-analysis that is based on 81 studies representing more than 6885 patients with advanced ovarian cancer14. This showed that whatever the dose of platinum chemotherapy used, the factor with the greatest impact on survival was maximal surgical cytoreduction. Second and most importantly, it appears that the definition of ‘optimal cytoreduction’ is of major importance. There is no difference in 5-year survival between patients having sub-optimal surgery and ‘optimal’ surgery that leaves residual tumour up to 2 cm in maximal diameter at the end of the procedure. So the term ‘optimal’ should be reserved for surgery resulting in residual tumour of less than 1 cm. Each 10% increase in maximal cytoreduction in Bristow's meta-analysis was associated with a 5.5% increase in median survival. ‘Optimal’ cytoreduction (to less than 1 cm) or ‘complete’ cytoreduction (to no visible disease) can be achieved in 20–90% of cases of advanced epithelial ovarian cancer. The former rate has been reported when the cases are managed by non-specialists and the latter when the cases are in the hands of highly experienced specialist surgeons.
The SCOTROC study was published in 2005 involving 1077 patients: 689 from the UK and 388 from Europe, United States, and Australasia. The study demonstrated that more extensive surgery was performed in non-UK patients, who were more likely to be optimally debulked (≤2 cm residual disease) than UK patients (71.3% v 58.4%, respectively; P < 0.001). Second, optimal debulking was associated with increased progression-free survival (PFS) mainly for patients with less extensive disease at the outset. Third, UK patients with no visible residual disease had a less favourable PFS compared with patients recruited from non-UK centres who were similarly debulked. However, the rate of large bowel resection, pelvic and para-aortic lymphadenectomy was statistically significantly higher in non-UK patients suggesting that a more radical surgical approach may improve survival16. Post-operative residual tumour is one of the most important independent prognostic factors for survival17. Surgery in centres with surgeons who performed comprehensive surgical debulking including retroperitoneal lymphadenectomy and peritoneal stripping was associated with higher rates of complete debulking compared to surgery in other centres (32.8% vs. 22.9%, P = 0.007). This resulted in a markedly improved overall survival (P = 0.045). This effect held true after adjustment for other prognostic factors such as grade and stage.
Tumour volume has no impact on survival if surgery achieves complete removal of all macroscopic disease. This important concept comes from several studies, one of which reports on a series of 408 patients. In this study, survival does not correlate with initial volume of disease but rather with complete cytoreduction rates12. This observation contradicts the concept that with high tumour load the benefits of radical surgery are limited. Treating the upper abdomen increases the rate of optimal surgery significantly from 50 to 76%18. Stripping the diaphragm, cholecystectomy, omentectomy and splenectomy are part of the surgical armamentarium required to achieve a complete result with cytoreduction.
Nearly 50% of nodes are involved in advanced ovarian cancer. Nodal disease does not respond very well to chemotherapy. Lymphadenectomy is justified to remove bulky nodes or in cases where complete macroscopic debulking has been achieved. Systematic lymphadenectomy improves progression-free but not overall survival in women with optimally debulked advanced ovarian carcinoma19. However, it may be seen that survival of women with advanced epithelial ovarian cancer correlates with the extent of lymphadenectomy20.
Neo-adjuvant chemotherapy followed by interval debulking surgery
This strategy results from the EORTC trial published in 1995 by Vanderburg et al.21. The study showed that patients who had sub-optimal primary surgery benefited from secondary cytoreduction if that second surgical effort was optimal. This trial was published around the same time as that of the results of the GOG trial presented by Maguire et al.22, looking at taxanes as a new approach to extending survival. Both trials lent support to the concept that chemotherapy up front would be preferable to standard management rendering surgery technically simpler and less morbid. It is true that this approach allows patients with very chemo-sensitive ovarian tumours to avoid certain procedures at the time of delayed primary surgery that would have been required at up front surgery. The problem is that the impact of this approach on survival is not known. Neoadjuvant chemotherapy represents a viable alternative management strategy for the limited number of patients felt to be optimally unresectable by an experienced ovarian cancer surgical team. A meta-analysis showed that each cycle of chemotherapy delivered before surgery decreased the median survival by 4.1 months23. Since we do not know the results of the ongoing EORTC trial (of surgery up front versus neo-adjuvant chemotherapy followed by delayed primary surgery), or the CHORUS Trial, primary surgery is still the standard of care and the outcome of surgery continues to be most important prognostic factor for advanced ovarian cancer. Thus, one should keep in mind that the impact of surgery may improve the 5-year survival rate of less than 20% to 50%24.
Surgical guidelines for advanced ovarian cancer
Preoperative imaging
Imaging is currently the best method for defining the extent of intraperitoneal disease pre-operatively. It is also useful in identifying extra-abdominal metastases as such a finding generally indicates that a neo-adjuvant approach to management is required. Imaging criteria of non-resectability were first defined by Nelson. Indicators of non-resectability included splenic involvement, mesenteric nodules greater than 2 cm in size, infiltration of the capsule or the hilum of the liver, infiltration of the diaphragm, supra-renal lymphadenopathy and pleural effusion25. From our experience, however, a deposit in the spleen or liver may well be resectable as is supra-renal lymphadeneopathy. Furthermore, imaging does not define the nature of peritoneal carcinomatosis and this is important as the appearance and nature of such carcinomatosis is very variable. At times it is superficial and on other occasions the deposits are markedly infiltrative. From our experience, it would appear that the bigger the deposits, the easier they are to remove. On the other hand, achieving complete cytoreduction of diffuse small volume disease is extremely difficult. Therefore, as recently published, pre-operative computed tomography (CT) predictors should be used with caution in choosing between surgical cytoreduction and neoadjuvant chemotherapy26.
Operative findings
Surgery currently provides the best method to explore the abdominal cavity and assess resectability. This can be done by laparoscopy or laparotomy. The benefit of laparoscopy is simply that its use avoids unnecessary laparotomy in those cases suitable for neo-adjuvant chemotherapy. The reliability of laparoscopy is a function of the experience of the surgeon and the extent of the laparoscopic assessment. It is preferable to insert the laparoscopic trochars along the midline (and therefore not through muscle) to decrease the risk of port site metastases27. Laparoscopy is reported to be accurate in assessing the resectability of peritoneal carinomatosis to achieve a complete or optimal result27,28.
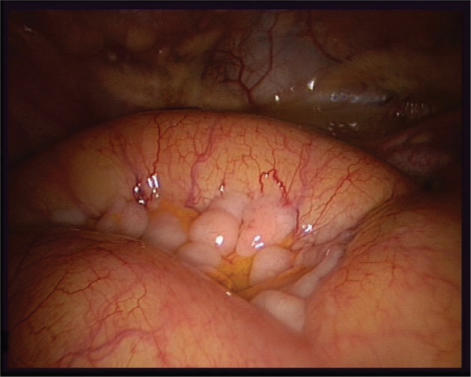
To define the distribution and extent of disease, it is recommended that a ‘map’ is used to document the pattern of carcinomatosis. This map is like a detailed photograph that allows an assessment of the surgical effort required if proceeding with primary surgery. Alternatively, it provides a baseline for determining the effect of neo-adjuvant chemotherapy at the time of the second surgical assessment. The peritoneal cancer index (PCI) is widely used29. Photographic or video evidence may be used to facilitate this (Fig. 1).
Figure 1.
Laparoscopic picture showing a diffuse carcinosis infiltrating the mesentery but respecting most of the serosa of the small bowel.
Surgical exploration must allow the surgeon to decide whether complete cytoreduction is possible or not. Pelvic disease, left hypochondrial disease and enlarged lymph nodes around the iliac or para-aortic regions are considered to be resectable. In our experience there are three independent factors that reduce the likelihood of achieving complete cytoreduction[30]:
small bowel involvement: it is important to distinguish metastases affecting the small bowel per se from metastases affecting the peritoneum of the small bowel mesentery as removing a superficial deposit from the mesentery is in general less of a problem. However, the critical issue is to identify involvement of the mesenteric peritoneum that encases the vessels as this is not resectable unless extensive bowel resection is performed. Multiple small bowel resections (>2) are not advised as this greatly increases morbidity. Permanent ileostomy is to be avoided from a quality of life perspective.
infiltration of the porta hepatis: gross infiltration in general precludes complete resection. However, dissection of the peritoneum starting on the anterior aspect of the hepatic pedicle may occasionally render this disease resectable.
right hemi diaphragm: diffuse infiltrative involvement of the right hemi diaphragm at the level of the suprahepatic vessels causing fixity of the liver is in general non-resectable.
Superficial disease (whatever the volume) including bulky disease can be resected. After dividing the falciform ligament, this is done by starting the dissection on the inferior vena cava and the right adrenal gland and continuing in that plane until reaching the right supra hepatic vein. This releases the right aspect of the liver and may leave part of Glisson's capsule in contact with the diaphragm. If necessary, up to half of the surface of the diaphragm can be resected with simple closure.
Operative procedures and strategy
Once histological proof of malignancy is available from frozen section, one must answer the following questions: is the disease technically resectable in its entirety and, if so, what is the extent of surgery required? One can define three types of surgery/procedure30:
Standard surgery comprises, as a minimum, hysterectomy, bilateral adnexectomy with excision of the pelvic peritoneum, total omentectomy including the supracolic omentum, appendicectomy, removal of bulky pelvic and lumbo-aortic nodes +/− simple peritonectomies.
Radical surgery comprising in addition to the above mentioned elements, en bloc removal of the uterus, both ovaries, the pelvic peritoneum and recto-sigmoid +/− simple peritonectomies.
- Supra-radical surgery, that is, a radical procedure plus at least one of the following:
- extensive peritonectomies including partial resection of the diaphragm
- resection of subcapsular liver metastases, cholecystectomy
- splenectomy, resection of the tail of the pancreas
- other bowel resection, partial gastrectomy, etc.
Type 2 and 3 procedures are mandatory in 50% of advanced ovarian cancer cases13,15. If inspection reveals that a type 3 procedure is unlikely to achieve complete cytoreduction, it is reasonable to recommend three cycles of chemotherapy before delayed primary surgery. When the surgical option is chosen, it is advisable to start the operation in the region considered to be the most challenging from a surgical perspective. A tumour initially considered to be resectable may prove to be inoperable during the procedure. In such a situation it is best not to proceed further but rather to reconsider chemotherapy as primary treatment.
Conclusion
Surgery has a major impact on survival in the management of malignant epithelial tumours of the ovary. Surgical staging can modify prognosis and treatment for early stage disease. For advanced stages of ovarian cancer, the surgical objective is maximal cytoreduction. The role of imaging is helpful to determine the extent of the disease and to plan the length and the type of surgery that may be required. In our opinion, cross sectional imaging may not clearly define resectability of intra-abdominal/retroperitoneal disease. It does not clearly preclude resectability or non-resectability in the abdomen. Ultimately surgical evaluation, either laparoscopically or at open operation, may be required to assess operability. The criteria for non-resectability are independent of the speciality of the surgeon. When initial surgical exploration considers peritoneal carcinomatosis to be unresectable, a neo-adjuvant chemotherapy/delayed primary surgery strategy should be considered. However, this strategy cannot be considered standard treatment for all cases as it may reduce overall survival. The potential morbidity inherent in extensive procedures must be considered and weighed against the option of debulking after three cycles of chemotherapy. The management of all cases of malignant epithelial tumours of the ovary requires the support of a multidisciplinary team with careful case selection.
References
- 1.Young RC, Walton LA, Elleberg SS, et al. Adjuvant therapy in stage 1 and stage Il epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med. 1990;322:1021–7. doi: 10.1056/NEJM199004123221501. [DOI] [PubMed] [Google Scholar]
- 2.Berek JS. Practical gynecologic oncology. Baltimore, MD: Williams and Wilkins; 1988. Epithelial ovarian cancer; pp. 327–64. [Google Scholar]
- 3.Averette HE, Hoskins W, Nguyen HN, et al. National survey of ovarian carcinoma. A patient care evaluation study of the American College of Surgeons. Cancer. 1993;71(suppl 4):1629–38. [PubMed] [Google Scholar]
- 4.Mayer AR, Chambers SK, Graves E, et al. Ovarian cancer staging: does it require a gynecologic oncologist ? Gynecol Oncol. 1992;47:223–7. doi: 10.1016/0090-8258(92)90110-5. [DOI] [PubMed] [Google Scholar]
- 5.Trimbos JB, Vergote I, Bolis G, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst. 2003;95:113–25. [PubMed] [Google Scholar]
- 6.Leblanc E, Querleu D, Narducci F, et al. Surgical staging of early invasive epithelial ovarian tumors. Semin Surg Oncol. 2000;19:36–41. doi: 10.1002/1098-2388(200007/08)19:1<36::aid-ssu6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Fontanelli R, Paladini D, Raspagliesi F, Di Re E. The role of appendicectomy in surgical procedure for ovarian cancer. Gynecol Oncol. 1992;46:42–4. doi: 10.1016/0090-8258(92)90193-m. [DOI] [PubMed] [Google Scholar]
- 8.Di Re F, Baiocchi G. Value of lymph node assessment in ovarian cancer: status of the art at the end of the second millennium. Int J Gynecol Cancer. 2000;10:435–42. doi: 10.1046/j.1525-1438.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 9.Morice P, Joulie F, Camatte S, et al. Lymph node involvement in epithelial ovarian cancer: analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J Am Coll Surg. 2003;197:198–205. doi: 10.1016/S1072-7515(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 10.Maggioni A, Benedetti Panici P, Dell'Anna T, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. 2006;95:699–704. doi: 10.1038/sj.bjc.6603323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson CN. Surgical treatment of ovarian cancer. Gynecol Oncol. 1973;1:370–8. [Google Scholar]
- 12.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–6. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 13.Aletti GD, Long HJ, Podratz KC, Cliby WA. Is time to chemotherapy a determinant of prognosis in advanced-stage ovarian cancer? Gynecol Oncol. 2007;104:212–6. doi: 10.1016/j.ygyno.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Gadducci A, Sartori E, Landoni F, et al. Relationship between time interval from primary surgery to the start of taxane- plus platinum-based chemotherapy and clinical outcome of patients with advanced epithelial ovarian cancer: results of a multicenter retrospective Italian study. J Clin Oncol. 2005;23:751–8. doi: 10.1200/JCO.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 15.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 16.Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005;23:8802–11. doi: 10.1200/JCO.2005.02.1287. [DOI] [PubMed] [Google Scholar]
- 17.Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A for the AGO-OVAR. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR) Gynecol Oncol. 2007;106:69–74. doi: 10.1016/j.ygyno.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Chi DS, Franklin CC, Levine DA, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94:650–4. doi: 10.1016/j.ygyno.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti Panici P, Maggioni A, Hacker N, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97:560–6. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]
- 20.Chan JK, Urban R, Hu JM, et al. The potential therapeutic role of lymph node resection in epithelial ovarian cancer: a study of 13 918 patients. Br J Cancer. 2007;96:1817–22. doi: 10.1038/sj.bjc.6603803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Burg MEL, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. N Engl J Med. 1995;332:629–34. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 22.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 23.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2007;106:274–5. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Bristow RE, Berek JS. Surgery for ovarian cancer: how to improve survival. Lancet. 2006;367:1558–60. doi: 10.1016/S0140-6736(06)68671-6. [DOI] [PubMed] [Google Scholar]
- 25.Nelson BE, Rosenfield AT, Schwartz PE. Preoperative abdominopelvic computed tomographic prediction of optimal cytoreduction in epithelial ovarian carcinoma. J Clin Oncol. 1993;11:166–72. doi: 10.1200/JCO.1993.11.1.166. [DOI] [PubMed] [Google Scholar]
- 26.Axtell AE, Lee MH, Bristow RE, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol. 2007;25:384–9. doi: 10.1200/JCO.2006.07.7800. [DOI] [PubMed] [Google Scholar]
- 27.Deffieux X, Castaigne D, Pomel C. Role of laparoscopy to evaluate candidates for complete cytoreduction in advanced stages of epithelial ovarian cancer. Int J Gynecol Cancer. 2006;16(Suppl 1):35–40. doi: 10.1111/j.1525-1438.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- 28.Fagotti A, Fanfani F, Ludovisi M, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol. 2005;96:729–35. doi: 10.1016/j.ygyno.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Harmon RL, Sugarbaker P. Prognostoc indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005;2:3. doi: 10.1186/1477-7800-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pomel C, Dauplat J. Management of malignant epithelial tumors of the ovary. J Chir (Paris) 2004;141:277–84. doi: 10.1016/s0021-7697(04)95334-3. [DOI] [PubMed] [Google Scholar]