Abstract
Primary benign and malignant neoplasm of the small bowel are rare. Malignant tumours often present late symptoms resulting in a poor prognosis. Early detection of small bowel neoplasms is desirable but challenging for both clinicians and radiologists. Conventional double contrast enteroclysis was the method of choice in small bowel imaging but is increasingly being replaced by cross-sectional imaging methods as computed tomography (CT) and magnetic resonance imaging (MRI). Multidetector CT (MDCT) produces high-resolution cross-sectional imaging of the abdomen and the small bowel. It allows multiplanar visualisation of small bowel tumours, demonstrates signs of small bowel obstruction as well as the mural and extramural extent of small bowel malignancies. This aids planning for surgical resection. In addition, liver metastases or peritoneal seeding can be detected with CT. The best visualisation of small bowel neoplasms is achieved with CT enteroclysis or enterography and this review discusses these techniques and MDCT characteristics of small bowel tumours.
Keywords: Computed tomography, small bowel tumour
Introduction
Primary neoplasms of the small bowel are quite uncommon, representing only about 3% of all neoplasms of the digestive tract, although the small bowel accounts for more than 90% of the intestinal mucosal surface1. The clinical symptoms are non-specific. Most small bowel tumours are not diagnosed until complications, such as bleeding, bowel obstruction, or perforation, occur. Other clinical symptoms are lassitude, weight loss, and abdominal pain and discomfort. Neuroendocrine tumours present with symptoms related to their hormonal activity. Carcinoid syndrome is a typical example, and can be characterised by clinically apparent signs that appear late in the disease, when the tumour has already spread to the liver2. This small index of clinical suspicion makes the detection of small bowel tumours a challenge for all clinicians. Most malignant small bowel tumours have already metastasised when diagnosed, which is responsible for the poor prognosis3.
Radiologists assume a major role in the detection of small bowel tumours. Endoscopy provides the advantage of obtaining biopsies, but only the terminal ileum, the duodenum, and the proximal ileum can be sufficiently explored in routine procedures. Ileoscopy is further compromised by the occasional inability to reach the caecum or to intubate the ileum during colonoscopy. Enteroscopy plays a significant role in the diagnosis of small bowel tumours, but may cover only a limited length of the small intestine and is not commonly available. The push enteroscopy is a promising endoscopic alternative; however, the procedure is time-consuming and complicated and rarely available4,5. Video capsule endoscopy provides an excellent alternative for the imaging and detection of small bowel disease. To date, definitive results are controversial and video capsule endoscopy is limited by complications, such as the capsule being lost, getting stuck, or by a low battery supply6. Another limitation of all endoscopic methods is the lack of information about the extramural extent of small bowel tumours.
For many years, ‘conventional’ double contrast enteroclysis has been suggested as the technique of choice for the evaluation of the small intestine7. Adequate distension of the small bowel allows imaging of mucosal abnormalities and provides functional information by defining free peristaltic contraction or fixation of the small bowel loops. The principal disadvantage of conventional enteroclysis is the limited information about the state of the bowel wall and extramural extension of tumour disease. Thus, it has largely been replaced by computed tomography (CT).
CT has the advantage of visualising the entire gastrointestinal tract as well as the extramural structures and abdominal organs. The introduction of multidetector-row scanners (MDCT) and advanced three-dimensional imaging capabilities have renewed interest in using CT to detect and stage small bowel tumours8. The best visualisation of small lesions is achieved with CT enteroclysis. In this method, a negative enteral contrast medium (methylcellulose solution) is administered through a nasojejunal tube, providing small bowel distension and optimal imaging of the small bowel wall9.
CT imaging
Optimal imaging is achieved with the use of multidetector CT (MDCT). The currently available devices, with 16–64 detector rows, allow the acquisition of data sets with near isotropic voxels, which leads to high-resolution imaging and is used for further multiplanar reconstructions (MPR). CT is often the first modality ordered in a patient with suspected obstruction or unclear abdominal symptoms. In cases where a suspected endocrine tumour, such as a carcinoid, is active, the scan protocol should be extended to detect possible hypervascular liver metastases. This provides tumour detection and staging in a one-stop-procedure.
Dedicated small bowel CT is typically requested in patients with known small bowel malignancies or if conventional imaging or endoscopy failed to demonstrate any abnormalities in patients with ongoing clinical symptoms10. Good results in tumour detection are achieved with CT enteroclysis11,12. CT enteroclysis is performed in a standardised fashion. After nasojejunal tube placement under fluoroscopic guidance, 1500 ml of 0.5% methylcellulose solution (MCS) are administered via a roller pump at an injection rate of 100–120 ml/min to provide adequate and uniform small bowel distension. Immediately after the administration of the designated or tolerated volume of methylcellulose, patients are transferred from the fluoroscopy suite to the CT unit, where 20 mg of hyoscine-N-butylbromide (Buscopan®, Boehringer Ingelheim, Germany) are injected i.v. for minimisation of potential artefacts by peristaltic bowel movement. While lying on the CT table, an additional 500 ml of MCS are administered at a flow rate of 80 ml/min to maintain optimal distension of the upper small jejunal loops during CT examination. CT enterography provides an alternative to CT enteroclysis, when tube placement is not available or has failed. Small bowel distension is achieved after oral application of low-density fluid, mixed with different types of water-binding substances to impair resorption13,14. CT enterography requires high patient compliance, because a total of 1800–2000 ml of fluid has to be consumed before the CT investigation.
In general, negative oral contrast agents are now given for CT enteroclysis (i.e. methylcellulose) or for CT enterography (i.e. water, methylcellulose, or PEG solution), because they provide better contrast between the low-density lumen and contrast-enhanced tumours of the small bowel. Oral contrast material of high density such as water soluble iodine solution has been suggested as a contrast material to visualise small bowel obstruction15. However, this approach may impair visualisation of the structure of the small bowel wall.
Suggested CT parameters for a 64-row scanner are as follows: 120 kV, 200 mAs and automatic dose modulation, detector configuration 64 × 0.6 (depending on the device), reconstructed slice thickness/interval of 3/2 mm and 1/0.7 mm for coronal MPR. 120–140 ml of non-ionic contrast material (at 300 mg/ml iodine) can be injected (at 4–5 ml/s) for a single phase scan or a biphasic scan in the arterial and venous phase. For CT enteroclysis and enterography usually a shorter delay of approx. 30–40 s would result in excellent mucosal enhancement. If a tumour is suspected and liver staging is sought, then a biphasic scan in the arterial and venous phase may be advantageous.
Benign tumours
Benign neoplasms account for approximately 1–2% of all GI tract tumours, with leiomyomas and adenomas the most common types of tumours. Other types of benign tumours, such as lipomas and hamartomas, are less frequently encountered15. Clinical presentation is non-specific, and 50% of patients remain asymptomatic.
Leiomyoma
Leiomyomas originate from the muscle coat of the small intestine and present mostly as solitary lesions. They are the most common benign tumour of the small bowel (with incidences ranging between 22 and 43%) and appear distributed throughout the jejunum and the ileum, with a slight predilection for the jejunum16. They may be located submucosally, intramurally, or subserosally and appear in CT as a sharply defined spherical mass from 1 cm to 10 cm diameter with homogenous tissue and show uniform contrast enhancement. Larger lesions may display dense focal calcifications. When located submucosally, they may present with small, focal, mucosal pressure ulcers. The typical findings and the absence of mesenteric changes and metastases helps in the diagnosis and mostly rules out malignant differential diagnoses.
Adenoma
Adenomas account for 14–20% of benign small bowel tumours17. They are proliferative epithelial neoplasms and are further classified like colon adenomas. They appear mainly as tubular adenomatous polyps, although villous adenomas are more common in the small bowel than in the colon. Adenomatous polyps demonstrate as intraluminal masses of small size (less than 2 cm). When multiple, they usually affect a single intestinal segment. In the case of familiar polyposis, the polyps are distributed throughout the entire small intestine. Villous adenomas are slightly larger, with an average diameter of over 3 cm, often broad-based. Adenomas are usually asymptomatic. If pedunculated, they may intussuscept and cause small bowel obstruction.
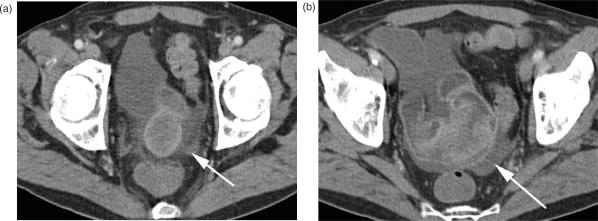
At CT, adenomas appear as a well-defined soft tissue mass showing moderate enhancement after intravenous contrast application, with clear fat planes around the tumour (Fig. 1). Inflammatory fibroid polyps, predominantly located in the ileum, are an essential consideration in the differential diagnosis.
Figure 1.
Duodenal adenoma. (a) Axial MDCT of a polypoid intraluminal mass (arrow) in the duodenum with distinct contrast enhancement. A fat rim allows good delineation of the bowel wall, there are no signs of bowel wall infiltration. (b) Coronal and (c) sagittal reformation.
Lipoma
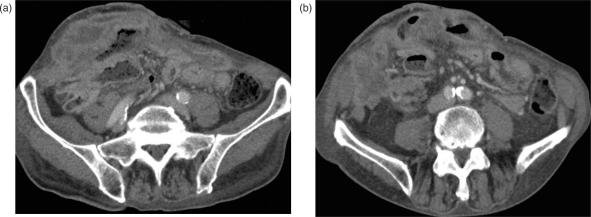
Lipomas represent the third most common benign lesion of the small intestine (8–20%). They are typically located in the duodenum or distal small bowel and commonly present with intussusceptions or bleeding. They mostly grow in a solitary fashion, and to a large size, then undergo necrosis, cystic degeneration, and calcification18. On CT, lipomas appear as sharply defined, ovoid masses with fat equivalent attenuation values of −40 to −100 HU. In the case of intussusception of a lipoma, CT can identify the lead point as a mass of fat tissue density (Fig. 2).
Figure 2.
Lipoma of the small intestine. (a) Axial and (b) coronal MDCT images of an intraluminal mass (arrow) in the terminal ileum with fat-equivalent attenuation values.
Haemangioma
Intestinal haemangiomas are mostly located in the jejunum and usually present with gastrointestinal bleeding. On CT, detection is difficult, unless the haemangioma is a large, well-defined, contrast-enhancing mass within the small bowel wall. Thin-section MPRs can sometimes enable the depiction of a feeding vessel19.
Polyposis syndromes
Polyposis syndromes include the Peutz–Jeghers syndrome, Cowden's disease, juvenile polyposis, Cronkhite–Canada syndrome, Gardner's syndrome, and familial adenomatous polyposis (FAP)20. The small polyps can be seen as several soft-tissue masses spread throughout the entire small bowel. Smaller lesions are easily detected on CT enteroclysis. In FAP and Gardner's syndrome, the polyps may grow in the small intestine after resection of the colon.
Malignant tumours
Up to 70% of all symptomatic small bowel tumours are found to be malignant. Risk factors for the development of malignant small bowel tumours include all forms of inflammatory bowel disease (Crohn's and celiac disease), FAP, neurofibromatosis and AIDS. At the time of diagnosis, more than 50% of the malignancies are not amenable to surgical resection, resulting in a very poor prognosis for neoplasms of the GI tract.
Adenocarcinoma
Adenocarcinoma is documented to be the most common malignant neoplasm of the small intestine, although it represents only 1% of all primary GI malignancies21. The duodenum is the most frequently involved site, with the proximal jejunum second, primarily in the first 25–30 cm beyond the ligament of Treitz. Adenocarcinoma as a complication of Crohn's disease arises mostly in the terminal ileum22.
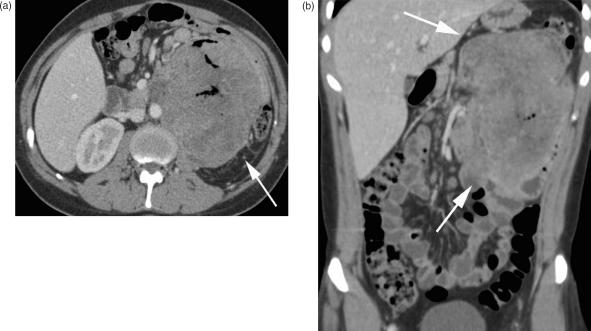
On CT, adenocarcinoma may have a variable appearance (Fig. 3). It typically appears as a focal wall thickening involving a short segment that causes luminal stenosis and small bowel obstruction (Fig. 4). Mucosal ulceration is typical and can be depicted on CT enteroclysis. CT typically demonstrates heterogeneous attenuation and moderate contrast enhancement. Adenocarcinoma tends to infiltrate the entire bowel wall and to extend into the surrounding mesenteric fat tissue, inciting a local desmoplastic reaction. CT is further valuable for imaging tumour extension, involvement of the adjacent viscera, and to stage distant metastases, focal, or distant lymph node involvement and peritoneal carcinosis. Typically, liver metastases of small bowel adenocarcinomas are seen as low attenuation focal lesions, visualised during the portal venous phase of enhancement23. Particular findings, such as a solitary lesion, high-grade small bowel obstruction, the involvement of only a short segment, and the rather proximal location, are imaging characteristics that help to differentiate small bowel adenocarcinoma from other small bowel malignancies.
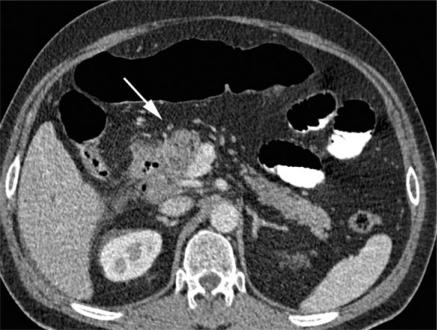
Figure 3.
Duodenal adenocarcinoma. MDCT shows a contrast-enhanced mass of the descending part of the duodenum infiltrating the surrounding mesenterial fat and the pancreas head (arrow). It is difficult to differentiate between duodenal cancer invading the pancreatic head and (the much more common situation of) pancreatic cancer invading the duodenum.
Figure 4.
Duodenal adenocarcinoma (horizontal part). (a) Axial and (b) coronal CT images with contrast-enhanced circumferential wall thickening of the horizontal part of the duodenum (arrow) causing stenosis with prestenotic dilatation, typical for adenocarcinoma.
Neuroendocrine tumours (NET)
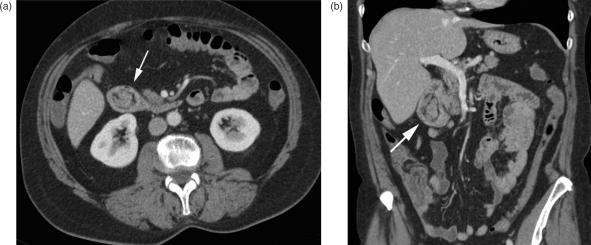
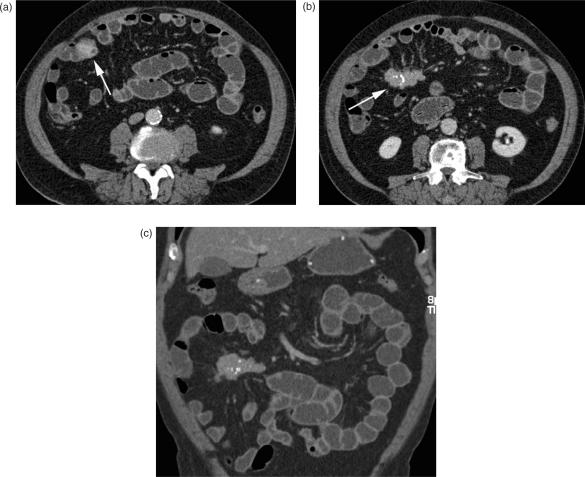
Neuroendocrine tumours (NET), formerly known as carcinoids, originate from the enterochromaffine cells at the base of the Lieberkuhn crypts within the bowel wall. After adenocarcinoma, these tumours are the next most frequently found common small bowel malignancies, representing up to 25% of all primary small bowel tumours. NETs are mainly located in the appendix (50%), but 33% can be found in the small bowel with a predilection for the ileum24. They are slow-growing neoplasms, first appearing as submucosal nodules best detected on CT enteroclysis (Fig. 5a). In later stages, a carcinoid resembles a mural contrast-enhancing mass extending into the adjacent mesentery, causing a soft-tissue density mass, often with spiculated margins due to the desmoplastic reaction. Calcifications are seen in up to 70% (Fig. 5b,c). The desmoplastic reaction is described as surrounding and encasing mesenteric vessels, and, in the worst case, leading to ischaemia of the affected small bowel loop. Early clinical symptoms are rare. Primary tumours are often less than 2 cm in size at diagnosis and may remain asymptomatic until the development of liver metastases, which are responsible for the hormonal activity that causes the carcinoid syndrome. Cutaneous flushing, intermittent hypertension, diarrhoea, and palpitations are the typical symptoms, sometimes induced by alcohol consumption25. Small bowel obstruction secondary to the desmoplastic reaction is a recognized complication, and mesenteric lymph node enlargement and peritoneal seeding are signs of an advanced tumour stage. The desmoplastic reaction typically occurs with lymph node disease in the mesentery. Liver metastases are typically hypervascular and best demonstrated on arterial phase imaging, appearing as bright, contrast-enhancing masses. A mesenteric soft tissue mass with calcifications, a desmoplastic reaction, and avid contrast enhancement, accompanied by the presence of hypervascular liver metastases, are the characteristic findings of neuroendocrine tumours and should prompt the search for the primary lesion in the bowel.
Figure 5.
Neuroendocrine tumour (NET). (a) Axial MDCT image with contrast-enhanced focal nodular wall thickening of the ileum (arrow); (b) the desmoplastic reaction within the adjacent mesenterial fat with typical calcifications below (arrow); (c) coronal reformation.
Lymphoma
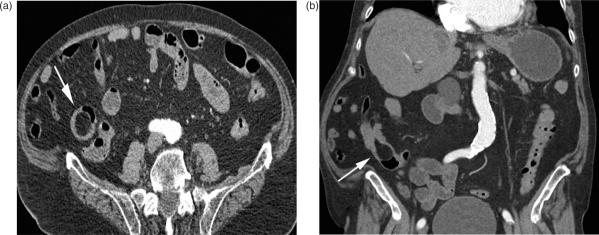
Lymphoma affects the small intestine either as a primary neoplasm that arises focally from the mucosa-associated lymphoid tissue (MALT) or as a part of systemic lymphatic disease. It is described to be the third most common primary malignant tumour of the small intestine, representing 15–20% of all small bowel malignancies. Lymphoma of the small bowel may also occur as a complication of coeliac disease26.
Clinical symptoms are mostly non-specific, and affected patients complain about weight loss, fever, diarrhoea, and non-specific abdominal pain. Lymphoma is typically located in the ileum. On CT, it appears with several growth patterns: multiple contrast-enhancing mucosal nodules that affect the small bowel multifocally or along a segment of several centimetres (Fig. 6); or, a single mass lesion that varies in size, with segmental, contrast-enhancing wall thickening and destruction of the mucosal folds, as well as infiltration into surrounding mesenteric fat. Lymphatic tumours are mostly soft, and are unlikely to cause small bowel obstruction, although luminal narrowing and intussusception can also occur27. The finding of a rather longer segmental small bowel wall thickening or a mass without severe obstruction located in the more distal part of the small bowel marks the difference between adenocarcinoma and other malignancies.
Figure 6.
Small bowel lymphoma. (a) Segmental wall thickening of an ileal loop (arrow). Typically there are no signs of luminal narrowing. (b) The same lesion on coronal reformation.
Gastrointestinal stroma tumour (GIST)
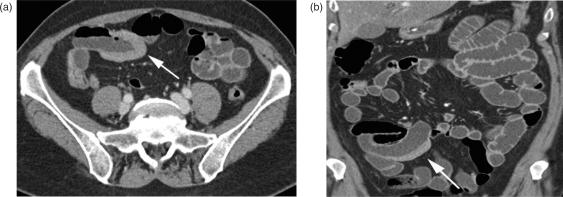
GIST is a common mesenchymal tumour of the gastrointestinal tract, primarily located in the stomach. Some authors consider GIST a benign neoplasm with the potential for malignant transformation, but most authors definitely classify GIST as a malignant tumour of the GI tract. It arises from the smooth mussel cells within the small bowel wall and, if not located in the stomach, mostly occurs in the jejunum28. Pathologically, GIST can be classified as benign, borderline, low, or highly malignant. Unless obvious metastases are present, there are no reliable CT signs in imaging to distinguish malignant from benign GIST. Therefore, surgical resection of a suspected GIST lesion is recommended. On CT, GIST typically appears as an exophytic, sometimes bulky mass, with moderate contrast enhancement, and tends to show central necrosis and calcification. Tumours can extend to a size of several centimetres, displacing the adjacent bowel loops, making the origin of the tumour difficult to appreciate (Figs. 7, 8). In the case of malignancy, GIST typically causes liver metastases and peritoneal seeding. Liver metastases mostly appear as hypoattenuating lesions.
Figure 7.
Gastrointestinal stroma tumour (GIST). (a) A contrast-enhanced ileal mass in the lower abdomen, infiltrating into the mesenteric fat (arrow). Adjacent to the mass, there are small extraluminal air bubbles as a sign of perforation; mesenteric stranding indicates local peritonitis. (b) Coronal reformation and small mesenteric lymph nodes.
Figure 8.
Low grade GIST of the jejunum. (a) Axial and (b) coronal MDCT images of another patient with a large rim-enhanced mass in the jejunum histologically verified as low grade GIST.
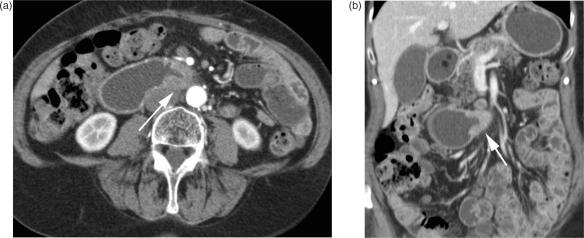
Leiomyosarcoma
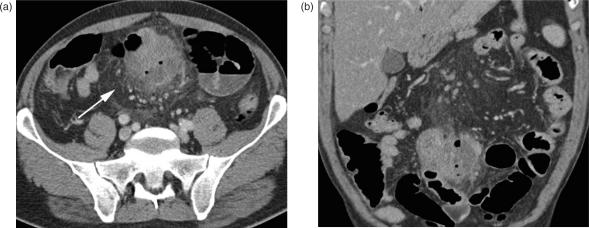
This malignant neoplasm arises from the intestinal smooth muscle of the bowel wall or the small intestinal blood vessels and accounts for less than 15% of primary intestinal malignancies. As a rule, leiomyosarcomas present as a single lesion and are located in the jejunum and ileum, but most commonly in a Meckel's diverticulum. Tumours grow slowly and predominantly extraluminally and the onset of clinical symptoms (i.e., bowel obstruction) is late.
On CT images, leiomyosarcomas appear primarily as large heterogeneous masses, with central necrosis and cystic portions, and show a typical rim enhancement after contrast administration (Fig. 9) Leiomyosarcomas may also be associated with ulceration, cavitation, or fistula29. CT also can depict liver and peritoneal metastases, which are typically large and necrotic with rim enhancement.
Figure 9.
Leiomyosarcoma of the small bowel. (a,b) Axial MDCT images of the lower abdomen with segmental contrast-enhanced wall thickening, histologically verified as a leiomyosarcoma. The tumour is mostly located intraluminal; distinct perifocal stranding suggests mesenteric infiltration.
Metastases
Metastases of the small bowel can be caused by intraperitoneal seeding, direct extension from adjacent tumours, or by haematogenous spread30. Colon and ovarian carcinoma are the most frequent site of origin of intraperitoneal metastases that can affect the small bowel wall. Typical CT findings are small contrast-enhancing nodules along the serosal surface. In advanced disease, these nodules encase the small bowel and cause obstruction (Fig. 10). Advanced stages of pancreatic, biliary, or colonic malignancies tend to infiltrate adjacent small bowel loops.
Figure 10.
Peritoneal carcinomatosis. (a,b) Extensive peritoneal seeding in a patient with end-stage ovarian carcinoma. There are multifocal contrast enhancing nodular masses infiltrating the small bowel wall and the ventral abdominal wall.
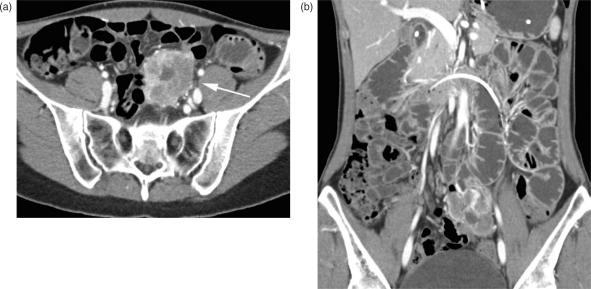
Haematogenous metastases commonly originate from bronchial carcinoma, breast carcinoma, melanoma, and renal cell carcinoma. There are no pathognomonic findings for any metastases, but, typically, they present as short, segmental, contrast-enhancing, wall thickening or as masses, causing transient intussusception or ulceration with bleeding (Fig. 11).
Figure 11.
Small bowel metastasis. (a) Axial and (b) coronal CT images of a large mass in the left lower part of the abdomen. Small central air bubbles are the only sign of an intestinal origin. The mass was verified as a metastasis of a seminoma.
Conclusion
The detection of neoplasms of the small intestine poses a significant diagnostic challenge to both clinician and radiologist. The diagnosis is affected by the low incidence of small bowel tumours and the mostly late onset of clinical symptoms. Due to the limitations of capsule endoscopy to assess the small bowel in patients with suspected obstruction, the high-resolution images provided by 64-row MDCT, in combination with the technique of CT enteroclysis or enterography, have renewed interest in using CT for small bowel tumour detection. The appearance, the typical locations, and the different propensity to seed metastases allow the spectrum of differential diagnoses to be narrowed (see Table 1). Radiologists, therefore, should be familiar with the CT appearance of various intestinal neoplasms.
Table 1.
Differential diagnosis of the most common malignant primary small bowel neoplasms
| Location | Typical signs | |
|---|---|---|
| Adenocarcinoma | Duodenum, proximal jejunum | Short segment affected; high-grade small bowel obstruction |
| Carcinoid | Ileum (appendix) | Desmoplastic reaction mesentery; calcification; hypervascular liver metastases |
| Lymphoma | Ileum | Long segment affected; luminal narrowing without small bowel obstruction; lymphadenopathy |
| Malignant GIST | Jejunum (stomach) | Large mass, exophytic; central necrosis; calcification |
| Leiomyosarcoma | Jejunum, ileum, Meckel's diverticulum | Solitary large mass; cavitation, ulceration, fistula |
References
- 1.Good CA. Tumors of the small intestine. AJR. 1963;89:685–705. [PubMed] [Google Scholar]
- 2.Ashley SW, Wells SA. Tumors of the small intestine. Semin Oncol. 1988;15:116–28. [PubMed] [Google Scholar]
- 3.Wilson JM, Melvin DB, Gray GF, et al. Primary malignancies of the small bowel: a report of 96 cases and review of the literature. Ann Surg. 1974;180:175–79. doi: 10.1097/00000658-197408000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis BS, Kornbluth A. Small bowel tumors: yield of enteroscopy. Gut. 1991;32:763–5. doi: 10.1136/gut.32.7.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May A, Nachbar L, Schneider M, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility, diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/s0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 6.Hara A, Leighton J, Heigh R, et al. Crohn's disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through and ileoscopy. Radiology. 2006;238:128–34. doi: 10.1148/radiol.2381050296. [DOI] [PubMed] [Google Scholar]
- 7.Herlinger H. Barium examinations. In: Gore RM, Levine MS, Laufer I, editors. Textbook of gastrointestinal radiology. Philadelphia, PA: Saunders; 1994. pp. 768–88. [Google Scholar]
- 8.Ramachandran I, Sinha R, Rajesh A, et al. Multidetector row CT of small bowel tumours. Clin Radiol. 2007;62:607–14. doi: 10.1016/j.crad.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Rajesh A, Maglinte DD. Multislice CT enteroclysis: technique and clinical applications. Clin Radiol. 2006;61:31–9. doi: 10.1016/j.crad.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Horton KM, Fishman EK. Multidetector-row computed tomography and 3-dimensional computed tomography imaging of small bowel neoplasms: current concept in diagnosis. J Comput Assist Tomogr. 2004;28:106–16. doi: 10.1097/00004728-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Romano S, De Lutio E, Rollandi GA, et al. Multidetector computed tomography enteroclysis (MDCT-E) with neutral enteral and IV contrast enhancement in tumor detection. Eur Radiol. 2005;15:1178–83. doi: 10.1007/s00330-005-2673-5. [DOI] [PubMed] [Google Scholar]
- 12.Schober E, Turetschek K, Schima W, et al. Boston: American Roentgen Ray Society (ARRS); 1997. May, Methyl cellulose enteroclysis spiral-CT: technique, examination quality and complications – experiences in 140 patients; p. 36. Book of Abstracts. [Google Scholar]
- 13.Reittner P, Goritschnig T, Petrisch W, et al. Multiplanar spiral CT enterography in patients with Crohn's disease using a negative oral contrast material: initial results of a noninvasive imaging approach. Eur Radiol. 2002;12:2253–7. doi: 10.1007/s00330-002-1361-y. [DOI] [PubMed] [Google Scholar]
- 14.Wold PB, Fletscher JG, Johnson CD, Sandborn WJ. Assessment of small bowel Crohn disease: Noninvasive peroral CT enterography compared with other imaging methods and endoscopy – feasibility study. Radiology. 2003;229:275–81. doi: 10.1148/radiol.2291020877. [DOI] [PubMed] [Google Scholar]
- 15.Gourtsoyiannis N, Mako E. Imaging of primary small intestinal tumours by enteroclysis and CT with pathological correlation. Eur Radiol. 1997;7:625–42. doi: 10.1007/BF02742916. [DOI] [PubMed] [Google Scholar]
- 16.Gourtsoyiannis NC, Bays D, Malamas M, et al. Radiological appearance of small intestine leiomyomas. Clin Radiol. 1992;45:94–103. doi: 10.1016/s0009-9260(05)80063-7. [DOI] [PubMed] [Google Scholar]
- 17.Elsayed AM, Sobin LH. Pathology of small intestinal neoplasms. In: Gourtsoyiannis NC, Nolan DJ, editors. Imaging of small intestine neoplasms. Amsterdam: Elsevier; 1997. pp. 249–82. [Google Scholar]
- 18.Taylor AJ, Stewart ET, Dodds WJ. Gastrointestinal lipomas: a radiologic and pathologic review. Am J Roentgenol. 1990;155:1205–10. doi: 10.2214/ajr.155.6.2122666. [DOI] [PubMed] [Google Scholar]
- 19.Lappas JC. Benign tumors. In: Gore RM, Levine MS, Laufer I, editors. Textbook of gastrointestinal radiology, Vol. 1. Philadelphia, PA: WB Saunders; pp. 892–9. [Google Scholar]
- 20.Harnek RK, Buck JL, Sobin LH. The hamartomous polyposis syndromes: clinical and radiologic features. Am J Roentgenol. 1995;165:565–71. doi: 10.2214/ajr.164.3.7863873. [DOI] [PubMed] [Google Scholar]
- 21.Nelson RL. Adenocarcinoma of the small intestine. In: Nelson RL, Nyhus LM, editors. Surgery of the small intestine. Norwalk, CT: Appleton and Lange; pp. 223–30. [Google Scholar]
- 22.Miller TL, Skucas J, Cudex D, et al. Bowel cancer characteristics in patients with regional enteritis. Gastrointest Radiol. 1987;12:45–52. doi: 10.1007/BF01885102. [DOI] [PubMed] [Google Scholar]
- 23.Nerine D, Fishman EK, Jones B. CT of the small bowel and mesentery. Radiol Clin North Am. 1989;27:707–15. [PubMed] [Google Scholar]
- 24.Herlinger H, Maglinte DDT. Clinical imaging of small bowel intestine. New York: Springer-Verlag; 1999. [Google Scholar]
- 25.Jeffree MA, Barter JS, Hemingway AP, Nolan DJ. Primary carcinoid tumors of the ileum: the radiologic appearances. Clin Radiol. 1984;35:451–4. doi: 10.1016/s0009-9260(84)80044-6. [DOI] [PubMed] [Google Scholar]
- 26.Brady LW, Asbell SO. Malignant lymphoma of the gastrointestinal tract. Radiology. 1980;137:291–8. doi: 10.1148/radiology.137.2.7001537. [DOI] [PubMed] [Google Scholar]
- 27.Buckley JA, Jones B, Fishman EK. Small bowel cancer: imaging features and staging. Radiol Clin North Am. 1997;35:381–402. [PubMed] [Google Scholar]
- 28.Bruenton JN. In: Imaging of gastrointestinal tract tumors. Bruenton JN, editor. Berlin, Heidelberg: Springer-Verlag; 1990. pp. 130–7. [Google Scholar]
- 29.McLeod AJ, Zornoza J, Shirkoda A. Leiomyosarcoma: computed tomographic findings. Radiology. 1984;152:133–6. doi: 10.1148/radiology.152.1.6729102. [DOI] [PubMed] [Google Scholar]
- 30.Seth S, Horton KM, Garland MR, et al. Mesenteric neoplasms: CT appearance of primary and secondary tumors and differential diagnosis. Radiographics. 1990;10:985–98. doi: 10.1148/rg.232025081. [DOI] [PubMed] [Google Scholar]