The papers by Fatt & Katz (1952) and del Castillo & Katz (1954) were watershed events in the history of synaptic physiology because they established that neurotransmitters are released from presynaptic terminals in discrete ‘quanta’. Bernard Katz and colleagues employed the frog neuromuscular junction, an accessible peripheral synapse that had already been used to establish some basic principles of synaptic action. Using the then-new technique of intracellular microelectrode recording, Katz and colleagues recorded the postsynaptic responses – termed the end-plate potential (EPP) – resulting from the action of acetylcholine (ACh) on the postsynaptic muscle cell.
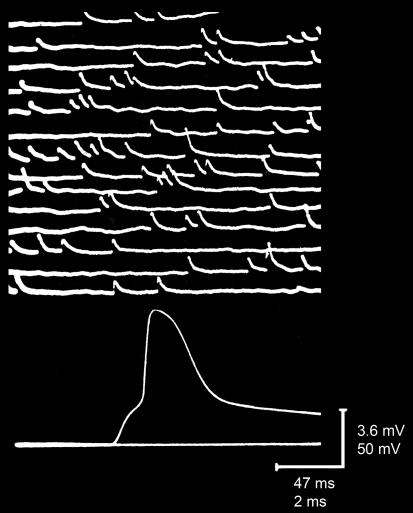
As described in the accompanying Classical Perspectives article by Nicholls (2007), an earlier paper by Fatt & Katz (1951) provided the first direct measurements of the EPP and divined some of its underlying mechanisms. Their insights into postsynaptic mechanisms then permitted Katz and colleagues to use EPPs as a sensitive monitor of ACh release from the presynaptic motor neuron, opening the door to dramatic advances in our understanding of neurotransmitter release. The first big advance came when Fatt & Katz (1952) described small, spontaneous depolarizations of the postsynaptic membrane potential that occurred even when even the motor neuron was not stimulated (Fig. 1, top). Because of the numerous similarities between these events and the EPPs evoked by presynaptic stimulation (Fig. 1, bottom) – such as waveform, spatial localization, and drug sensitivity –Fatt & Katz (1952) named these events ‘miniature end-plate potentials’ (usually referred to as ‘minis’ in modern parlance). Fatt & Katz (1952) reached the fundamental conclusion that minis result from the spontaneous release of ACh from the presynaptic motor neuron. Their experiments also established many important properties of spontaneous transmitter release; for example, the observation that mini frequency is extremely sensitive to osmotic pressure has led to today's widespread use of hypertonic solutions as a chemical means of triggering transmitter release (e.g. Rosenmund & Stevens, 1996).
Figure 1.
Recording of spontaneous minis (top) and an evoked EPP (bottom); the latter is suprathreshold and elicits an action potential in the postsynaptic muscle fibre Modified from Fatt & Katz (1952).
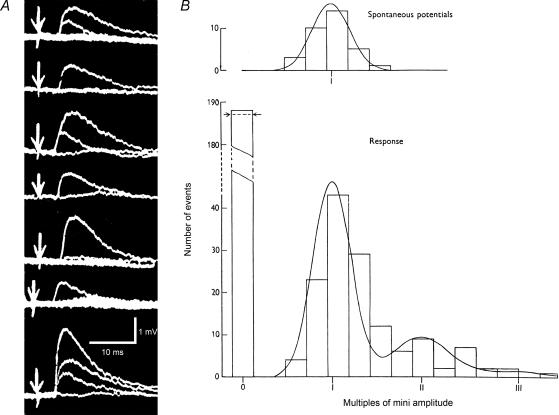
The next, and even more profound, advance came when del Castillo & Katz (1954) quantitatively examined the relationship between EPPs and minis. They focused on the observation of Fatt & Katz (1952) that incubating neuromuscular synapses in Ringer solution with a lower than normal concentration of calcium ions (and extra magnesium to further reduce calcium entry into the presynaptic terminal) caused EPPs to become very small and exhibit substantial trial-to-trial fluctuations in amplitude (Fig. 2A). These fluctuations appeared to be step-like, with each step roughly comparable in amplitude to a mini. del Castillo & Katz (1954) were able to substantiate this observation rigorously by analysing some of the EPP recordings published in the Fatt & Katz (1952) paper. They found that the statistical fluctuations in EPP amplitude were precisely as predicted from a Poisson series, with maxima in the distribution of EPP amplitudes occurring at multiples of the mean amplitude of minis (Fig. 2B). Further, the number of occasions when no EPPs occurred in response to stimulation of the motor neuron, termed synaptic ‘failures’, also could be predicted very accurately from the Poisson model (leftmost bin in Fig. 2B, bottom). Based on their statistical analyses, del Castillo & Katz (1954) concluded that the EPP consists of multiple, mini-like quanta of ACh. Further, they viewed the presynaptic terminal as possessing a pool of such quanta, with presynaptic stimulation causing the synchronous release of some part of this pool. Finally, their results indicated that calcium controls the probability of a given quantum being released. All three of these conclusions are now indisputable facts that have been confirmed at many other chemical synapses and serve as the foundation of our current understanding of neurotransmitter release mechanisms.
Figure 2.
Quantal transmitter release A, recordings of EPPs from a neuromuscular synapse bathed in a low calcium Ringer solution. Each trace represents several superimposed responses to stimulation of the presynaptic motor neuron (at arrows). Note the step-like fluctuations in EPP amplitude. From Fatt & Katz (1952). B, quantitative analysis of EPP amplitude fluctuations. The upper graph plots the distribution of amplitudes of minis, which can be described by a Gaussian function. The lower graph illustrates the pronounced trial-to-trial fluctuations in EPP amplitude, including ‘failures’ (leftmost histobar) where no EPP was elicited. Smooth curve represents the predictions of a Poisson series, assuming that EPPs represent multiple release of mini-like quanta. The number of quanta in each EPP is indicated by the roman numerals on the abscissa. Horizontal arrows indicate number of EPP failures predicted from Poisson model. Modified from del Castillo & Katz (1954).
While the concept of quantal release of neurotransmitters is beyond refute, there are some limitations to this view of neurotransmitter release. As pointed out by Bernard Katz, there is no easy explanation for ‘subminiature’ minis, events with abnormally small and skewed amplitudes that do not resemble the quanta of del Castillo & Katz (1954) yet can be detected at neuromuscular synapses in certain conditions (e.g. Erxleben & Kriebel, 1988). Also, calculation of the ‘quantal content’ of a synaptic response, namely the number of quanta released in response to a presynaptic stimulus, can be misleading for the case of ‘postsynaptic silent’ synapses, where released neurotransmitter does not activate postsynaptic receptors. For example, such calculations led to apparently incorrect conclusions about the locus of expression of long-term potentiation of hippocampal synapses (Liao et al. 1995; Isaac et al. 1995).
Despite these relatively minor limitations, these papers have stood the test of time exceedingly well. Indeed, it is impossible to overstate the enduring significance of the Fatt & Katz (1952) and del Castillo & Katz (1954) papers. In conjunction with Fatt & Katz (1951), this series of Journal of Physiology papers is on a par with the series published by Hodgkin & Huxley – which of course also appeared in The Journal of Physiology– in terms of their impact on the basic concepts of cellular neurophysiology. The work of Katz and colleagues on quantal release of neurotransmitters can be found in every contemporary neuroscience or physiology textbook and has guided subsequent generations of research discoveries. Most significantly, these papers still illuminate contemporary studies of presynaptic mechanisms and undoubtedly will continue to do so long into the future.
Supplementary Material
Original classic papers
The original classic papers reviewed in this article and published in The Journal of Physiology can be accessed online at:
DOI: 10.1113/jphysiol.2006.123224
http://jp.physoc.org/cgi/content/full/jphysiol.2006.123224/DC1
References
- del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erxleben C, Kriebel ME. Subunit composition of the spontaneous miniature end-plate currents at the mouse neuromuscular junction. J Physiol. 1988;400:659–676. doi: 10.1113/jphysiol.1988.sp017142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. An analysis of the end-plate potential recorded with an intra-cellular electrode. J Physiol. 1951;115:320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Nicholls JG. How acetylcholine gives rise to current at the motor end-plate. J Physiol. 2007;578:621–622. doi: 10.1113/jphysiol.2006.122143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.