Abstract
Perforated whole-cell configuration of patch clamp was used to determine the contribution of the electrogenic Na+/HCO3− cotransport (NBC) on the shape of the action potential in cat ventricular myocytes. Switching from Hepes to HCO3− buffer at constant extracellular pH (pHo) hyperpolarized resting membrane potential (RMP) by 2.67 ± 0.42 mV (n = 9, P < 0.05). The duration of action potential measured at 50% of repolarization time (APD50) was 35.8 ± 6.8% shorter in the presence of HCO3− than in its absence (n = 9, P < 0.05). The anion blocker SITS prevented and reversed the HCO3−-induced hyperpolarization and shortening of APD. In addition, no HCO3−-induced hyperpolarization and APD shortening was observed in the absence of extracellular Na+. Quasi-steady-state currents were evoked by 8 s duration voltage-clamped ramps ranging from −130 to +30 mV. A novel component of SITS-sensitive current was observed in the presence of HCO3−. The HCO3−-sensitive current reversed at −87 ± 5 mV (n = 7), a value close to the expected reversal potential of an electrogenic Na+/HCO3− cotransport with a HCO3−:Na+ stoichiometry ratio of 2: 1. The above results allow us to conclude that the cardiac electrogenic Na+/HCO3− cotransport has a relevant influence on RMP and APD of cat ventricular cells.
Na+/HCO3− cotransport (NBC) was first described by Boron & Boulpaep (1983) in the renal proximal tubule of the salamander, with a HCO3−/Na+ stoichiometry of 3: 1, which generates a net flux of negative charge across the cell membrane. In the heart, this mechanism was first reported to be present in sheep Purkinje fibres (Dart & Vaughan-Jones, 1992) and isolated guinea pig ventricular myocytes (Lagadic-Gossman et al. 1992) as an electroneutral transporter. However, the lack of electrogenicity of the NBC in myocardium was challenged by experiments performed in cat heart multicellular preparations (Camilión de Hurtado et al. 1995, 1996) and rat ventricular myocytes (Aiello et al. 1998) where the presence of an NBC with a HCO3−/Na+ stoichiometry of 2: 1 was suggested. More recently, the data proved by molecular biology supported the notion that one electroneutral (NBC3 or NBCn1) (Pushkin et al. 1999; Choi et al. 2000) and two electrogenic isoforms (NBC1b or hhNBC or NBCe1-B and NBC4 or NBCe2-c) (Choi et al. 1999; Pushkin et al. 2000; Sassani et al. 2002; Virkki et al. 2002) coexist in the myocardium. A recent work described in detail the functional diversity of the electrogenic NBC in ventricular myocytes from rat, rabbit and guinea pig (Yamamoto et al. 2005). Although we have previously suggested that the electrogenic NBC contributes to the modulation of the spike-like rat action potential (AP) waveform, the participation of this transporter in the configuration of the typical prolonged cardiac AP of larger mammals is an interesting issue that remains to be studied. Thus, in this study we present evidence for the presence of an electrogenic NBC in isolated cat ventricular myocytes, and for its contribution to the modulation of resting membrane potential (RMP) and AP duration (APD).
Methods
Cell isolation
All experiments were performed in accordance with the guidelines for Animal Care of the Scientific Committee of the University of La Plata School of Medicine. Cats (body weight 3–4 kg) were anaesthetized by intraperitoneal injection of sodium pentobarbitone (35 mg (kg body weight)−1). The chests were opened when plane three of phase III of anaesthesia was reached, verified by the loss of the corneal reflex and appearance of slow deep diaphragmatic breathing. The hearts were quickly removed, mounted in a Langendorff apparatus and retrogradly perfused with Krebs-Henseleit solution (K-H) containing (mm): NaCl 123, KCl 4.69, CaCl2 1.35, NaHCO3 20, NaH2PO4 1.2, MgSO4 1.2, glucose 11, pH 7.35 after gassing with 95% O2–5% CO2. Hearts were perfused at constant pressure for a stabilization period of 10–15 min. Single ventricular myocytes were isolated by an enzymatic dispersion technique in which hearts were perfused with nominally Ca2+-free K-H solution for 5 min before treatment with collagenase (74.5 u ml−1, Worthington Biochemical Corp., Lakewood, NJ, USA) in Ca2+-free K-H solution for 45 min. The left ventricle was then removed, placed in Ca2+-free solution, and cut into small pieces (2 × 2 mm). After a final wash, the tissues were kept in K-H at room temperature, and single myocytes were obtained by gentle trituration.
Patch-clamp recordings
Isolated cat ventricular myocytes were placed in a recording chamber and superfused with bath solution at a flow rate of 1.5 ml min−1. Only rod-shaped myocytes with clear and distinct striations and an obvious marked shortening and relaxation on stimulation were used. Experiments were performed at room temperature (20–22°C), at 30°C or at 37°C.
The nystatin perforated whole-cell configuration of the patch clamp technique (Korn et al. 1991) was used for voltage- and current-clamp recordings with a patch-clamp amplifier (Axopatch 200A, Axon Instruments, Union City, CA, USA). Patch pipettes were pulled with a PP-83 puller (Narishige, Tokyo, Japan) and fire-polished with a MF-83 Microforge (Narishige) to a final resistance of 0.5–1 MΩ when filled with a control pipette solution. Membrane voltage and whole-cell currents (sampling rate = 1 kHz; low pass filter = 1 kHz) were digitally recorded directly to hard disk via an analog-to-digital convertor (Digidata 1200, Axon Instruments) interfaced with an IBM clone computer running pClamp software (Axon Instruments). A pacing rate of 0.2 Hz was applied in the current-clamp recordings. Data analysis was performed with pCLAMP (Clampfit). A Ag/AgCl wire directly in contact with the extracellular solution was used as reference electrode. Since the pipette potential was nulled in external solution, all current-clamp tracings and voltage-clamp protocols required corrections for junction potential. This was accomplished by filling 20 pipettes with standard internal solution. They were then nulled in internal solution, and the difference in potential on immersion in external solution was recorded. The measured junction potential value was consistently −10 mV, and this value was used to correct all current-clamp data and voltage-clamp protocols. There were no significant differences in the value of junction potential among all the external solutions used in the present work. For each cell, capacitative current was recorded to determine the membrane capacitance, and the currents were normalized for cell capacitance. Average cell capacitance was 126.6 ± 11.1 pF (n = 52).
pHi measurements
After enzymatic isolation, myocytes were loaded with the membrane-permeant acetoxymethyl ester form of the fluorescent H+-sensitive indicator SNARF-1/AM. Cell suspensions (2 ml) were exposed to a final concentration of 4 μm SNARF-1/AM and 0.6% v/v DMSO. After 10 min, the myocytes were gently centrifuged for 2 min and resuspended in Hepes buffer and stored at room temperature until use. pHi and cell length were monitored on the stage of a modified inverted microscope, as previously described (Vila Petroff et al. 2000). After excitation at 530 ± 5 nm, the ratio of SNARF-1/AM emission at 590 ± 5 nm to that of 640 ± 5 nm was used as a measure of pHi according to an in vivo calibration, obtained from SNARF-1/AM-loaded myocytes exposed to solutions of varying pH values containing 140 mm KCl, 20 μm nigericin, 1 μm valinomycin, and 1 μm carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone at room temperature.
Cell shortening
In some experiments, cell shortening, as an index of contractility, was measured simultaneously with the perforated-patch recordings or the pHi measurements. Resting cell length and cell shortening were measured by a video-based motion detector (Crescent Electronics, UT, USA) and stored by software for an off-line analysis.
Solutions
The HCO3−-free external solution (Hepes-buffered) contained (mm): NaCl 133, KCl 5, MgSO4 1.2, MgCl2 0.8, glucose 10, CaCl2 1.35, and Hepes 10, pH 7.35 with 5 mm NaOH (total Na+ 138 mm). The HCO3−-buffered solution contained (mm): NaCl 118, KCl 5, MgSO4 1.2, MgCl2 0.8, glucose 10, CaCl2 1.35, Hepes 10, choline-Cl 15, and NaHCO3 20. The pH was titrated to 7.35 with TrisBase after gassing with 95% O2–5% CO2. In some experiments the extracellular solution contained 5 mm instead of 20 mm NaHCO3, and no gassing with O2/CO2 was assayed. This solution was prepared fresh for each experimental day, in order to minimize the CO2 hydration reaction. In the Na+-free experiments, the external NaCl was replaced completely with LiCl in both, Hepes- and HCO3−-buffered solutions (in the zero Na+ HCO3−-buffered solution, NaHCO3 was replaced with choline-HCO3, LiCl was 133 mm and no choline-Cl was added). The pH of the Na+-free Hepes-buffered solution was titrated to 7.35 with Tris base. NaHCO3 was replaced completely with choline-HCO3 in the HCO3−-buffered solution. The Nystatin pipette solution contained (mm): K-gluconate 130, KCl 10, NaCl 8, MgCl2 0.5, EGTA 1, Hepes 10, and Nystatin 0.3 mg ml−1. The pH was titrated to 7.15 with KOH. NaCl was replaced with choline-Cl in the pipette solution of the Na+-free experiments. In all the cases, the estimated equilibrium potential for Cl− was −52 mV.
Statistics
Data are expressed as mean ± s.e.m. and were compared with Student's t test for paired values, and repeated measures ANOVA followed by the Student-Newman-Keuls test. A value of P < 0.05 was considered statistically significant (two-tailed test).
Results
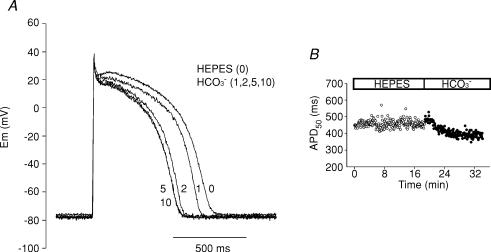
Figure 1A shows representative traces of cat ventricular APs before and after 1, 2, 5 and 10 min of replacing the extracellular Hepes-buffered solution (nominal HCO3−-free) with a CO2/HCO3−-buffered solution (HCO3− 20 mm) at constant pHo. The RMP hyperpolarized rapidly by approximately 3 mV in the presence of the physiological buffer. APD shortened gradually until steady-state was reached after 5 min in HCO3−. Single cardiac myocytes with long APDs frequently exhibit variability in APD from one beat to another, even when paced at a constant rate, possibly leading to a misinterpretation of our results. However, in the present work we did not detect substantial beat-to-beat APD variability, as shown in the time course of beat-to-beat APD, before and after HCO3−, depicted in Fig. 1B. Thus, it seems unlikely that the changes in APD detected in the presence of HCO3− were due to beat-to-beat APD variability.
Figure 1.
APD shortening after switching the extracellular solution from Hepes to HCO3−-buffered solution A, AP recordings under current-clamp mode before (Hepes) and after 1, 2, 5 and 10 min of superfusion of a cat ventricular myocyte with external HCO3−. The presence of HCO3− in the external solution produced a gradual APD shortening. B, typical time course of beat-to-beat APD50 measured before and after switching the extracellular solution from Hepes to HCO3−.
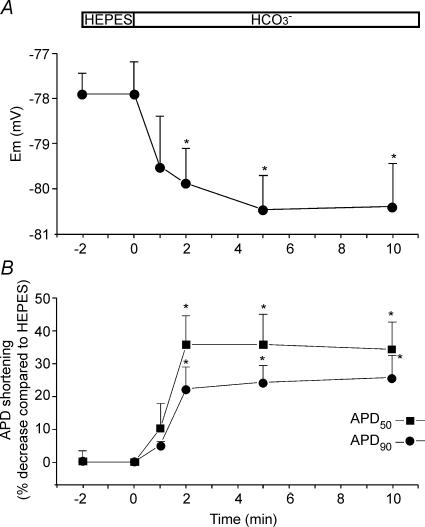
Figure 2A shows the time course of the average changes in RMP during the replacement of Hepes-buffered superfusate with HCO3−-buffered solution. On average, RMP hyperpolarized 2.67 ± 0.47 mV (n = 9) after 5 min in the presence of HCO3−. Figure 2B depicts percentage average changes in APD after 50% (APD50) and 90% (APD90) of repolarization time induced by the presence of HCO3− in the bath media. A significant and relevant APD50 (∼35%) and APD90 (∼25%) shortening was observed as early as 2 min after exposing the myocytes to HCO3−, remaining constant afterwards during the rest of the experiment. Although without reaching statistical significance, the effect of HCO3− was more pronounced for the APD50 than for the APD90, possibly reflecting the higher driving force for HCO3− influx through the NBC at plateau potentials than at more repolarized potentials.
Figure 2.
Hyperpolarization of RMP and shortening of the APD induced by external HCO3− A, time course of the average changes in RMP after switching the superfusate from Hepes- to HCO3−-buffered solution (n = 9). B, time course of the percentage average changes (with respect to the value in Hepes measured immediately before the switch to HCO3−) in APD50 and APD90 induced by external HCO3− (n = 9). External HCO3− induced a slight RMP hypepolarization and a significant and relevant shortening of the APD.
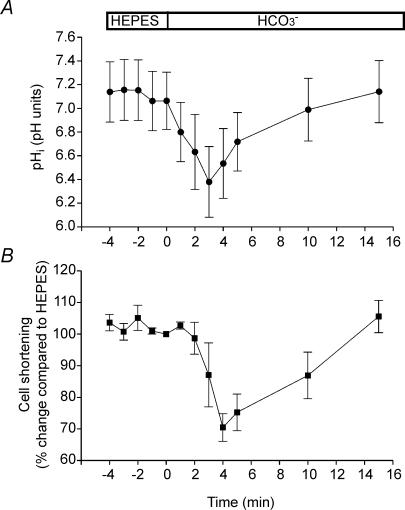
The change of a superfusate from a Hepes-buffered to a CO2/HCO3−-buffered solution induces a transient intracellular acidification due to the rapid CO2 permeation into the cell, followed by a recovery to control values within 5–10 min after changing from Hepes to CO2/HCO3− solutions (Dart & Vaughan-Jones, 1992, Camilión de Hurtado et al. 1995). Since membrane currents are sensitive to pHi changes, these effects may partially explain the APD alterations observed in CO2/HCO3−-buffered solution. In order to evaluate the time course of pHi changes induced by the switch from Hepes to CO2/HCO3− solution, we performed experiments in isolated cat myocytes in which pHi and cell length shortening were measured simultaneously. Figure 3A shows the average changes in pHi after the acid load induced by replacement of the Hepes-buffered superfusate with the HCO3−-buffered solution. An early decrease in pHi, followed by a recovery, can be seen. Initially, CO2 entry causes intracellular acidification but, within 2–4 min, pHi started to recover towards control values. After 10 min, pHi was completely recovered to values not different from those obtained before the replacement of the extracellular solution. The time course of the cell-length-shortening changes induced by the replacement of Hepes-buffered with CO2/HCO3−-buffered solution was associated to that of pHi (Fig. 3B). As previously observed in cat papillary muscles (Camilión de Hurtado et al. 1995), the changes in contractility were slightly delayed with respect to the changes in pHi, suggesting that the former are secondary to the latter, probably due to alterations in myofibrillar Ca2+ sensitivity and/or changes in sarcoplasmic reticulum Ca2+ content or fractional release (Bers, 2001).
Figure 3.
Simultaneous measurements of pHi and cell shortening in the absence and presence of external HCO3− A, average pHi values obtained in five myocytes during the switch of the extracellular solution from Hepes to HCO3−. B, average cell shortening (n = 5, expressed as percentage change with respect to the value in Hepes measured immediately before the switch to HCO3−) recorded simultaneously with pHi.
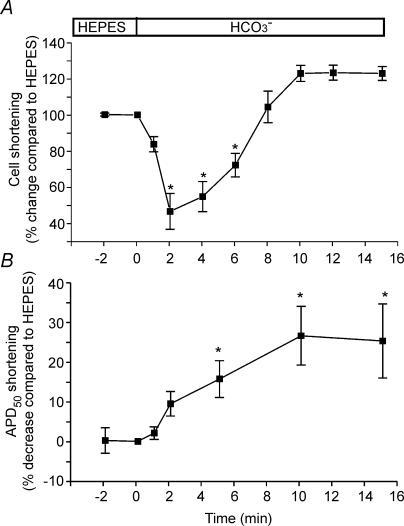
We next recorded cell length shortening and APs simultaneously as an indirect method to evaluate whether the time course of pHi changes observed in intact cat cardiomyocytes is similar to that of the perforated patch-clamped cat cardiomyocytes. As observed in the intact myocytes, when the superfusate was switched from Hepes to HCO3−-buffered solution, the perforated patch-clamped myocyte contractility transiently decreased, but within 6–8 min recovered towards control values, reaching steady-state after 8 min (Fig. 4A). These changes in contractility were faster than those observed in the epifluorescence setup, possibly due to slight differences in superfusion of the cells (i.e. different chamber sizes). However, if we consider that the changes in contractility are secondary to those of pHi (see above), we can speculate that pHi would be totally recovered after 6–8 min of switching the superfusate of the clamped myocytes from Hepes to CO2/HCO3−. Thus, after 6–8 min of switching to CO2/HCO3−, despite full recovery of pHi, APD remained shortened (Fig. 4B), indicating that both phenomena are not related. However, taking into account that it was previously reported that acidosis causes APD lengthening (Coraboeuf et al. 1976; Poole-Wilson & Langer, 1975; Spitzer & Hogan, 1979), we cannot rule out the possibility that the APD shortening would have been delayed during the first minute in CO2/HCO3− due to the transient intracellular acidification.
Figure 4.
Simultaneous measurements of cell shortening and APD in the absence and presence of external HCO3− A, average cell shortening of seven myocytes during the switch of the extracellular solution from Hepes to HCO3−. B, average APD50 (n = 7) recorded simultaneously with cell shortening. The values were expressed as percentage change with respect to the value in Hepes measured immediately before the switch to HCO3−. The HCO3−-induced APD shortening remained present despite the transient decrease and postponed recovery of cell contractility.
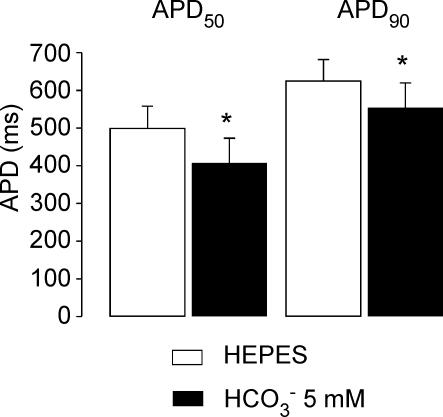
We next evaluated the effect on APD of a smaller concentration of HCO3− (5 mm) in the absence of CO2 bubbling (HCO3− added to the Hepes-buffered solution), which should minimize the CO2-induced transient intracellular acidification. Under these conditions RMP hyperpolarized from −73.8 ± 1.2 mV to −76 ± 1.2 mV (n = 9, P < 0.05) after 8 min of exposure of the myocytes to HCO3−. Figure 5 shows the average values of APD50 and APD90 in the absence of HCO3− and after 8 min in the presence of 5 mm HCO3− in the extracellular solution. This concentration of HCO3− induced an APD50 and APD90 shortening of 19.3 ± 4.6% and 11.9 ± 3.8% (n = 9) of the values in the absence of HCO3−, respectively, values that are lower than those observed with 20 mm HCO3− (Fig. 2B). These results demonstrate that the magnitude of the APD shortening observed in HCO3− is dependent on the extracellular concentration of HCO3−.
Figure 5.
APD shortening induced by 5 mm HCO3− Average values of APD50 and APD90 in Hepes and after 8–10 min with 5 mm HCO3− in the extracellular solution (n = 9). This concentration of HCO3− induced an APD50 and APD90 shortening of 19.3 ± 4.6% and 11.9 ± 3.8% (n = 9) of the values in the absence of HCO3−, respectively. *Significantly different from Hepes.
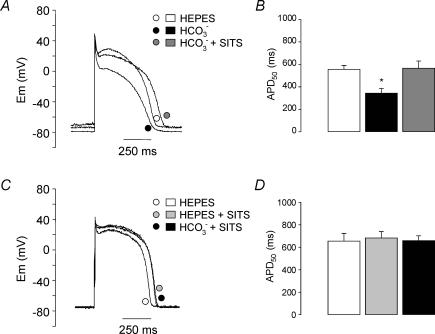
The APD shortening induced by exposure of the myocytes to 20 mm HCO3− was reversed (Fig. 6A and B) and prevented (Fig. 6C and D) by the non-specific blocker (general anionic blocker) of NBC, SITS (0.1 mm). As observed in Fig. 6, there is a tendency of APD to be slightly prolonged with SITS, but this effect did not attain statistical significance. The RMP hyperpolarization was also reversed (Hepes: −73.3 ± 2.2 mV; HCO3−: −76.4 ± 2.4 mV, P < 0.05; HCO3−+ SITS: −73.4 ± 2.3 mV; n = 6) and prevented by SITS (−75.3 ± 2.3 mV; Hepes + SITS: −75.6 ± 0.5 mV; HCO3−+ SITS: −76.5 ± 0.5 mV; n = 6).
Figure 6.
Effects of the anionic blocker SITS on the HCO3−-induced changes in APD A, representative traces of APs recorded from a myocyte exposed successively to Hepes, HCO3− (10 min) and HCO3−+ SITS (0.1 mm) (10 min). B, average values of APD50 obtained from six myocytes subjected to the conditions of A. C, representative traces of APs recorded from a myocyte exposed successively to Hepes, Hepes + SITS (0.1 mm) (10 min) and HCO3−+ SITS (0.1 mm) (10 min). D, average values of APD50 obtained from six myocytes subjected to the conditions of C. The APD shortening induced by 20 mm HCO3− was reversed and prevented by 0.1 mm SITS. *Significantly different from Hepes.
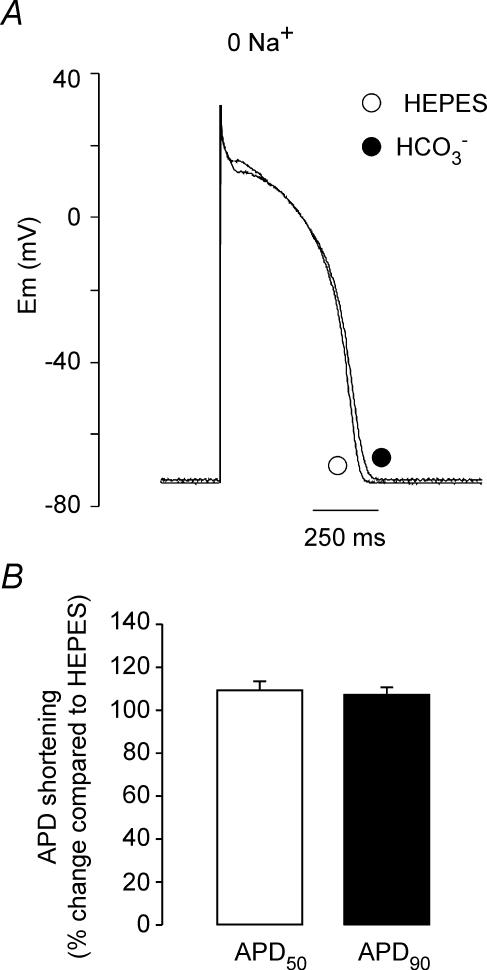
Figure 7A shows representative traces of APs recorded in Hepes zero Na+ and HCO3− zero Na+. Na+ was entirely replaced with Li+, a cation that can be carried by the Na+ channels (Le Guennec & Noble, 1994) and by the Na+/H+ exchanger (NHE) (Paris & Pouyssegur, 1983; Aronson, 1985), but can only minimally substitute for extracellular Na+ on NBC transport (Jentsch et al. 1985; Dart & Vaughan-Jones, 1992; Amlal et al. 1998; Sciortino & Romero, 1999). Changing the superfusate from Hepes- to HCO3−-buffered solution in the absence of extracellular Na+ did not produce significant changes on the AP (Fig. 7A and B). As shown on Fig. 7B, after 10 min in the presence of HCO3− the APD50 and APD90 were 109.6 ± 3.9% and 107.5 ± 3.1% of the value in Hepes (n = 6), respectively. These results indicate that the HCO3−-induced APD shortening is dependent on the presence of extracellular Na+, as expected for the involvement of the electrogenic NBC in this effect.
Figure 7.
Effects of Na+ deprivation on the HCO3−-induced changes in APD A, representative traces of AP recordings before and after 10 min of exposure of a cat myocyte to HCO3− in the absence of extracellular Na+. No HCO3−-induced effects on RMP and APD were observed under these conditions. B, average change in APD50 and APD90 after 10 min in HCO3− zero Na+ (n = 4), expressed as percentage change with respect to the value in Hepes (100%) measured immediately before the switch to HCO3−.
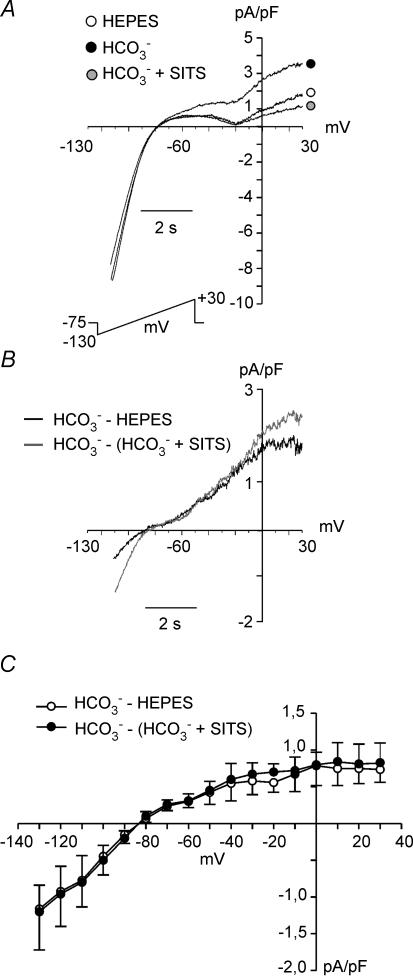
Figure 8A shows the effects of changing the extracellular superfusate from Hepes to HCO3− on steady-state currents evoked by 8 s duration voltage-clamped ramps from −130 to +30 mV, from a holding potential of −75 mV. A novel component of an outward current was detected in the presence of HCO3−, consistent with an electrogenic influx of this anion. This HCO3−-induced outward current was cancelled by addition of SITS to the extracellular medium (Fig. 8A). Figure 8B shows the difference currents, obtained by subtraction of the current in Hepes or the current in HCO3−+ SITS to the current in HCO3−. The average current–voltage relationship for these two difference currents is shown in Fig. 8C. Although there was a tendency for the SITS-sensitive current to be greater than the HCO3−-sensitive current, the difference did not attain statistical significance. The HCO3−-sensitive current and the SITS-sensitive current reversed at around −85 mV (HCO3−-sensitive current: −87 ± 4.8 mV, n = 7; SITS-sensitive current:88 ± 6 mV, n = 7). The reversal potential (ENBC) of the NBC current (INBC) with a HCO3−/Na+ stoichiometry of 2: 1 was calculated with the following equation:
Figure 8.
Effects of external HCO3− on steady-state currents, perforated whole-cell configuration A, steady-state currents evoked by 8 s-duration voltage-clamp ramps ranging from −130 to +30 mV, from a holding potential of −75 mV, recorded from a cardiomyocyte exposed successively to external Hepes, HCO3− (10 min), and HCO3− in the presence of SITS (10 min). B, HCO3−-sensitive difference current (HCO3−− Hepes) and SITS-sensitive difference current (HCO3−− (HCO3−+ SITS). C, average current–voltage relationship for the HCO3−-sensitive difference current (HCO3−− Hepes) (n = 7) and SITS-sensitive difference current (HCO3−− (HCO3−+ SITS) (n = 7). These two difference currents reversed at ∼−85 mV, a value close to the expected ENBC of an electrogenic NBC with a stoichiometry ratio of 2HCO3−: 1Na+.
Considering that Na+i = 8 mm, Na+o = 138 mm, HCO−3 i = 14.5 mm (pHi∼ 7.2), HCO−3 o = 20 mm (pHo = 7.35) and that the experiments were performed at 20–22°C, ENBC was calculated to be −89.4 mV, a similar value to the one measured in our experiments. Thus, these results suggest that the HCO3−-sensitive current and the SITS-sensitive current detected herein represents INBC.
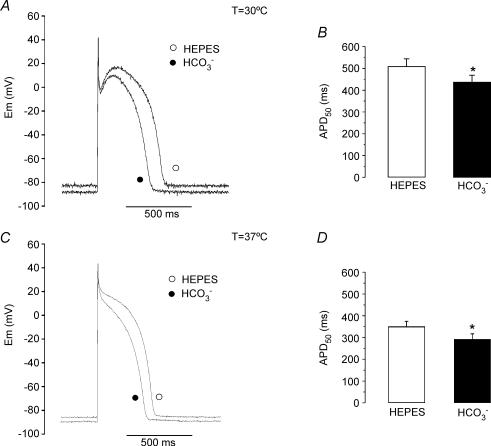
Finally, in order to investigate the physiological implication of the electrogenic NBC in the electrical properties of cat cardiomyocytes, the impact of HCO3− on APD was evaluated at 30°C and 37°C. Figure 9 shows representative traces of AP before and after 10 min of switching the extracellular solution from Hepes to HCO3− at 30°C (Fig. 9A) or 37°C (Fig. 9C). In both cases, RMP hyperpolarization and APD shortening were observed in the presence of the physiological buffer. Average values of APD50, before and after HCO3−, are shown in Fig. 9B (30°C) and D (37°C). On average, we detected a HCO3−-induced APD50 shortening of 14.2 ± 1.6% (n = 8) at 30°C and of 16.1 ± 2.3% at 37°C (n = 4). In Hepes, the average values of RMP were −77.5 ± 2.3 mV at 30°C and −80.7 ± 2.5 mV at 37°C (n = 4). After exposure of the myocytes to 10 min of HCO3−, RMP hyperpolarized to −81.1 ± 2.2 mV (n = 8, P < 0.05) and −84.1 ± 2.2 mV (n = 4, P < 0.05) at 30°C and 37°C, respectively. These data support the notion that the electrogenic NBC plays a relevant role in the electrical modelling of the ventricular AP at physiological temperatures.
Figure 9.
APD shortening induced by external HCO3− at 30°C and 37°C A, AP recordings before (Hepes) and after 10 min of superfusion of a cat ventricular myocyte with external HCO3− at 30°C. B, average APD50 (n = 8) measured before and after 10 min of HCO3−-buffered solution at 30°C. At 30°C, HCO3− induced an APD shortening of 14.2 ± 1.6% (n = 8). C, AP recordings before (Hepes) and after 10 min of superfusion of a cat ventricular myocyte with external HCO3− at 37°C. D, average APD50 (n = 4) measured before and after 10 min of HCO3−-buffered solution at 37°C. At 37°C, HCO3− induced an APD shortening of 16.1 ± 2.3% (n = 4).
Discussion
The experiments reported herein demonstrate that cat cardiac ventricular cells possess an electrogenic NBC that has a relevant impact on APD. Changing the myocytes' bathing superfusate from a HCO3−-free (Hepes-buffered) to a HCO3−-containing solution at constant pHo, induces RMP hyperpolarization, APD shortening, and development of an outward current, consistent with the influx of HCO3− into the cell. These changes are sensitive to the extracellular concentration of HCO3−, and are blunted by anionic blockade or by sodium deprivation. Accordingly, the RMP hyperpolarization and APD shortening induced by HCO3− seem to have an underlying anionic current which is dependent on the presence of extracellular Na+, sensitive to the extracellular concentration of HCO3− and reverses at a potential close to the estimated ENBC with a HCO3−: Na+ ratio of 2: 1. Thus, these results strongly suggest that the relevant HCO3−-induced changes in RMP and APD observed in the present study are due to the activation of the electrogenic NBC present in the cardiomyocyte.
The results presented here are in agreement with early experiments performed in canine Purkinje fibres by Spitzer & Hogan (1979). The authors reported that lowering extracellular HCO3− at constant pHo produced depolarization of RMP and APD lengthening. These authors suggested that these effects were due to changes in a background HCO3− current. In the light of the present results, it seems likely that the previously reported background HCO3− current of canine Purkinje fibres could have been carried by the electrogenic NBC.
Although the effects of acidosis on the cardiac action potential are relatively modest, they are manifested as a depolarization of RMP and prolongation of APD (Coraboeuf et al. 1976; Poole-Wilson & Langer, 1975; Spitzer & Hogan, 1979). In our experiments, the transient acidosis caused after changing the superfusate from Hepes- to HCO3−-buffered solution did not seem to have significant effects on the membrane currents that control RMP or AP configuration, since in the presence of SITS or in the absence of extracellular Na+, exposure of the myocytes to CO2/HCO3− did not significantly alter RMP or APD. It is important to note that in the SITS and the zero Na+ (replaced with Li+) experiments, the recovery of pHi upon changing from Hepes to CO2/HCO3− solution, would be partially impaired due to block of NBC, depending exclusively on the activity of the NHE. Thus, a persisting acidosis might be present at the time chosen to measure APD and RMP (10 min after changing the solutions). In order to evaluate the time course of pHi changes in the presence of SITS or in the absence of extracellular Na+, we measured pHi under these conditions. In the presence of SITS, the average pHi of four myocytes decreased from 7.31 ± 0.16 (Hepes) to 6.68 ± 0.23 at 3–4 min in CO2/HCO3−, recovering to 6.96 ± 0.23 at 10 min in the continuous presence of the physiological buffer. In the absence of extracellular Na+, the average values of pHi of six myocytes were 7.23 ± 0.04 in Hepes, and 6.59 ± 0.10 and 6.91 ± 0.09 at 3–5 min and 10 min in CO2/HCO3−, respectively. Thus, the fact that APD in Hepes was not significantly different from that measured after 10 min in HCO3− plus SITS (Fig. 6) or HCO3− zero Na+ (Fig. 7), indicates that a decrease of 0.3 pH units in basal cat cardiac pHi is not sufficient to modify the AP configuration.
The HCO3−-induced hyperpolarization was small but consistent and, with the obvious cell-to-cell variability, it was present in most of the experiments performed. Moreover, just a small hyperpolarization is expected because at RMP values the driving force for HCO3− influx through the NBC with a stoichiometry ratio of 2 HCO3−: 1 Na+ is not large.
In the presence of external HCO3−, we were able to record in isolated cat myocytes a SITS-sensitive current which reverses at approximately −85 mV. This value is very close to the estimated ENBC, with a HCO3−/Na+ stoichiometry ratio of 2: 1. The same stoichiometry was reported for native INBC recorded in rat (Aiello et al. 1998; Yamamoto et al. 2005), rabbit and guinea pig (Yamamoto et al. 2005) ventricular myocytes, amphibian optic nerve and mouse cerebral astrocytes (Astion & Orkand, 1988; Brookes & Turner, 1994), leech glial cells (Deitmer & Schlue, 1989) and frog retinal epithelium (Hughes et al. 1989). In contrast, a stoichiometry of 3: 1 has been estimated for mammalian proximal tubule (Boron & Boulpaep, 1983) and salamander retinal glia (Newman, 1991). This variation in stoichiometry may reflect differences in cotransporter function in different systems. A cotransporter stoichiometry of 2: 1 in ventricular myocytes suggests that HCO3− is normally transported in an inward direction in these cells, generating an outward current that contributes to hyperpolarizing RMP and shortening APD.
An electrically silent NBC was reported to be present in sheep Purkinje fibres and isolated guinea pig myocytes, by Dart & Vaughan-Jones (1992) and Lagadic-Gossman et al. (1992), respectively. The authors failed to record INBC using a standard whole-cell configuration of patch-clamp technique in guinea pig ventricular myocytes (Lagadic-Gossman et al. 1992). However, a recent work did report the presence of INBC in guinea pig, rat and rabbit myocytes, using perforated-patch instead of standard whole-cell techniques (Yamamoto et al. 2005). Since our previous (Aiello et al. 1998) and present experiments were also performed with perforated patch, it seems mandatory that minimal altered intracellular milieu conditions (perforated-patch configuration) are necessary to detect this novel anionic current.
Yamamoto et al. (2005) reported that rat INBC recorded at 0 mV and 36°C was two-fold and five-fold greater than INBC recorded in guinea pig and rabbit cardiomyocytes, respectively. Thus, it seems likely that INBC is much smaller in larger animals, compared to the rat. However, in disagreement with this hypothesis, the magnitude of INBC of cat cardiomyocytes recorded herein at 0 mV and room temperature was almost two-fold higher than the one reported for rat cardiomyocytes by Yamamoto et al. (2005). Interestingly, if we consider the density of INBC recorded at +30 mV, cat INBC (Fig. 8) was similar to that of rat INBC (Yamamoto et al. 2005), due to the strong rectification of cat INBC at potentials positive to 0 mV, in comparison to rat INBC, which seems to rectify at more positive potentials (Yamamoto et al. 2005). Although these modest variations in the biophysical properties of INBC among these two species could be explained by the disparity in the recording temperature, it is also possible that the rectification at less positive voltages could be typical of larger mammals, since a similar phenomenon was observed in guinea pig and rabbit INBC (Yamamoto et al. 2005). Although both electrogenic isoforms of NBC, NBC1 and NBC4, were reported to be present in normal human heart (Choi et al. 1999; Pushkin et al. 2000), no studies have yet shown the presence of native INBC in human cardiomyocytes. Thus, the evaluation of human cardiac INBC and its influence on APD and RMP represents a relevant matter to be addressed in the near future.
We presented herein that the HCO3−-induced APD shortening was also present at physiological temperatures. However, although the NBC activity was reported to be slowed by reduced temperature (Ch'en et al. 2003), the magnitude of the HCO3−-induced APD shortening at physiological temperatures was not greater, but actually smaller, than at room temperature. Although we do not have data that can certainly explain the reason for this difference, we can speculate that the expected decrease in membrane resistance and increase in other ionic currents at higher temperatures could account for the smaller effect of INBC on APD.
Yamamoto et al. (2005) have shown that the cardiac NBC activity is associated with a net Na+ influx to the cell. After the acidification induced by ischaemia, Na+-coupled acid extrusion during ischaemia and/or reperfusion contributes to net H+ efflux. The associated Na+ entry may contribute to a rise in [Na+]i and therefore Ca2+ overload via Na+/Ca2+ exchange. Vandenberg et al. 1993) showed that the NBC contributes to about 20% to the total pHi recovery during reperfusion. Schafer et al. 2000) demonstrated that during reoxygenation of rat myocytes exposed to 70 min of anoxia, the NBC also plays an important role in pHi recovery (approximately 50% of the total pHi recovery). Furthermore, these authors showed that the calcium oscillations that cause hypercontracture of the cells were diminished by DIDS, a blocker of the NBC (Schafer et al. 2000). More recently, Khandoudi et al. (2001) have shown in perfused rat hearts that the presence of a selective antibody against cardiac NBC1b significantly improved the post-ischaemic functional recovery. These authors also showed that NBC1b but not NBC3 protein expression in human myocardium from patients with heart failure was markedly increased in comparison to control hearts (Khandoudi et al. 2001). In addition, an upregulation of NBC1 mRNA and protein was reported to be present after myocardial infarction in the rat heart (Sandmann et al. 2001). Finally, since the NBC1b is a Na+-loading mechanism, the overexpression of this transporter might contribute to increase the incidence of arrhythmias in the failing heart (Verdonck et al. 2003). Accordingly, the contribution of INBC to the shape of the AP in pathophysiological states represents an interesting forthcoming issue to be resolved.
In summary, the present study demonstrates the presence of an electrogenic NBC in isolated cat ventricular myocytes that participates in the modulation of RMP and APD. These effects originate from the presence of a SITS-sensitive outward current that can be seen only when HCO3− and Na+ are present in the media. Although HCO3− is the physiological buffer, HCO3−-buffered solutions are not usually employed in patch-clamp experiments, masking the observation of these effects. From these results it is clear that INBC should be considered as part of the currents contributing to the configuration of the cardiac AP.
Acknowledgments
This work was partly supported by a grant from the Agencia Nacional de Promoción Científica y Tecnológica (PICT 25495 to E.A.A.). The authors wish to thank Mónica Rando for excellent technical assistance.
References
- Aiello EA, Vila Petroff MG, Mattiazzi AR, Cingolani HE. Evidence for an electrogenic Na+/HCO3− symport in rat cardiac myocytes. J Physiol 512. 1998;1:137–148. doi: 10.1111/j.1469-7793.1998.137bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlal H, Wang Z, Burnham C, Soleimani M. Functional characterization of a cloned human kidney Na+:HCO3− cotransporter. J Biol Chem. 1998;273:16810–16815. doi: 10.1074/jbc.273.27.16810. [DOI] [PubMed] [Google Scholar]
- Aronson PS. Kinetic properties of the plasma membrane Na+–H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Astion MK, Orkand RK. Electrogenic Na+/HCO3− cotransport in neuroglia. Glia. 1988;1:355–357. doi: 10.1002/glia.440010508. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac inotropy and Ca mismanagement. In: Bers DM, editor. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht: Kluwer Academic Publishers; 2001. pp. 273–331. [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. J General Physiol. 1983;8:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N, Turner RJ. K+-induced alkanization in mouse cerebral astrocytes mediated by reversal of electrogenic Na+–HCO3− cotransport. Am J Physiol Cell Physiol. 1994;267:C1633–C1640. doi: 10.1152/ajpcell.1994.267.6.C1633. [DOI] [PubMed] [Google Scholar]
- Camilión de Hurtado MC, Alvarez BV, Pérez NG, Cingolani HE. Role of an electrogenic Na+/HCO3− cotransport in determining myocardial pHi after an increase in heart rate. Circ Res. 1996;79:698–704. doi: 10.1161/01.res.79.4.698. [DOI] [PubMed] [Google Scholar]
- Camilión de Hurtado MC, Pérez NG, Cingolani HE. An electrogenic sodium-bicarbonate cotransport in the regulation of myocardial intracellular pH. J Mol Cell Cardiol. 1995;27:231–242. [PubMed] [Google Scholar]
- Ch'en FFT, Dilworth E, Swietach P, Goddard RS, Vaughan-Jones RD. Temperature dependence of Na+–H+ exchange, Na+–HCO3− co-transport, intracellular buffering and intracellular pH in guinea-pig ventricular myocytes. J Physiol. 2003;552:715–726. doi: 10.1113/jphysiol.2003.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+–HCO−3 cotransporter isoform (hhNBC) Am J Physiol Cell Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E, Deroubaix E, Hoerter J. Control of ionic permeabilities in normal and ischemic heart. Circ Res. 1976;38:I92–I98. [PubMed] [Google Scholar]
- Dart C, Vaughan-Jones RD. Na+–HCO3− symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BA, Adorante JS, Miller SS, Lin H. Apical electrogenic NaHCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. J Gen Physiol. 1989;94:125–150. doi: 10.1085/jgp.94.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Schill BS, Schwartz P, Matthes H, Keller SK, Wiederholt M. Kidney epithelial cells of monkey origin (BSC-1) express a sodium bicarbonate cotransport. Characterization by 22Na+ flux measurements. J Biol Chem. 1985;260:15554–15560. [PubMed] [Google Scholar]
- Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, Bevensee MO, Boron WF, Bril A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res. 2001;52:387–396. doi: 10.1016/s0008-6363(01)00430-8. [DOI] [PubMed] [Google Scholar]
- Korn SJ, Marty A, Connor JA, Horn R. Perforated patch recording. Meth Neurosciences. 1991;4:364–373. [Google Scholar]
- Lagadic-Gossman DK, Buckler J, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guennec JV, Noble DJ. Effects of rapid changes of external Na+ concentration at different moments during the action potential in guinea-pig myocytes. J Physiol. 1994;478:493–504. doi: 10.1113/jphysiol.1994.sp020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Sodium-bicarbonate cotransport in retinal glia of the salamander. J Neuroscience. 1991;11:3972–3983. doi: 10.1523/JNEUROSCI.11-12-03972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, Pouyssegur J. Biochemical characterization of the amiloride sensitive Na+/H+ antiport in Chinese hamster lung fibroblasts. J Biol Chem. 1983;258:3503–3508. [PubMed] [Google Scholar]
- Poole-Wilson PA, Langer GA. Effect of pH on ionic exchange and function in rat and rabbit myocardium. Am J Physiol. 1975;229:570–581. doi: 10.1152/ajplegacy.1975.229.3.570. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta. 2000;1493:215–218. doi: 10.1016/s0167-4781(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Sandmann SYuM, Kaschina E, Blume A, Bouzinova E, Aalkjaer C, Unger T. Differential effects of angiotensin AT1 and AT2 receptors on the expression, translation and function of the Na+–H+ exchanger and Na+–HCO3− symporter in the rat heart after myocardial infarction. J Am Coll Cardiol. 2001;37:2154–2165. doi: 10.1016/s0735-1097(01)01287-6. [DOI] [PubMed] [Google Scholar]
- Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol. 2002;282:C408–C416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- Schafer C, Ladilov YV, Siegmund B, Piper HM. Importance of bicarbonate transport for protection of cardiomyocytes against reoxygenation injury. Am J Physiol Heart Circ Physiol. 2000;278:H1457–H1463. doi: 10.1152/ajpheart.2000.278.5.H1457. [DOI] [PubMed] [Google Scholar]
- Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+–HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol. 1999;277:F611–F623. doi: 10.1152/ajprenal.1999.277.4.F611. [DOI] [PubMed] [Google Scholar]
- Spitzer KW, Hogan PM. The effects of acidosis and bicarbonate on action potential repolarization in canine cardiac Purkinje fibers. J Gen Physiol. 1979;73:199–218. doi: 10.1085/jgp.73.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg JI, Metcalfe JC, Grace AA. Mechanisms of pHi recovery after global ischemia in the perfused heart. Circ Res. 1993;72:993–1003. doi: 10.1161/01.res.72.5.993. [DOI] [PubMed] [Google Scholar]
- Verdonck F, Volders PGA, Vos MA, Sipido KR. Intracellular Na+ and altered Na+ transport mechanisms in cardiac hypertrophy and failure. J Mol Cell Cardiol. 2003;35:5–25. doi: 10.1016/s0022-2828(02)00280-8. [DOI] [PubMed] [Google Scholar]
- Vila Petroff MG, Aiello EA, Palomeque J, Salas MA, Mattiazzi AR. Subcellular mechanisms of the positive inotropic effect of angiotensin II in cat myocardium. J Physiol. 2000;529:189–203. doi: 10.1111/j.1469-7793.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+–HCO3− cotransporter (NBCe2) Am J Physiol Cell Physiol. 2002;282:C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Swietach P, Rossini A, Loh SH, Vaughan-Jones RD, Spitzer KW. Functional diversity of electrogenic Na+–HCO3− cotransport in ventricular myocytes from rat, rabbit and guinea pig. J Physiol. 2005;562:455–475. doi: 10.1113/jphysiol.2004.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]