Abstract
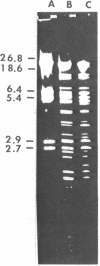
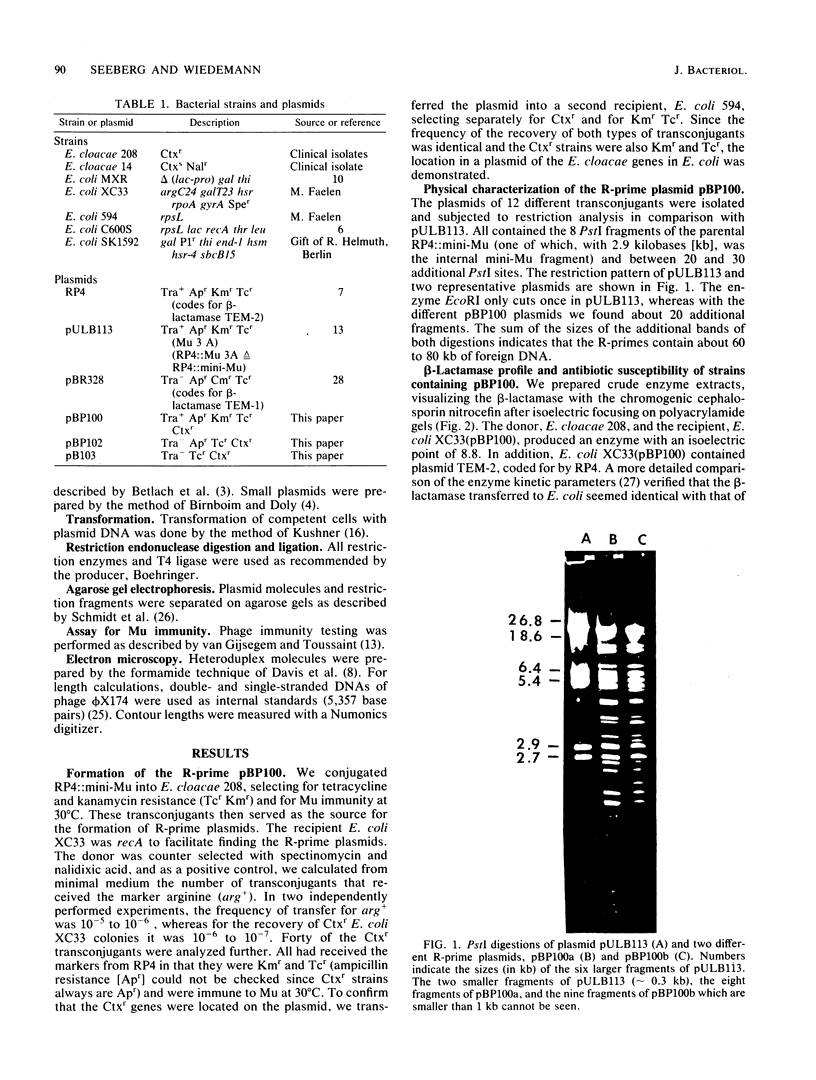
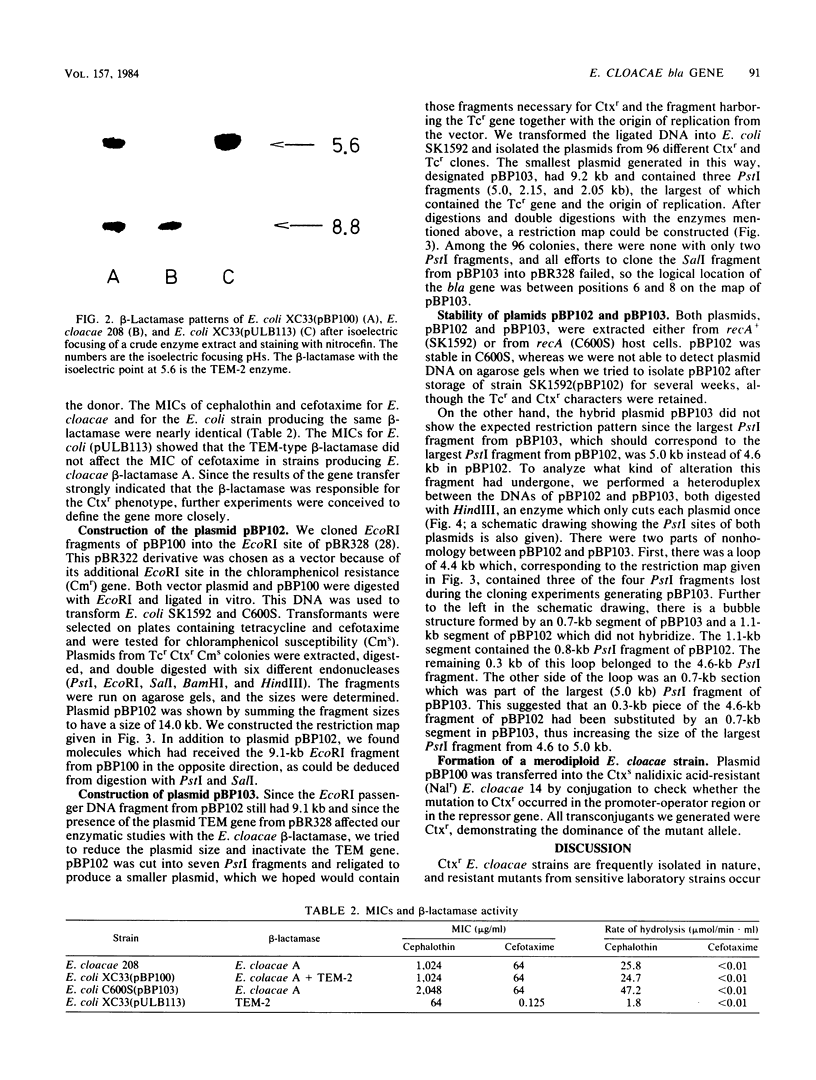
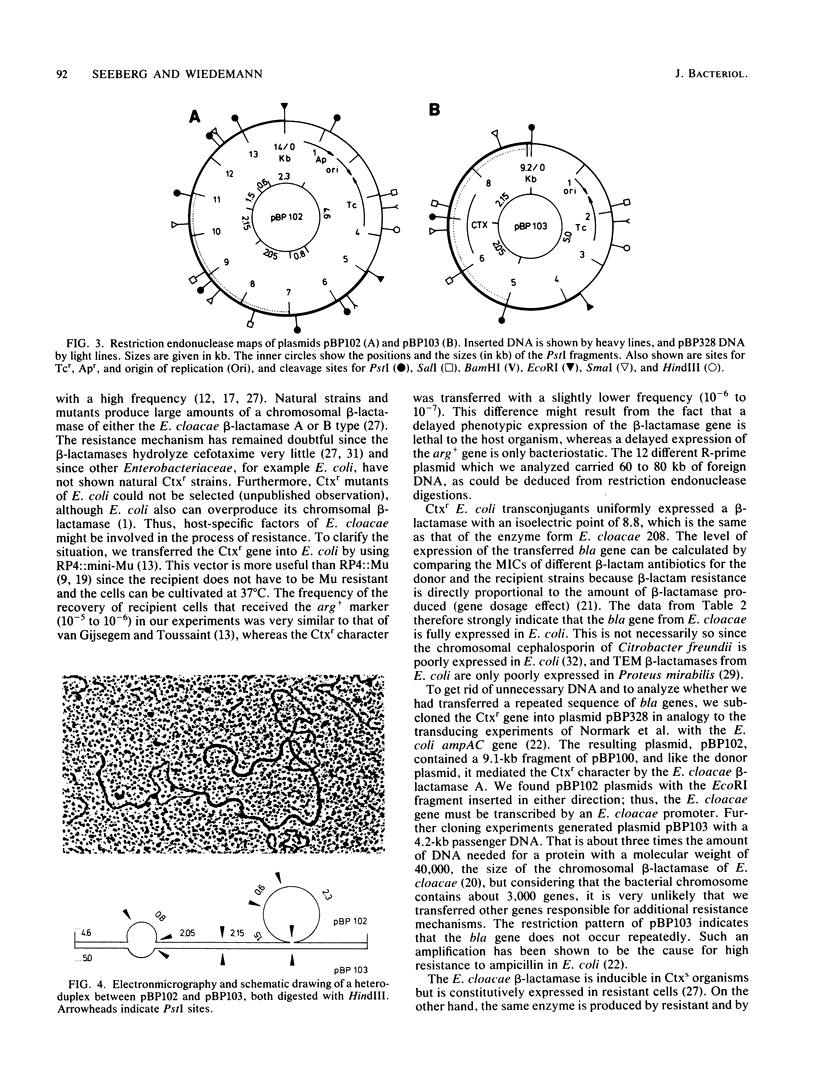
The resistance gene for beta-lactamase-stable cephalosporins from Enterobacter cloacae was transferred to Escherichia coli by the aid of RP4::mini-Mu. The R-prime plasmids generated carried 60 to 80 kilobases (kb) of E. cloacae DNA and coded for the chromosomal E. cloacae beta-lactamase. The gene was fully expressed in the recipient. Restriction endonuclease EcoRI fragments of the R-prime plasmid pBP100 were cloned into the vector pBP328, yielding the plasmid pBP102 with a size of 14 kb. A restriction map of this plasmid was constructed. By digesting pBP102 into seven PstI fragments, ligating the fragments, and looking for the smallest plasmid generated, pBP103 was isolated. It consisted of three PstI fragments, two of them (together 4.2 kb) necessary for resistance. During the experiment (performed in a recA+ background) the largest PstI fragment had undergone a substitution of a 0.3-kb segment of pBP102 by a 0.7-kb segment in pBP103 (as deduced by heteroduplex analysis). The bla gene of resistant E. cloacae strains was dominant over the gene of susceptible organisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström S., Normark S. Beta-lactam resistance in clinical isolates of Escherichia coli caused by elevated production of the ampC-mediated chromosomal beta-lactamase. Antimicrob Agents Chemother. 1979 Oct;16(4):427–433. doi: 10.1128/aac.16.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Olsson O., Normark S. Common evolutionary origin of chromosomal beta-lactamase genes in enterobacteria. J Bacteriol. 1982 May;150(2):528–534. doi: 10.1128/jb.150.2.528-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlach M., Hershfield V., Chow L., Brown W., Goodman H., Boyer H. W. A restriction endonuclease analysis of the bacterial plasmid controlling the ecoRI restriction and modification of DNA. Fed Proc. 1976 Jul;35(9):2037–2043. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causey S. C., Brown L. R. Transconjugant analysis: limitations on the use of sequence-specific endonucleases for plasmid identification. J Bacteriol. 1978 Sep;135(3):1070–1079. doi: 10.1128/jb.135.3.1070-1079.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faelen M., Resibois A., Toussaint A. Mini-mu: an insertion element derived from temperate phage mu-1. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1169–1177. doi: 10.1101/sqb.1979.043.01.132. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Krause J. M. Relationship Between beta-Lactamase Activity and Resistance of Enterobacter to Cephalothin. Infect Immun. 1970 Nov;2(5):610–616. doi: 10.1128/iai.2.5.610-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findell C. M., Sherris J. C. Susceptibility of Enterobacter to cefamandole: evidence for a high mutation rate to resistance. Antimicrob Agents Chemother. 1976 Jun;9(6):970–974. doi: 10.1128/aac.9.6.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T., Muraoka Y., Fujii A., Naganawa H., Takita T., Umezawa H. Chemistry of bleomycin. XXV. Reductive methylation of bleomycin, a chemical proof for the presence of the free secondary amine in bleomycin. J Antibiot (Tokyo) 1980 Jan;33(1):114–117. doi: 10.7164/antibiotics.33.114. [DOI] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lampe M. F., Allan B. J., Minshew B. H., Sherris J. C. Mutational enzymatic resistance of Enterobacter species to beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Apr;21(4):655–660. doi: 10.1128/aac.21.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S., Inoue M., Mitsuhashi S. Purification and properties of a cephalosporinase from Enterobacter cloacae. Antimicrob Agents Chemother. 1980 Dec;18(6):853–857. doi: 10.1128/aac.18.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Takizawa N., Harada T. Introduction of bacteriophage Mu into bacteria of various genera and intergeneric gene transfer by RP4::Mu. J Bacteriol. 1981 Jan;145(1):358–368. doi: 10.1128/jb.145.1.358-368.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Burman L. G. Resistance of Escherichia coli to penicillins: fine-structure mapping and dominance of chromosomal beta-lactamase mutations. J Bacteriol. 1977 Oct;132(1):1–7. doi: 10.1128/jb.132.1.1-7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Edlund T., Grundström T., Bergström S., Wolf-Watz H. Escherichia coli K-12 mutants hyperproducing chromosomal beta-lactamase by gene repetitions. J Bacteriol. 1977 Dec;132(3):912–922. doi: 10.1128/jb.132.3.912-922.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. L., Turner J. R., Mahoney D. F. Lack of correlation between beta-lactamase production and susceptibility to cefamandole or cefoxitin among spontaneous mutants of Enterobacteriaceae. Antimicrob Agents Chemother. 1979 Jan;15(1):14–19. doi: 10.1128/aac.15.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V. In vitro antagonism of beta-lactam antibiotics by cefoxitin. Antimicrob Agents Chemother. 1982 Jun;21(6):968–975. doi: 10.1128/aac.21.6.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Schmidt F., Van Treeck U., Wiedemann B. Multimerization and replication of plasmid pBP11. Plasmid. 1982 Sep;8(2):126–140. doi: 10.1016/0147-619x(82)90051-8. [DOI] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gijsegem F., Toussaint A. Chromosome transfer and R-prime formation by an RP4::mini-Mu derivative in Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae, and Proteus mirabilis. Plasmid. 1982 Jan;7(1):30–44. doi: 10.1016/0147-619x(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Murayama S. Y., Sawai T. Cloning and expression of the gene(s) for cephalosporinase production of Citrobacter freundii. Mol Gen Genet. 1983;190(1):85–91. doi: 10.1007/BF00330328. [DOI] [PubMed] [Google Scholar]