Abstract
Many human diseases are caused by mutations in ion channels. Dissecting the pathogenesis of these ‘channelopathies’ has yielded important insights into the regulation of vital biological processes by ions and has become a productive tool of modern ion channel biology. One of the best examples of a synergism between the clinical and basic science aspects of a modern biological topic is cystic fibrosis. Not only did the identification of the ion channel mutated in cystic fibrosis pinpoint the root cause of this disease, but it also has significantly advanced our understanding of basic biological processes as diverse as protein folding and epithelial fluid and electrolyte secretion. The list of confirmed ‘channelopathies’ is growing and several members of the TRP family of ion channels have been implicated in human diseases such as mucolipidosis type IV (MLIV), autosomal dominant polycystic kidney disease (ADPKD), familial focal segmental glomerulosclerosis (FSG), hypomagnesemia with secondary hypocalcaemia (HSH), and several forms of cancer. Analysing pathogenesis of the diseases linked to TRP dysregulation provides an exciting means of identifying novel functions of TRP channels.
Pathogenesis of some ‘TRPpathies’, such as HSH, appears straightforward. HSH manifests as low blood Mg2+ due to deficient renal and intestinal Mg2+ re-absorption (reviewed in Konrad et al. 2004). HSH has been linked to several mutations in the gene TRPM6, which codes for the TRPM6 channel (Schlingmann et al. 2002; Walder et al. 2002) (chromosomal localization of this and other TRP coding discussed in this review can be found in Table 1). This Mg2+-selective channel is predominantly expressed in the intestinal epithelium and in the kidney (Schlingmann et al. 2002; Walder et al. 2002), and some of the mutations shown to induce HSH result in an inactive channel in recombinant system (Voets et al. 2004). Some other HSH-linked mutations affect TRPM6 interaction with TRPM7 (Chubanov et al. 2004), its close relative linked to Guamanian amyotrophic lateral sclerosis and parkinsonism dementia (Hermosura et al. 2005). Since many TRP channels hetero-multimerize in order to form functional channels (Strubing et al. 2001; Goel et al. 2002; Strubing et al. 2003), the loss of TRPM6–TRPM7 interaction results in the loss of channel function (Chubanov et al. 2004).
Table 1.
TRP channels implicated in genetic disorders
| TRP channel | Gene | Chromosomal localization (human/mouse) | Associated disorder | Effect of mutation | Causative relationship | References |
|---|---|---|---|---|---|---|
| TRP channels linked to human diseases | ||||||
| TRP-ML1 | MCOLN1 | 19p13.3-p13.2 | MLIV | Down/change in selectivity or localization | Yes | (Bassi et al. 2000; Sun et al. 2000; Bach, 2001; Slaugenhaupt, 2002; LaPlante et al. 2004; Manzoni; et al. 2004,Raychowdhury et al. 2004; Cantiello et al. 2005; Kiselyov et al. 2005) |
| 8 A1.1 | ||||||
| TRPP2 | PKD2 | 4q21-q23 | ADPK2 | Down | Yes | (Koptides & Deltas, 2000; Boucher & Sandford, 2004) |
| 5 E5 | ||||||
| TRPC6 | TRPC6 | 11q21-q22 | Prostate cancer | Up | ? | (Buess et al. 1999; Thebault et al. 2006) |
| 9 A1 | ||||||
| — | — | — | FSG | Up/? | Yes/? | (Reiser et al. 2005; Winn et al. 2005) |
| TRPV6 | TRPV6 | 7q33-q34 | Prostate cancer | Up | Increased proliferation | (Peng et al. 2001; Wissenbach et al. 2001; Fixemer et al. 2003; Wissenbach et al. 2004; Schwarz et al. 2006) |
| TRPM1 | TRPM1 | 15q13-q14 | Cutaneous melanoma | Down | ? | (Duncan et al. 1998; Fang & Setaluri, 2000) |
| 7 C | ||||||
| TRPM6 | TRPM6 | 9q21.13 | Hypomagnesemiawith secondary hypocalcaemia | Down or disrupted interaction with TRPM7 | Yes | (Schlingmann et al. 2002; Walder et al. 2002; Chubanov et al. 2004; Voets et al. 2004; Schlingmann et al. 2005) |
| 19 B | ||||||
| TRPM7 | TRPM7 | 15q21 | Guamanian amyotrophic lateral sclerosis, parkinsonism dementia | Mutations that increase inhibition by Mg | Yes | (Hermosura et al. 2005) |
| 2 F2 | ||||||
| TRPM8 | TRPM8 | 2q37.1 | Prostate, breast, colon, lung, and skin tumers | Up/Down | Increased cell survival | (Tsavaler et al. 2001; (Fuessel et al. 2003; Henshall et al. 2003; Zhang & Barritt, 2004, 2006) |
| 1 D | ||||||
| Phenotypes in mice with altered TRP channels | ||||||
| TRPC2 | TRPC2 | 11p15.4-p15.3 | Altered sexual (pseudogene) | Knock-out and social behaviour | Pheromone recognition | (Leypold, 2002; Stowers et al. 2002) |
| 7 F1 | ||||||
| TRPC3 | TRPC3 | 4q27 | Cardiac hypertrophy or cardiomyopathy in mouse heart | Transgenic expression of human TRPC3 | Ca2+ entry | (Nakayama et al. 2006) |
| 3 B | ||||||
| TRPC4 | TRPC4 | 13q13.1-q13.2 | Impaired regulation of vascular tone and vascular permeability | Knock-out | Ca2+ entry | (Freichel et al. 2001; Tiruppathi et al. 2002; Freichel et al. 2004) |
| 3 D | ||||||
| TRPC6 | TRPC6 | 11q21-q22 | Blood pressure regulation | Knock-out | Regulation of smooth muscle contractility in mice | (Dietrich et al. 2005b) |
| 9 A1 | ||||||
| TRPM5 | TRPM5 | 11p15.5 | Suppressed taste; impaired thermal sensitivity of taste | Knock-out | Taste sensation, Thermosensation | (Zhang et al. 2003; Talavera et al. 2005; Damak et al. 2006) |
| 7 F5 | ||||||
| — | — | — | Beckwith-Wiedemann syndrome (BWS) | ? | Aberrant imprinting in the gene cluster containing TRPM5* | (Enklaar et al. 2000; Prawitt et al. 2000) |
| TRP-ML3 | MCOLN3 | 1p22.3 | Pigmentation defects, hearing loss due to hair cell degeneration | Mutations (knock-out is perinatal lethal) | ? | (Di Palma et al. 2002) |
| 3 H2 | ||||||
| TRPP2 | PKD2 | 4q21-q23 | Kidney cysts, liver lesions, cerebral arterial lesions | Knock-out | Mechanically induced Ca2+ influx | (Wu et al. 1998; Qian et al. 2003; Thomson et al. 2003; Gallagher et al. 2006) |
| 5 E5 | ||||||
| TRPP3 | PKD2L1 | 10q24 | Kidney and retinal defects | Deletion in the region containing PKD2L1** | ? | (Nomura et al. 1998) |
| 19 C3 | ||||||
| TRPV1 | TRPV1 | 17p13.3 | Abnormal bladder. contractions. Diminished heat sensation, thermal hyperalgesia, fever production,allodynia, neuropathic pain | Knock-out, Down | Mechanosensation?, Thermosensation | (Caterina et al. 2000; Davis et al. 2000; Kamei et al. 2001; Birder et al. 2002; Walker et al. 2003; Karai et al. 2004; Iida et al. 2005; Jhaveri et al. 2005; Kanai et al. 2005; Christoph et al. 2006) |
| 3 B | ||||||
| TRPV2 | TRPV2 | 17p11.2 | Dystrophic patients and animal models | Increase in sarcolemma | Mechanosensation, Thermosensation | (Iwata et al. 2003) |
| 11 B2 | ||||||
| TRPV3 | TRPV3 | 17p13.3 | Deficient response to noxious and non-noxious heat; dermatitis and hairlessness | Knock-out/? | (Smith et al. 2002; Xu et al. 2002; Moqrich et al. 2005; Asakawa et al. 2006) | |
| 11 B4 | ||||||
| TRPV4 | TRPV4 | 12q24.1 | Hearing impairments, Abnormal thermal selection and osmotic regulation | Knock-out | Mechanosensation, Thermosensation | (Mizuno et al. 2003; Todaka et al. 2004; Lee et al. 2005; Tabuchi et al. 2005) |
| 5 F | ||||||
| TRPV5 | TRPV5 | 7q35 | Hypercalciuria | Knock-out | Ca2+ reabsorbtion | (Hoenderop et al. 2003) |
| TRPA1 | TRPA1 | 8q13 | Impaired cold, mechanical and chemical nociception | Antisense knockdown, Knock-out | Thermosensation, mechanosensation | (Story et al. 2003; Obata et al. 2005; Bautista et al. 2006; Katsura et al. 2006; Kim et al 2006;. Kwan et al. 2006) |
| 1 A3 | ||||||
Only the channels with confirmed involvement in human diseases or the channels whose mouse knock-out models show clear aberrant physiological function are listed.
Although TRPM5 is known to reside within a cluster of genes affected in BWS, no clear connection between BWS and TRPM5 up/down-regulation has been shown.
Krd (kidney and retinal defects) mice have a deletion in the region containing PKD2L1 gene and show kidney agenesis or cysts and retinal degeneration. TRPP3 is deleted in Krd mice.
Perhaps the best-known example of a pathologically relevant TRP channel is TRPV1. Cloned as a result of a search for molecular determinants of perception of heat and the noxious compound in hot pepper, capsaicin (Caterina et al. 1997; Tominaga et al. 1998), TRPV1 seems to be involved in an array of functions involving perception of heat and chemical pain (Table 1, see also (Clapham, 2003; Nilius et al. 2005)). TRPV1 is clearly a promising target for pharmacological interventions into pain, cough, inflammation and urinary problems (reviewed in Nagy et al. 2004; Szallasi & Appendino, 2004; Jia et al. 2005).
The causal relationships between dysregulation of TRP channels and the corresponding genetic diseases remain obscure for several human TRPpathies. Some diseases, such as ADPKD, attracted enormous interest in recent years, which resulted in a significant degree of understanding of the physiology of the corresponding TRP channels if not of the exact connection between the channel dysregulation and the disease. As discussed below, the same is largely true for TRPC6, TRPV6 and cancer as well as for TRPC6 and FSG. Very limited information exists about localization, permeation properties or regulation of TRPM1, despite clearly documented links to skin cancer. Several recent reports, focused on the function of the lysosomal ion channel TRP-ML1, mutations in which are responsible for lysosomal storage disorder MLIV, have suggested several possible roles of this ion channel in regulating lysosomal function. The present review will focus on these TRPpathies, and will specifically discuss some unanswered questions pertaining to pathogenesis of these diseases and the roles of the corresponding ion channels in maintaining normal cellular function.
To illustrate the broad physiological roles of TRP channels, Table 1 lists the physiological functions of TRP channels derived from genetic diseases, in in vitro experiments and from knock-out mice. Additional information on the biology, physiological functions and therapeutic potential of TRP channels can be found in several excellent recent reviews (Clapham, 2003; Nilius et al. 2005) and a series of reviews published in Cell Calcium (volume 33, issues 5–6, 2003) and Pflugers Archiv (volume 451, number 1, 2005).
Mucolipin 1 and MLIV
MLIV is a neurodegenerative disorder with an early onset. Patients with MLIV display severe psychomotor retardation and a developmental delay (reviewed in Bach, 2001; Slaugenhaupt, 2002). Other clinical manifestations of MLIV include achloridia and hypergastrinaemia (Schiffmann et al. 1998; Lubensky et al. 1999). At the cellular level, MLIV is a classic lysosomal storage disease with accumulation, in virtually all tissues and cells, of electron-dense vesicles and membranous inclusions containing phospholipids (Bach & Desnick, 1988; Bargal & Bach, 1989) and gangliosides (Zeigler & Bach, 1986). Proteolysis defects have not been shown in MLIV.
MLIV is caused by nonsense or missense mutations in the gene MCOLN1, which codes for TRP-ML1, a member of the TRP-ML subfamily (Bassi et al. 2000; Sun et al. 2000). The mutations result in deletion or affect cellular localization or ion selectivity and permeability of TRP-ML1 (LaPlante et al. 2004; Manzoni et al. 2004; Raychowdhury et al. 2004; Cantiello et al. 2005; Kiselyov et al. 2005).
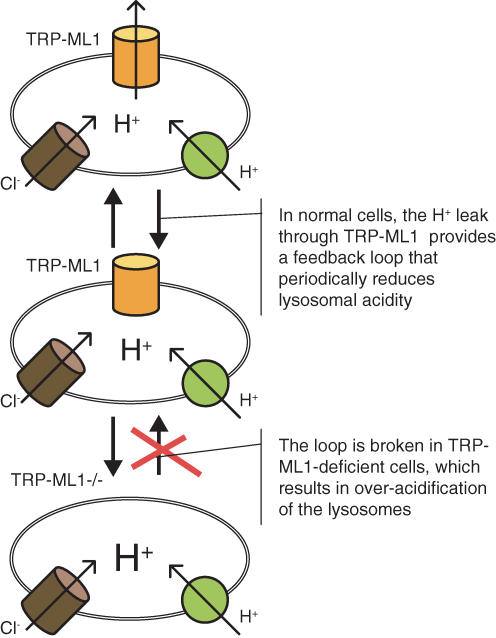
TRP-ML1 is a lysosomal ion channel (Manzoni et al. 2004; Kiselyov et al. 2005; Miedel et al. 2006; Soyombo et al. 2006; Vergarajauregui & Puertollano, 2006), and is therefore expected to regulate lysosomal ion content, although its role in lysosomal function is still not known in full. TRP-ML1 was reported to be a Ca2+ channel (LaPlante et al. 2002, 2004), or an outwardly rectifying monovalent cation channel regulated by either Ca2+ (Cantiello et al. 2005) or pH (Raychowdhury et al. 2004). Our recent work shows that TRP-ML1 limits lysosomal acidification by providing a lysosomal H+ leak pathway (Soyombo et al. 2006) (Fig. 1). H+ is a critical lysosomal ion that regulates numerous lysosomal functions. The acidification of the lysosomal lumen is mediated by a vacuolar H+ pump (Beyenbach & Wieczorek, 2006) and ClC family Cl− channels (Jentsch et al. 2005). A H+ leak mechanism that limits lysosomal acidification has been proposed but not identified. We suggest that in the absence of TRP-ML1 the lysosomes are chronically overacidified. In contrast with our findings, Bach et al. (1999) reported normal lysosomal pH in MLIV fibroblasts. The reason for the different findings is not known. However, we note that (a) we used two different techniques to estimate lysosomal pH, (b) MLIV cells are particularly sensitive to the weak base chloroquine (Goldin et al. 1999; Soyombo et al. 2006), which can only be if their lysosomes are more acidic than the normal lysosomes, (c) TRP-ML1 is permeable to H+ and (d), the MLIV phenotype can be reversed by dissipating lysosomal pH (Soyombo et al. 2006).
Figure 1.
Function of TRP-ML1 as a lysosomal H+ leak valve Lysosomal acidification is mediated by V-type H+ pumps and ClC Cl− channels. At moderate lysosomal pH (top), TRP-ML1 provides a H+ leak to limit lysosomal acidification. Cleavage of TRPML1 by a Cathapsin B-mediated mechanism leads to further acidification of the lysosomes (middle). Arrival of a new TRP-ML1 increases lysosomal H+ leak to reestablish the moderate acidic state (top). The cycle is repeated resulting in oscillation in lysosomal pH between the moderate and acidic states. In the absence of TRP-ML1, the H+ leak valve does not work, which results in chronically over-acidified lysosomes.
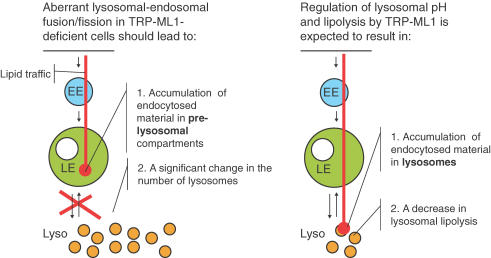
TRP-ML1 has also been suggested to modulate lysosomal biogenesis by mediating fusion of lysosomes with late endosomes or fission of lysosomes from hybrid organelles (LaPlante et al. 2004; Piper & Luzio, 2004; Treusch et al. 2004). The biogenesis model integrates results obtained in human MLIV fibroblasts and in C. elegans deficient in the TRP-ML1 homologue CUP-5. An exchange between lysosomal and late endosomal content was reported to be affected in MLIV fibroblasts (LaPlante et al. 2002), which was taken to indicate that down-regulation of TRP-ML1 impedes the fusion of lysosomes with endosomes. On the other hand, the ablation of CUP-5 in C. elegans increased retention of lysosomal markers in late endosomes, which could be reversed by knocking-in the human TRP-ML1 (Treusch et al. 2004). This was interpreted as delayed reformation of lysosomes from the late endosomes in TRP-ML1-deficient cells and TRP-ML1 was suggested to promote the fission of lysosomes from late endosomes. Although an elegant hypothesis, several findings are not consistent with a primary role of TRP-ML1 in regulation of fusion/fission of endosomes/lysosomes. A primary role of TRP-ML1–membrane interaction events predicts that the loss of TRP-ML1 has to result in (a) accumulation of undigested lipids in pre-lysosomal compartments and (b) a marked change in the number of lysosomes (Fig. 2). Several reports, however, showed accumulation of lipids in the lysosomes of MLIV cells (Chen et al. 1998; Jansen et al. 2001; Soyombo et al. 2006), with no dramatic change in the lysosomal numbers (Treusch et al. 2004; Soyombo et al. 2006). It is likely that abnormal membrane trafficking in MLIV is secondary to accumulation of undigested material in these cells.
Figure 2.
A comparison of the expected consequences of a role of TRP-ML1 in regulation of lysosomal biogenesis (left) or reduction in lysosomal acidity and hydrolytic activity (right) The biogenesis model proposes that TRP-ML1 regulates fusion of lysosomes (Lyso) with late endosomes (LE) or reformation of lysosomes from hybrid organelles and their accumulation in the cytoplasmic pool. Consequently, in the absence of TRP-ML1 the exchange of endocytosed material between late endosomes and the cytoplasmic pool of lysosomes would be impaired and the endocytosed material would accumulate in late endosomes. This should lead to a significant change in the numbers of lysosomes. Regulation of lysosomal pH by TRP-ML1 leads to an alternative model in which lysosomal overacidification in the absence of TRP-ML1 affects lysosomal lipolysis and perhaps proteolysis. This model predicts accumulation of endocytosed material in lysosomes and a significant decrease in lysosomal hydrolytic activity.
A particularly interesting aspect of TRP-ML1 physiology is its proteolytic cleavage in the lysosomes (Kiselyov et al. 2005; Miedel et al. 2006; Vergarajauregui & Puertollano, 2006). Since the full length TRP-ML1 seems to be the active form (Raychowdhury et al. 2004; Kiselyov et al. 2005), it is reasonable to propose that the cleavage is the major form of regulation of the channel. The full-length TRP-ML1 appears to be constitutively active (Raychowdhury et al. 2004; Kiselyov et al. 2005). The ‘always-on’ modus operandi of TRP-ML1 is consistent with its role in H+ leak, or a shunt-like activity. Thus, the cleavage may be a mechanism to limit its activity to a selective subset of organelles in the lysosomal degradation pathway. The only other form of TRP-ML1 regulation discovered so far is by divalent ions (Cantiello et al. 2005; Soyombo et al. 2006), raising the possibility that changes in cytoplasmic Ca2+ ([Ca2+]i) during cell stimulation may acutely regulate channel activity.
Polycystin 2 and ADPKD
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited late onset renal disorder characterized by formation of kidney cysts leading to renal failure. ADPKD also has extrarenal effects that include formation of cysts in the pancreas, liver and spleen, hypertension and brain aneurisms. About 20% of the cases of ADPKD are associated with mutations in the gene PKD2, which codes for the ion channel polycystin 2 (TRPP2) (reviewed in Koptides & Deltas, 2000; Boucher & Sandford, 2004). TRPP2 physically interacts with polycystin 1 (PC1), which is probably involved in cell–cell communication and establishment of cellular junctions. Mutations in the gene coding for PC1 are responsible for the remaining cases of ADPKD.
TRPP2 is a cation channel with limited selectivity for Ca2+ (Gonzalez-Perrett et al. 2001; Koulen et al. 2002). Depending on cell type and expression system, TRPP2 localizes at the endoplasmic reticulum (Koulen et al. 2002), the primary cilia (Yoder et al. 2002; Nauli et al. 2003; Raychowdhury et al. 2005; Geng et al. 2006), the apical pole (Gonzalez-Perrett et al. 2001) or the basolateral surface of epithelial cells (Foggensteiner et al. 2000).
Why mutations in TRPP2 lead to formation of the fluid-filled kidney cysts is unclear. Localization of TRPP2 in the primary cilia led to a model in which TRPP2 reads the mechanical disturbance of the cilial apparatus in response to flow (Nauli et al. 2003; Ong & Wheatley, 2003; Nauli & Zhou, 2004). TRPP2 responds to mechanical stimulation (Montalbetti et al. 2005) and the association of TRPP2 with the cytoskeletal elements tropomyosin-1 (Li et al. 2003a), troponin I (Li et al. 2003b), α-actinin (Li et al. 2005a) and HS1-Associated Protein X1 (Hax-1) Gallagher et al. 2000) further supports the role of TRPP2 in mechanotransduction. The sensitivity of TRPP2 to mechanical stimuli is probably regulated by hormones, neurotransmitters and growth factors since stimulation of phospholipase C-coupled receptors activates TRPP2 (Ma et al. 2005).
The mechanotransduction model postulates that activation of TRPP2 by mechanical deflection of the cilia induces local Ca2+ influx, which propagates into the cell interior by Ca2+-induced Ca2+ release through activation of ryanodine- and inositol (1,4,5) trisphosphate receptors (Nauli et al. 2003), or perhaps by activation of the endoplasmic reticulum resident TRPP2 (Koulen et al. 2002). The flow-induced Ca2+ responses in tubular epithelial cells mediated by TRPP2 are probably necessary for reporting changes in flow rate and fluid osmolarity. This can explain the particular susceptibility to ADPKD of the kidney, pancreatic and biliary duct and spleen, all of which experience large fluctuations in fluid flow and osmolarity.
How the TRPP2-mediated Ca2+ fluxes translate to the downstream cellular response has not been elucidated. The TRPP2-mediated Ca2+ fluxes probably have acute and long-term effects. The latter may include gene activation and regulation of cell proliferation.
TRPP2 was shown to be a cofactor in PC-1-dependent cell cycle arrest induced by activation of the JAK-STAT signalling pathway (Bhunia et al. 2002). Furthermore, the C terminus of TRPP2 binds the transcriptional suppressor Id2 (Li et al. 2005b), a member of an ‘inhibitor of DNA binding’ (Id) helix–loop–helix transcription factor subfamily. The Id family proteins lack DNA binding domains but they bind helix–loop–helix transcription factors, and inhibit their binding to DNA. Depending on cell type, this may inhibit or promote cell differentiation and proliferation (reviewed in Perk et al. 2005). In the absence of TRPP2 or PC1 most of Id2 is found in the nucleus. TRPP2 sequesters Id2 in the cytosol (Li et al. 2005b), which up-regulates a cyclin-dependent kinase (CDK) inhibitor p21 and down-regulates Cdk2. These findings suggest that the predominantly nuclear localization of Id2 in TRPP2- (or PC1-) deficient cells affects the p21-CDK cell cycle regulation cascade and results in aberrant cell growth (Li et al. 2005b). The role of TRPP2 Ca2+ transport function in either of these processes is unknown. Both effects, however, can account for the inverse relation between the levels of TRPP2 in kidney tissues and cell growth rates (Chang et al. 2006; Grimm et al. 2006).
Melastatin and cutaneous melanomas
A search for molecular correlates of the metastatic potential of human cutaneous melanomas has led to the identification of another TRP member, melastatin 1 (TRPM1). A loss of TRPM1 mRNA in metastasizing skin cancer is as robust a predictor of melanoma progression as any of the commonly accepted criteria (Duncan et al. 1998).
Normal and benign melanocytes express the full-length TRPM1 mRNA of approximately 5.4 kb along with some shorter products (Duncan et al. 1998; Fang & Setaluri, 2000). Metastatic melanomas and pigmented metastatic melanoma cell lines lack the full-length transcript, but express several short fragments of TRPM1 mRNA (Duncan et al. 1998; Fang & Setaluri, 2000). The anticancer drug hexamethylene bisacetamide (HMBA) reverses the loss of the full-length TRPM1 mRNA (Fang & Setaluri, 2000). Although the latter observation links melanoma progression to the loss of TRPM1, it does not establish a causative relation between the two.
TRPM1 mRNA levels in melanocytes and melanoma cell lines seem to depend on the melanocyte-specific transcription factor MITF as the TRPM1 promoter is under the MITF control and overexpression of MITF increases expression of TRPM1 (Miller et al. 2004; Zhiqi et al. 2004).
The channel properties and physiological function of TRPM1 have not been explored methodically. The only recording of TRPM1 activity was obtained with recombinant channel expressed in HEK 293 cells, where expression of the full-length TRPM1 dramatically increased resting [Ca2+]i (Xu et al. 2001). When expressed alone, the full-length channel was targeted to the plasma membrane, while coexpression of the full-length and the short isoforms resulted in retainment of the full-length TRPM1 in the endoplasmic reticulum (Xu et al. 2001). It is currently unknown whether expression of TRPM1 in metastasizing lines inhibits their growth. The situation is further complicated by the fact that the native TRPM1 protein has not been identified and it was reported that normal melanocytes do not express noticeable levels of the predicted full-length TRPM1. A series of smaller products is detected instead, which was attributed to proteolysis of the full length protein (Zhiqi et al. 2004).
The properties and cellular localization of other TRP channels involved in cancer are somewhat better established. TRPC6 is a plasma membrane channel permeable to monovalent cations, modestly selective for Ca2+ and involved in Ca2+ influx induced by activation of G protein-coupled receptors (Estacion et al. 2004, 2006). TRPC6 was reported to be down-regulated in a murine autocrine tumour model (Buess et al. 1999) and to mediate the Ca2+ influx that maintains growth of prostate cancer epithelial cells (Thebault et al. 2006).
The highly Ca2+-selective TRP channel, TRPV6, a vanilloid receptor homologue, is up-regulated in prostate cancer (Peng et al. 2001; Wissenbach et al. 2001, 2004; Fixemer et al. 2003; Schwarz et al. 2006) and in breast, thyroid, colon, and ovarian carcinomas (Zhuang et al. 2002). Chronic overexpression of TRPV6 reversibly increases proliferation of HEK 293 cells (Schwarz et al. 2006).
Another TRP channel associated with cancer is TRPM8. TRPM8 is a non-selective cation channel, that mediates cold sensation and response to menthol in neuronal cells (Peier et al. 2002) is also up-regulated in prostate cancer (Tsavaler et al. 2001; Fuessel et al. 2003; Henshall et al. 2003; Zhang & Barritt, 2006). Strikingly, TRPM8 seems to be lost at the very advanced stages of prostate cancer (Henshall et al. 2003). TRPM8 expression is regulated by androgen (Henshall et al. 2003; Zhang & Barritt, 2004; Bidaux et al. 2005) and is required for the survival of the androgen-sensitive LNCaP cell line (Zhang & Barritt, 2004).
Although a connection between apoptosis, cancer and [Ca2+]i has been established, it is not clear why up- or down-regulation of these specific TRP channels induce cell transformation towards the cancerous phenotype. The remarkable plasticity of the cellular Ca2+ signalling machinery (Zhao et al. 2001) would probably allow the cells to adapt to a change in TRP channel activity in order to maintain normal Ca2+ signalling. It is likely that these TRP channels have a specific role in a regulatory protein complex in which they reside. For example, by colocalizing at the cell junctional complexes, TRP channels may participate in the regulation of cell adhesion and neighbour sensing. Studying the role of the cancer-associated TRP channels in their cellular context should test these possibilities.
TRPC6 and familial focal segmental glomerulosclerosis
Recent studies linked mutations in TRPC6 to familial focal segmental glomerulosclerosis (FSG), a form of nephropathy due to aberrant glomerular filtration (Reiser et al. 2005; Winn et al. 2005). FSG causes proteinuria, nephritic syndrome and a progressive loss of renal function, often resulting in end-stage renal disease (Daskalakis & Winn, 2006). Glomerular filtration is regulated by the slit diaphragm, which forms the renal filtration barrier. The glomerular podocytes with their foot processes are central component of the slit diaphragm (Somlo & Mundel, 2000). The podocytes are contractile cells that actively regulate glomerular permeability. Several structural proteins of podocytes participate in assembly or regulation of the slit diaphragm. Among them are nephrin, a 185 kDa single transmembrane spanning protein, localizing at signalling domains in the podocyte foot structure (Ruotsalainen et al. 1999) and podocin, a 42 kDa single transmembrane spanning protein that is found at the base of the podocyte foot structure (Roselli et al. 2002). Podocin interacts with nephrin and with a CD2-associated protein CD2AP (Schwarz et al. 2001). Mutations in these structural proteins and in α-actinin 4 (Mathis et al. 1998; Kaplan et al. 2000) have been linked to the familial forms of FSG. Mutations in NPHS1 that codes for nephrin and in NPHS2 that codes for podocin are responsible for the autosomal recessive forms of FSG (Kestila et al. 1998), whereas mutations in ACTN4 coding for α-actinin 4 cause autosomal dominant form of FSG (Mathis et al. 1998).
Another autosomal dominant form of FSG was, unexpectedly, linked to mutations in TRPC6. In the glomerulus, TRPC6 is expressed at high levels in the podocyte foot structure, which determines glomerular permeability to macromolecules, including proteins. In the podocytes, TRPC6 interacts with nephrin and podocin, but not with CD2AP (Reiser et al. 2005; Winn et al. 2005). Mutations in TRPC6 associated with FSG were found in several cohorts and appear to fall into two categories: mutations that result in channel activation (Reiser et al. 2005; Winn et al. 2005), and mutations that had no apparent effect on channel activity (Reiser et al. 2005). The activating mutation P112Q was shown to increase the surface expression of TRPC6 (Reiser et al. 2005; Winn et al. 2005). The mechanism by which the R895C and E897K increase TRPC6 activity is not known.
It is possible that the activating mutations in TRPC6 result in an increased basal [Ca2+]i to cause a tonic contraction of the podocytes and a persistent increase in glomerular permeability to macromolecules. The cause and effect for the mutations that do not increase TRPC6 activity is still to be determined. These mutations may boost TRPC6 sensitivity to stimulation, alter protein turnover or alter interaction with nephrin, podocin or with other TRPC channels. Podocytes express TRPC1, TRPC5 and TRPC6 (Reiser et al. 2005). TRPC6 function within a hetero-multimeric TRPC channels complex (Strubing et al. 2003; Bandyopadhyay et al. 2005; Dietrich et al. 2005a). It is thus possible that mutations that did not affect the activity of heterologously expressed TRPC6 may affect Ca2+ influx when TRPC6 is present in hetero-multimers.
It is unclear why some of the probands analysed by Reiser et al. were positive for such mutations, but did not show the kidney pathology (Reiser et al. 2005). The latter, and the lack of a clear kidney phenotype in the TRPC6 knock-out mice (Dietrich et al. 2005b), suggests that an unidentified genetic modifier is also involved in FSG pathogenesis. Indeed, it has been known since the 1970s that a circulating humoral factor is associated with FSG (Shalhoub, 1974). The involvement of a circulating factor was postulated based on the observations of recurrent proteinuria in transplant patients who were diagnosed with FSG (Ohta et al. 2001), induction of proteinuria in rats injected with serum from patients with FSG (Zimmerman, 1984) and remission of the proteinuria by removal of serum proteins by treatment with protein A–Sepharose (Dantal et al. 1994). It is possible that variations in the levels of the circulating factors between patients or with time may account for variability of manifestations of the disease and its timing among patients carrying mutations in TRPC6.
In conclusion, several human diseases highlight the key role that TRP channels play in cellular physiology. Tracing mutations in ion channels that are associated with human diseases provides an excellent tool to reveal their cellular function. Such studies can also identify the signalling pathway that interprets signals initialed by the ion channels in the specific cellular environment in which they function. An invaluable advantage of these systems is that they have very distinct phenotypes, which facilitates testing novel models and hypotheses.
Acknowledgments
The authors wish to thank Dr Oxana Ibraghimov-Beskrovnaya for critical comments.
References
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, Sakata T. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126:2664–2672. doi: 10.1038/sj.jid.5700468. [DOI] [PubMed] [Google Scholar]
- Bach G. Mucolipidosis type IV. Mol Genet Metab. 2001;73:197–203. doi: 10.1006/mgme.2001.3195. [DOI] [PubMed] [Google Scholar]
- Bach G, Chen CS, Pagano RE. Elevated lysosomal pH in mucolipidosis type IV cells. Clin Chim Acta. 1999;280:173–179. doi: 10.1016/s0009-8981(98)00183-1. [DOI] [PubMed] [Google Scholar]
- Bach G, Desnick RJ. Lysosomal accumulation of phospholipids in mucolipidosis IV cultured fibroblasts. Enzyme. 1988;40:40–44. doi: 10.1159/000469140. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, Ambudkar IS. Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J Biol Chem. 2005;280:12908–12916. doi: 10.1074/jbc.M410013200. [DOI] [PubMed] [Google Scholar]
- Bargal R, Bach G. Phosphatidylcholine storage in mucolipidosis IV. Clin Chim Acta. 1989;181:167–174. doi: 10.1016/0009-8981(89)90184-8. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21 (waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Bidaux G, Roudbaraki M, Merle C, Crepin A, Delcourt P, Slomianny C, Thebault S, Bonnal JL, Benahmed M, Cabon F, Mauroy B, Prevarskaya N. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr Relat Cancer. 2005;12:367–382. doi: 10.1677/erc.1.00969. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Boucher C, Sandford R. Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2) Eur J Hum Genet. 2004;12:347–354. doi: 10.1038/sj.ejhg.5201162. [DOI] [PubMed] [Google Scholar]
- Buess M, Engler O, Hirsch HH, Moroni C. Search for oncogenic regulators in an autocrine tumor model using differential display PCR: identification of novel candidate genes including the calcium channel mtrp6. Oncogene. 1999;18:1487–1494. doi: 10.1038/sj.onc.1202445. [DOI] [PubMed] [Google Scholar]
- Cantiello HF, Montalbetti N, Goldmann WH, Raychowdhury MK, Gonzalez-Perrett S, Timpanaro GA, Chasan B. Cation channel activity of mucolipin-1: the effect of calcium. Pflugers Arch. 2005;451:304–312. doi: 10.1007/s00424-005-1448-9. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chang MY, Parker E, Ibrahim S, Shortland JR, El Nahas M, Haylor JL, Ong AC. Haploinsufficiency of Pkd2 is associated with increased tubular cell proliferation and interstitial fibrosis in two murine Pkd2 models. Nephrol Dial Transplant. 2006;21:2078–2084. doi: 10.1093/ndt/gfl150. [DOI] [PubMed] [Google Scholar]
- Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc Natl Acad Sci U S A. 1998;95:6373–6378. doi: 10.1073/pnas.95.11.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, Grunweller A, Mika J, Schafer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–243. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Chubanov V, Waldegger S, Mederos Y, Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Dantal J, Bigot E, Bogers W, Testa A, Kriaa F, Jacques Y, Hurault De Ligny B, Niaudet P, Charpentier B, Soulillou JP. Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N Engl J Med. 1994;330:7–14. doi: 10.1056/NEJM199401063300102. [DOI] [PubMed] [Google Scholar]
- Daskalakis N, Winn MP. Focal and segmental glomerulosclerosis: varying biologic mechanisms underlie a final histopathologic end point. Semin Nephrol. 2006;26:89–94. doi: 10.1016/j.semnephrol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Rost BR, Gudermann T. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflugers Arch. 2005a;451:72–80. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/– mice. Mol Cell Biol. 2005b;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A. 2002;99:14994–14999. doi: 10.1073/pnas.222425399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- Enklaar T, Esswein M, Oswald M, Hilbert K, Winterpacht A, Higgins M, Zabel B, Prawitt D. Mtr1, a novel biallelically expressed gene in the center of the mouse distal chromosome 7 imprinting cluster, is a member of the Trp gene family. Genomics. 2000;67:179–187. doi: 10.1006/geno.2000.6234. [DOI] [PubMed] [Google Scholar]
- Estacion M, Li S, Sinkins WG, Gosling M, Bahra P, Poll C, Westwick J, Schilling WP. Activation of human TRPC6 channels by receptor stimulation. J Biol Chem. 2004;279:22047–22056. doi: 10.1074/jbc.M402320200. [DOI] [PubMed] [Google Scholar]
- Estacion M, Sinkins WG, Jones SW, Applegate MA, Schilling WP. Human TRPC6 expressed in HEK 293 cells forms non-selective cation channels with limited Ca2+ permeability. J Physiol. 2006;572:359–377. doi: 10.1113/jphysiol.2005.103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Setaluri V. Expression and up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun. 2000;279:53–61. doi: 10.1006/bbrc.2000.3894. [DOI] [PubMed] [Google Scholar]
- Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol. 2000;11:814–827. doi: 10.1681/ASN.V115814. [DOI] [PubMed] [Google Scholar]
- Freichel M, Philipp S, Cavalie A, Flockerzi V. TRPC4 and TRPC4-deficient mice. Novartis Found Symp. 2004;258:189–199. Discussion 199203, 263186. [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/– mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Fuessel S, Sickert D, Meye A, Klenk U, Schmidt U, Schmitz M, Rost AK, Weigle B, Kiessling A, Wirth MP. Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int J Oncol. 2003;23:221–228. [PubMed] [Google Scholar]
- Gallagher AR, Cedzich A, Gretz N, Somlo S, Witzgall R. The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton. Proc Natl Acad Sci U S A. 2000;97:4017–4022. doi: 10.1073/pnas.97.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AR, Hoffmann S, Brown N, Cedzich A, Meruvu S, Podlich D, Feng Y, Konecke V, De Vries U, Hammes HP, Gretz N, Witzgall R. A truncated polycystin-2 protein causes polycystic kidney disease and retinal degeneration in transgenic rats. J Am Soc Nephrol. 2006;17:2719–2730. doi: 10.1681/ASN.2005090979. [DOI] [PubMed] [Google Scholar]
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Goldin E, Cooney A, Kaneski CR, Brady RO, Schiffmann R. Mucolipidosis IV consists of one complementation group. Proc Natl Acad Sci U S A. 1999;96:8562–8566. doi: 10.1073/pnas.96.15.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm DH, Karihaloo A, Cai Y, Somlo S, Cantley LG, Caplan MJ. Polycystin-2 regulates proliferation and branching morphogenesis in kidney epithelial cells. J Biol Chem. 2006;281:137–144. doi: 10.1074/jbc.M507845200. [DOI] [PubMed] [Google Scholar]
- Henshall SM, Afar DE, Hiller J, Horvath LG, Quinn DI, Rasiah KK, Gish K, Willhite D, Kench JG, Gardiner-Garden M, Stricker PD, Scher HI, Grygiel JJ, Agus DB, Mack DH, Sutherland RL. Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 2003;63:4196–4203. [PubMed] [Google Scholar]
- Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci U S A. 2005;102:11510–11515. doi: 10.1073/pnas.0505149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JG, Van Leeuwen JP, Van Der Eerden BC, Kersten FF, Van Der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112:1906–1914. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen SM, Groener JE, Bax W, Poorthuis BJ. Delayed lysosomal metabolism of lipids in mucolipidosis type IV fibroblasts after LDL-receptor-mediated endocytosis. J Inherit Metab Dis. 2001;24:577–586. doi: 10.1023/a:1012467827719. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Poet M, Fuhrmann JC, Zdebik AA. Physiological functions of CLC Cl− channels gleaned from human genetic disease and mouse models. Annu Rev Physiol. 2005;67:779–807. doi: 10.1146/annurev.physiol.67.032003.153245. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Elmes SJ, Kendall DA, Chapman V. Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naive, carrageenan-inflamed and neuropathic rats. Eur J Neurosci. 2005;22:361–370. doi: 10.1111/j.1460-9568.2005.04227.x. [DOI] [PubMed] [Google Scholar]
- Jia Y, McLeod RL, Hey JA. TRPV1 receptor: a target for the treatment of pain, cough, airway disease and urinary incontinence. Drug News Perspect. 2005;18:165–171. doi: 10.1358/dnp.2005.18.3.892761. [DOI] [PubMed] [Google Scholar]
- Kamei J, Zushida K, Morita K, Sasaki M, Tanaka S. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol. 2001;422:83–86. doi: 10.1016/s0014-2999(01)01059-7. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Nakazato E, Fujiuchi A, Hara T, Imai A. Involvement of an increased spinal TRPV1 sensitization through its up-regulation in mechanical allodynia of CCI rats. Neuropharmacology. 2005;49:977–984. doi: 10.1016/j.neuropharm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–123. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein - nephrin - is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Kim H, Mittal DP, Iadarola MJ, Dionne RA. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Medical Genet. 2006;43:e40. doi: 10.1136/jmg.2005.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A. TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem. 2005;280:43218–43223. doi: 10.1074/jbc.M508210200. [DOI] [PubMed] [Google Scholar]
- Konrad M, Schlingmann KP, Gudermann T. Insights into the molecular nature of magnesium homeostasis. Am J Physiol Renal Physiol. 2004;286:F599–F605. doi: 10.1152/ajprenal.00312.2003. [DOI] [PubMed] [Google Scholar]
- Koptides M, Deltas CC. Autosomal dominant polycystic kidney disease: molecular genetics and molecular pathogenesis. Hum Genet. 2000;107:115–126. doi: 10.1007/s004390000347. [DOI] [PubMed] [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- LaPlante JM, Falardeau J, Sun M, Kanazirska M, Brown EM, Slaugenhaupt SA, Vassilev PM. Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett. 2002;532:183–187. doi: 10.1016/s0014-5793(02)03670-0. [DOI] [PubMed] [Google Scholar]
- LaPlante JM, Ye CP, Quinn SJ, Goldin E, Brown EM, Slaugenhaupt SA, Vassilev PM. Functional links between mucolipin-1 and Ca2+-dependent membrane trafficking in mucolipidosis IV. Biochem Biophys Res Commun. 2004;322:1384–1391. doi: 10.1016/j.bbrc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Dai Y, Guo L, Liu Y, Hao C, Wu G, Basora N, Michalak M, Chen XZ. Polycystin-2 associates with tropomyosin-1, an actin microfilament component. J Mol Biol. 2003a;325:949–962. doi: 10.1016/s0022-2836(02)01333-5. [DOI] [PubMed] [Google Scholar]
- Li Q, Montalbetti N, Shen PY, Dai XQ, Cheeseman CI, Karpinski E, Wu G, Cantiello HF, Chen XZ. Alpha-actinin associates with polycystin-2 and regulates its channel activity. Hum Mol Genet. 2005a;14:1587–1603. doi: 10.1093/hmg/ddi167. [DOI] [PubMed] [Google Scholar]
- Li Q, Shen PY, Wu G, Chen XZ. Polycystin-2 interacts with troponin I, an angiogenesis inhibitor. Biochemistry. 2003b;42:450–457. doi: 10.1021/bi0267792. [DOI] [PubMed] [Google Scholar]
- Li X, Luo Y, Starremans PG, McNamara CA, Pei Y, Zhou J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat Cell Biol. 2005b;7:1202–1212. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
- Lubensky IA, Schiffmann R, Goldin E, Tsokos M. Lysosomal inclusions in gastric parietal cells in mucolipidosis type IV: a novel cause of achlorhydria and hypergastrinemia. Am J Surg Pathol. 1999;23:1527–1531. doi: 10.1097/00000478-199912000-00010. [DOI] [PubMed] [Google Scholar]
- Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni M, Monti E, Bresciani R, Bozzato A, Barlati S, Bassi MT, Borsani G. Overexpression of wild-type and mutant mucolipin proteins in mammalian cells: effects on the late endocytic compartment organization. FEBS Lett. 2004;567:219–224. doi: 10.1016/j.febslet.2004.04.080. [DOI] [PubMed] [Google Scholar]
- Mathis BJ, Kim SH, Calabrese K, Haas M, Seidman JG, Seidman CE, Pollak MR. A locus for inherited focal segmental glomerulosclerosis maps to chromosome 19q13. Kidney Int. 1998;53:282–286. doi: 10.1046/j.1523-1755.1998.00828.x. [DOI] [PubMed] [Google Scholar]
- Miedel MT, Weixel KM, Bruns JR, Traub LM, Weisz OA. Posttranslational cleavage and adaptor protein complex-dependent trafficking of mucolipin-1. J Biol Chem. 2006;281:12751–12759. doi: 10.1074/jbc.M511104200. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Du J, Rowan S, Hershey CL, Widlund HR, Fisher DE. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res. 2004;64:509–516. doi: 10.1158/0008-5472.can-03-2440. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- Montalbetti N, Li Q, Gonzalez-Perrett S, Semprine J, Chen XZ, Cantiello HF. Effect of hydro-osmotic pressure on polycystin-2 channel function in the human syncytiotrophoblast. Pflugers Arch. 2005;451:294–303. doi: 10.1007/s00424-005-1458-7. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Nagy I, Santha P, Jancso G, Urban L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. Faseb J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T, Peters J. Sci STKE 2005. 2005. TRP channels in disease; p. re8. [DOI] [PubMed] [Google Scholar]
- Nomura H, Turco AE, Pei Y, Kalaydjieva L, Schiavello T, Weremowicz S, Ji W, Morton CC, Meisler M, Reeders ST, Zhou J. Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J Biol Chem. 1998;273:25967–25973. doi: 10.1074/jbc.273.40.25967. [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Kawaguchi H, Hattori M, Komatsu Y, Akioka Y, Nagata M, Shiraga H, Ito K, Takahashi K, Ishikawa N, Tanabe K, Yamaguchi Y, Ota K. Effect of pre-and postoperative plasmapheresis on posttransplant recurrence of focal segmental glomerulosclerosis in children. Transplantation. 2001;71:628–633. doi: 10.1097/00007890-200103150-00008. [DOI] [PubMed] [Google Scholar]
- Ong AC, Wheatley DN. Polycystic kidney disease – the ciliary connection. Lancet. 2003;361:774–776. doi: 10.1016/S0140-6736(03)12662-1. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peng JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, Hediger MA, Freeman MR. CaT1 expression correlates with tumor grade in prostate cancer. Biochem Biophys Res Commun. 2001;282:729–734. doi: 10.1006/bbrc.2001.4638. [DOI] [PubMed] [Google Scholar]
- Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- Piper RC, Luzio JP. CUPpling calcium to lysosomal biogenesis. Trends Cell Biol. 2004;14:471–473. doi: 10.1016/j.tcb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Prawitt D, Enklaar T, Klemm G, Gartner B, Spangenberg C, Winterpacht A, Higgins M, Pelletier J, Zabel B. Identification and characterization of MTR1, a novel gene with homology to melastatin (MLSN1) and the trp gene family located in the BWS-WT2 critical region on chromosome 11p15.5 and showing allele-specific expression. Hum Mol Genet. 2000;9:203–216. doi: 10.1093/hmg/9.2.203. [DOI] [PubMed] [Google Scholar]
- Qian Q, Hunter LW, Li M, Marin-Padilla M, Prakash YS, Somlo S, Harris PC, Torres VE, Sieck GC. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet. 2003;12:1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- Raychowdhury MK, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, Chasan B, Goldmann WH, Stahl S, Cooney A, Goldin E, Cantiello HF. Molecular pathophysiology of mucolipidosis type IV: pH dysregulation of the mucolipin-1 cation channel. Hum Mol Genet. 2004;13:617–627. doi: 10.1093/hmg/ddh067. [DOI] [PubMed] [Google Scholar]
- Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem. 2005;280:34718–34722. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann R, Dwyer NK, Lubensky IA, Tsokos M, Sutliff VE, Latimer JS, Frei KP, Brady RO, Barton NW, Blanchette-Mackie EJ, Goldin E. Constitutive achlorhydria in mucolipidosis type IV. Proc Natl Acad Sci U S A. 1998;95:1207–1212. doi: 10.1073/pnas.95.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingmann KP, Sassen MC, Weber S, Pechmann U, Kusch K, Pelken L, Lotan D, Syrrou M, Prebble JJ, Cole DE, Metzger DL, Rahman S, Tajima T, Shu SG, Waldegger S, Seyberth HW, Konrad M. Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J Am Soc Nephrol. 2005;16:3061–3069. doi: 10.1681/ASN.2004110989. [DOI] [PubMed] [Google Scholar]
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz EC, Wissenbach U, Niemeyer BA, Strauss B, Philipp SE, Flockerzi V, Hoth M. TRPV6 potentiates calcium-dependent cell proliferation. Cell Calcium. 2006;39:163–173. doi: 10.1016/j.ceca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- Slaugenhaupt SA. The molecular basis of mucolipidosis type IV. Curr Mol Med. 2002;2:445–450. doi: 10.2174/1566524023362276. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Appendino G. Vanilloid receptor TRPV1 antagonists as the next generation of painkillers. Are we putting the cart before the horse? J Med Chem. 2004;47:2717–2723. doi: 10.1021/jm030560j. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Suzuki M, Mizuno A, Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci Lett. 2005;382:304–308. doi: 10.1016/j.neulet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen'kyi V, Slomianny C, Beck B, Mariot P, Bonnal JL, Mauroy B, Shuba Y, Capiod T, Skryma R, Prevarskaya N. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–2047. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- Thomson RB, Mentone S, Kim R, Earle K, Delpire E, Somlo S, Aronson PS. Histopathological analysis of renal cystic epithelia in the Pkd2WS25/- mouse model of ADPKD. Am J Physiol Renal Physiol. 2003;285:F870–F880. doi: 10.1152/ajprenal.00153.2003. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4−/– mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Treusch S, Knuth S, Slaugenhaupt SA, Goldin E, Grant BD, Fares H. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc Natl Acad Sci U S A. 2004;101:4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- Vergarajauregui S, Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Nilius B, Hoefs S, Van Der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Niemeyer B, Himmerkus N, Fixemer T, Bonkhoff H, Flockerzi V. TRPV6 and prostate cancer: cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem Biophys Res Comms. 2004;322:1359–1363. doi: 10.1016/j.bbrc.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A. 2001;98:10692–10697. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Zeigler M, Bach G. Internalization of exogenous gangliosides in cultured skin fibroblasts for the diagnosis of mucolipidosis IV. Clin Chim Acta. 1986;157:183–189. doi: 10.1016/0009-8981(86)90224-x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao XS, Shin DM, Liu LH, Shull GE, Muallem S. Plasticity and adaptation of Ca2+ signaling and Ca2+-dependent exocytosis in SERCA2+/− mice. EMBO J. 2001;20:2680–2689. doi: 10.1093/emboj/20.11.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiqi S, Soltani MH, Bhat KM, Sangha N, Fang D, Hunter JJ, Setaluri V. Human melastatin 1 (TRPM1) is regulated by MITF and produces multiple polypeptide isoforms in melanocytes and melanoma. Melanoma Res. 2004;14:509–516. doi: 10.1097/00008390-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Laboratory Invest. 2002;82:1755–1764. doi: 10.1097/01.lab.0000043910.41414.e7. [DOI] [PubMed] [Google Scholar]
- Zimmerman SW. Increased urinary protein excretion in the rat produced by serum from a patient with recurrent focal glomerular sclerosis after renal transplantation. Clin Nephrol. 1984;22:32–38. [PubMed] [Google Scholar]