Abstract
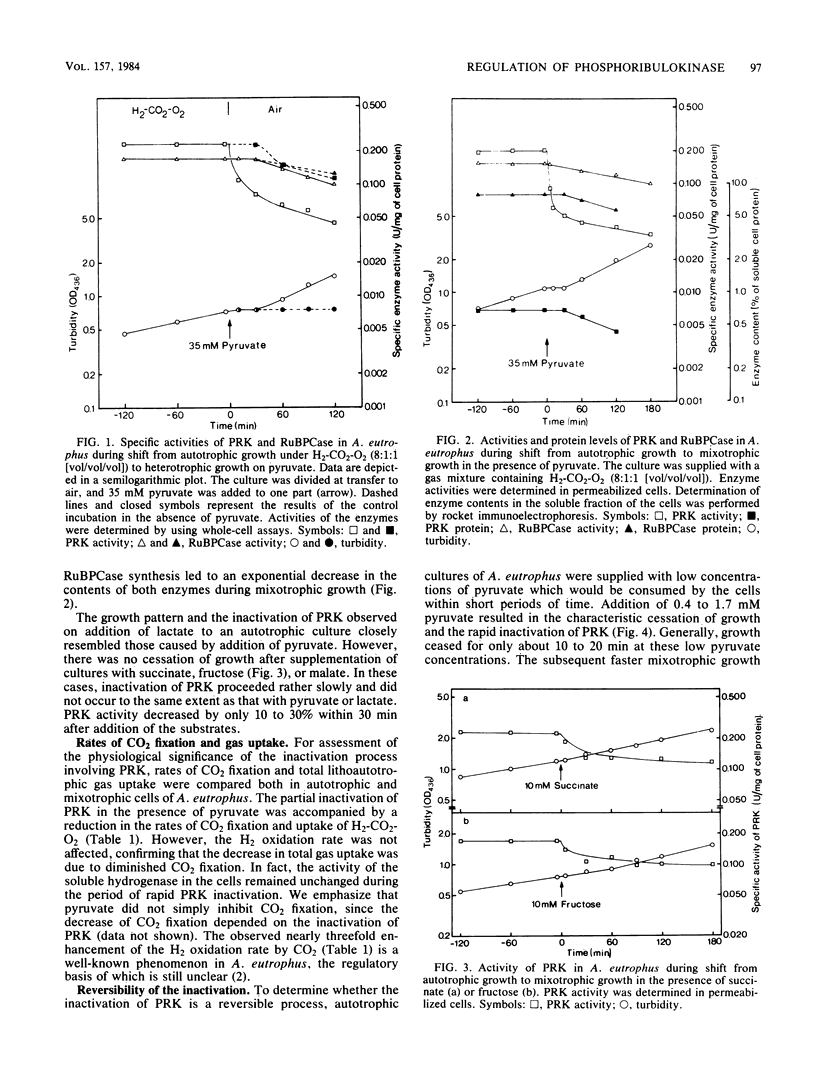
Phosphoribulokinase in Alcaligenes eutrophus was partially inactivated when an autotrophic culture was shifted to heterotrophic growth with pyruvate as the sole source of carbon and energy. A similar response was observed on addition of various organic substrates to autotrophic cultures during the transition to mixotrophic growth. The extent of inactivation depended on the added substrate. Pyruvate or lactate caused the strongest inactivation among the tested substrates. Up to 75% of the phosphoribulokinase activity found in the autotrophic cells was lost within 30 min after supplementation of the cultures with either of these two substrates. This loss of enzyme activity was not the result of degradation of enzyme protein. Inactivation of phosphoribulokinase was accompanied by a decrease in the CO2 fixation rate of the cells. Reactivation of the enzyme occurred after exhaustion of pyruvate from the medium. Neither inactivation nor reactivation required de novo protein synthesis; however, continued energy conversion was necessary for the inactivation to occur. We suggest that the pyruvate metabolism of A. eutrophus is involved in these regulatory processes which act on phosphoribulokinase. They appear to contribute to the control of autotrophic CO2 assimilation in this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Schlegel H. G. Purification and regulatory properties of phosphoribulokinase from Hydrogenomonas eutropha H 16. Biochem J. 1974 Jun;139(3):481–489. doi: 10.1042/bj1390481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHA R. [Physiological studies on the chemolithotropic metabolism of recently isolated Hydrogenomonas strains]. Arch Mikrobiol. 1962;41:313–350. [PubMed] [Google Scholar]
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Flügge U. I., Stitt M., Freisl M., Heldt H. W. On the Participation of Phosphoribulokinase in the Light Regulation of CO(2) Fixation. Plant Physiol. 1982 Jan;69(1):263–267. doi: 10.1104/pp.69.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. Tandem-crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:57–59. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PONTREMOLI S., MANGIAROTTI G. A simple method for the preparation of D-ribulose 5-phosphate. J Biol Chem. 1962 Mar;237:643–645. [PubMed] [Google Scholar]
- SCHLEGEL H. G., GOTTSCHALK G., VON BARTHA R. Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature. 1961 Jul 29;191:463–465. doi: 10.1038/191463a0. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schlesier M., Friedrich B. In vivo inactivation of soluble hydrogenase of Alcaligenes eutrophus. Arch Microbiol. 1981 Apr;129(2):150–153. doi: 10.1007/BF00455352. [DOI] [PubMed] [Google Scholar]
- Siebert K., Schobert P., Bowien B. Purification, some catalytic and molecular properties of phosphoribulokinase from Alcaligenes eutrophus. Biochim Biophys Acta. 1981 Mar 13;658(1):35–44. doi: 10.1016/0005-2744(81)90247-3. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]


