Abstract
Exposure to microgravity alters the distribution of body fluids and the degree of distension of cranial blood vessels, and these changes in turn may provoke structural remodelling and altered cerebral autoregulation. Impaired cerebral autoregulation has been documented following weightlessness simulated by head-down bed rest in humans, and is proposed as a mechanism responsible for postspaceflight orthostatic intolerance. In this study, we tested the hypothesis that spaceflight impairs cerebral autoregulation. We studied six astronauts ∼72 and 23 days before, after 1 and 2 weeks in space (n = 4), on landing day, and 1 day after the 16 day Neurolab space shuttle mission. Beat-by-beat changes of photoplethysmographic mean arterial pressure and transcranial Doppler middle cerebral artery blood flow velocity were measured during 5 min of spontaneous breathing, 30 mmHg lower body suction to simulate standing in space, and 10 min of 60 deg passive upright tilt on Earth. Dynamic cerebral autoregulation was quantified by analysis of the transfer function between spontaneous changes of mean arterial pressure and cerebral artery blood flow velocity, in the very low- (0.02–0.07 Hz), low- (0.07–0.20 Hz) and high-frequency (0.20–0.35 Hz) ranges. Resting middle cerebral artery blood flow velocity did not change significantly from preflight values during or after spaceflight. Reductions of cerebral blood flow velocity during lower body suction were significant before spaceflight (P < 0.05, repeated measures ANOVA), but not during or after spaceflight. Absolute and percentage reductions of mean (±s.e.m.) cerebral blood flow velocity after 10 min upright tilt were smaller after than before spaceflight (absolute, −4 ± 3 cm s−1 after versus−14 ± 3 cm s−1 before, P = 0.001; and percentage, −8.0 ± 4.8% after versus−24.8 ± 4.4% before, P < 0.05), consistent with improved rather than impaired cerebral blood flow regulation. Low-frequency gain decreased significantly (P < 0.05) by 26, 23 and 27% after 1 and 2 weeks in space and on landing day, respectively, compared with preflight values, which is also consistent with improved autoregulation. We conclude that human cerebral autoregulation is preserved, and possibly even improved, by short-duration spaceflight.
Astronauts have reduced orthostatic tolerance on return to Earth (Hoffler et al. 1974; Blomqvist & Stone, 1983; Bungo et al. 1985; Fritsch-Yelle et al. 1994; Buckey et al. 1996; Levine et al. 1997). The mechanisms responsible for orthostatic intolerance are disputed and are probably several. However, the final event leading to syncope must be a reduction of cerebral blood flow, sufficient to cause loss of consciousness (Töyry et al. 1997). Indeed, the brain has such a high metabolic rate that if its blood flow is sharply reduced, even for a short time, syncope will occur.
The mechanism most commonly invoked to explain orthostatic intolerance after spaceflight is that cerebral blood flow declines secondary to hypotension. However, in a previous spaceflight study (Buckey et al. 1996), two of 14 astronauts had significant symptoms after spaceflight and could not remain standing, but did not exhibit hypotension. One possible explanation for this is that spaceflight and microgravity impair cerebral autoregulatory mechanisms. Several studies conducted with analogues of spaceflight support this possibility. For example, a study conducted in rats (Wilkerson et al. 1999) showed that cerebral arteries hypertrophy in response to microgravity simulated by tail-suspension. Other studies (Bondar et al. 1995; Zhang et al. 1997, 1998b) show that cerebral blood flow velocity declines during lower body suction after prolonged head-down bed rest, without significant reductions of arterial pressure. These findings support the possibility that spaceflight impairs cerebral autoregulation, and that this impairment contributes to orthostatic intolerance after spaceflight, notwithstanding adequate autonomic blood pressure control (Levine et al. 2002).
Autoregulation maintains a steady oxygen supply to the brain by adjusting cerebral arteriolar caliber and resistance to minimize the effects of changes of perfusion pressure. ‘Static’ cerebral autoregulation maintains cerebral blood flow at relatively constant levels, despite sustained perfusion pressure variations between 60 and 150 mmHg (Paulson et al. 1990). A less traditional concept of ‘dynamic’ cerebral autoregulation holds that the effectiveness of cerebral vascular responses is also determined importantly by the time period over which and frequency at which blood pressure changes occur (Giller, 1990; Blaber et al. 1997; Panerai et al. 1999; Zhang et al. 1998a, b, 2000, 2002; Lipsitz et al. 2000).
We tested the hypothesis that abnormalities of static or dynamic cerebral autoregulation develop during spaceflight, and that these impair the ability of autoregulatory mechanisms to maintain cerebral blood flow constant in the upright position upon return to Earth, even when blood pressure is well maintained. To test this hypothesis, we measured cerebral blood flow velocity by transcranial Doppler ultrasound before, during and after the 16 day Neurolab (STS-90) space shuttle mission. This study follows four earlier articles from the Neurolab mission (Cox et al. 2002; Ertl et al. 2002; Levine et al. 2002; Fu et al. 2002) and reports data obtained from the same astronauts.
Methods
Subjects
Six male crew members of the 16 day Neurolab mission, described in detail by Cox et al. (2002), were studied. Their mean age (±s.e.m.) was 40 ± 2 years (range, 38–44 years), height 187 ± 2 cm and weight 87 ± 5 kg. All were in excellent health, as documented by comprehensive National Aeronautics and Space Administration (NASA) Class III physical examinations. No subject smoked or used any medication regularly. This study was conducted under guidelines developed by the NASA Johnson Space Center Human Research Policies and Procedures Committee, in accordance with the Declaration of Helsinki. All subjects signed an informed consent form approved by the NASA Human Subjects Review Committee and the institutional review boards of principal investigators' institutions (The University of Texas Southwestern Medical Center at Dallas; Vanderbilt University; DLR-Institute for Aerospace Medicine; and the Medical College of Virginia). All six astronauts participated in both pre- and postflight sessions, and four participated in two in-flight sessions (days 7–8 and 12–13). The number of subjects is indicated for each measurement; because of NASA restrictions, not all astronauts were studied with all protocols.
Preflight experiments (n= 6)
Experiments were performed 73–70 and 24–21 days preflight, to determine the reliability and repeatability of measurements (Hopkins, 2000). The error, expressed as a coefficient of variation in cerebral blood flow velocity, was 10%; that in mean arterial pressure was 6%; and that in transfer function gain in the low-frequency range was 12%; indicating good repeatability of these measurements. Data from 24–21 day preflight experiments were used for preflight comparisons because of their temporal proximity to the mission. Subjects refrained from performing high-intensity exercise or taking over-the-counter medications within 24 h of all studies. Subjects were studied at least 2 h after a meal, and more than 12 h after the last caffeinated or alcoholic beverage. Experiments were conducted in a quiet, environmentally controlled laboratory with an ambient temperature of 25°C, at Johnson Space Center, Houston, TX, USA. Subjects were studied supine in a custom-built rigid plastic chamber designed to allow both lower body suction and upright tilt. The right leg was supported in the fully extended position by removable foot boards, customized for each subject. The left leg was elevated 15 cm from the bottom of the chamber with the hip flexed for microneurography (Levine et al. 2002).
In-flight experiments (after 1 and 2 weeks, n= 4)
Four of the six astronauts participated in two in-flight experiments on flight days 7 or 8 and 12 or 13. Subjects were positioned in a custom-designed collapsible fabric chamber for lower body suction. Ambient carbon dioxide levels were always below 0.1% in the space shuttle.
Postflight landing day experiments (n= 6)
No subject took medication during the 48 h prior to landing. However, just prior to re-entry while still in space, three astronauts consumed 1.5 l of hyposmolar glucose and electrolyte solution, and three astronauts consumed about 8 g of salt and 912 ml of water, according to NASA fluid-loading protocols (Bungo et al. 1985), which induced vomiting in one of the latter three. Immediately after the space shuttle landing at Kennedy Space Center, Cape Canaveral, FL, USA, astronauts were transported to the laboratory in the supine position, where they remained until the experiments were completed. (Two of the six subjects first sat upright for less than 30 min, to complete another experiment.) Subjects were studied in pairs; the first pair was studied within 1 h, the second within 3 h and the third within 5 h of landing on Earth. All astronauts were allowed free access to water from the time of landing until the beginning of the protocol. Subjects were placed in the supine position on a commercial tilt bed (OT9001, Omni Technologies, Valley City, ND, USA), with the right leg extended. The left leg was elevated for microneurography. Wide straps were placed over the right thigh near the knees and at the waist to minimize body motion, and to prevent knee flexion during tilting.
Postflight recovery experiments (24 h after landing, n= 6)
Experiments were performed 1 day after return to Earth. Details were the same as during preflight experiments.
Instrumentation
Heart rate was measured from the electrocardiogram (HP 78801 B, Hewlett-Packard, Andover, MA, USA), and beat-by-beat arterial pressure was estimated by finger photoplethysmography (on Earth, Finapres, Ohmeda, Englewood, CO, USA; in space, Finapres, as modified by TNO Biomedical Instrumentation, Amsterdam, The Netherlands, for the European Space Agency). Arm blood pressure was measured intermittently by electrosphygmomanometry (on Earth, Suntech Medical Instruments 4240, Raleigh, NC, USA; in space, Puritan Bennett D500, Pleasanton, CA, USA), with a microphone placed over the brachial artery to detect Korotkoff sounds. An adjustable arm support held the finger at heart level in both supine and upright tilt positions.
Middle cerebral artery blood flow velocity was measured continuously using transcranial Doppler ultrasound. A 2 MHz probe (Multiflow, DWL, Sipplingen, Germany) was placed over the subject's temporal window. The probe was placed in the same position both in-flight and on Earth, and fixed at a constant angle with a polymer holder made to fit each subject's facial bone structure and ear (Giller & Giller, 1997). The signal was obtained according to standard techniques (Åaslid et al. 1982), with the Doppler sample volume adjusted to obtain the maximum proximal middle cerebral artery blood flow velocity. End-tidal carbon dioxide concentrations were measured with a mass spectrometer (Marquette MGA1100 Mass Spectrometer, St Louis, MO, USA).
Protocol
Left and right antecubital veins were cannulated for the injection and blood drawing. After at least 20 min of quiet supine rest, plasma and blood volumes were measured with the Evans Blue dye technique (Foldager & Blomqvist, 1991). Cardiac output was measured with acetylene rebreathing (Triebwasser et al. 1977) every 5 min until it reached a stable level, defined as two consecutive measurements within 500 ml min−1 of each other. To induce or simulate orthostatic stress, upright tilt was used during preflight and postflight landing day sessions, and lower body suction was used during preflight, in-flight and one postflight session (24 h after return to Earth), after a battery of tests of autonomic function, which included controlled-frequency breathing, static handgrip, the Valsalva manoeuvre and the cold-pressor test. Sufficient time was allowed between each intervention for the haemodynamic state and sympathetic nerve activity to return to baseline levels. A detailed schedule for Neurolab experiments is given in the Appendix of the article by Cox et al. (2002).
Lower body suction (preflight, after 1 and 2 weeks in space, and 24 h after return to Earth)
Each subject lay supine in the lower body chamber sealed at the iliac crests (Ertl et al. 2002). After 7 min of baseline recording, steady suction was applied at −15 and −30 mmHg in fixed order, for 7 min each. Mean arterial pressures and cerebral blood flow velocities were averaged for 5 min, after a 2 min stabilization period, during the 2nd to 7th minute of each 7 min period.
Upright tilt (preflight, landing day)
After a minimum 10 min recovery period following the preceding intervention, baseline data collection was repeated for 5 min in the supine position. Then, the astronaut was tilted smoothly to a 60 deg upright position, over 10–15 s, to remain upright for 10 min. Automated cuff blood pressure and cerebral blood flow velocity recordings during tilt were analysed after a 2 min stabilization period, during the 2nd to 4th minute. Then, cardiac output was measured and blood was drawn for plasma noradrenaline determinations. Data were analysed during the 7th to 9th minute, for comparisons between pre- and postflight conditions.
Spectral analysis
The analog arterial pressure and peak envelope of Doppler cerebral blood flow velocity were sampled simultaneously at 100 Hz and digitized at 12 bits. Real-time beat-by-beat values of mean arterial pressure and mean cerebral blood flow velocity were calculated as true electronic averages of each integrated waveform and stored in the memory of the Multiflow device.
To evaluate the relation between pressure and flow as ‘dynamic’, cerebral autoregulation, beat-by-beat mean arterial pressures and cerebral blood flow velocities during quiet spontaneous breathing were integrated for each cardiac cycle, from the 5 min of baseline recordings for lower body suction preflight, after 1 and 2 weeks in space, and 24 h after landing, and from the 5th minute of baseline recordings during upright tilt on landing day. Beat-by-beat mean arterial pressure and cerebral blood flow velocity were then linearly interpolated and resampled at 4 Hz for spectral analysis. Spectra of mean arterial pressure and cerebral blood flow velocity were calculated with fast Fourier transforms, and the transfer function between these two variables was calculated with a cross-spectral method to assess dynamic cerebral blood flow autoregulation (Zhang et al. 1998a, 2000, 2002). Spectral power, mean transfer function gain, phase and coherence functions were calculated in very low- (0.02–0.07 Hz), low- (0.07–0.20 Hz) and high-frequency (0.20–0.35 Hz) ranges.
These ranges were chosen to reflect different patterns of the dynamic pressure–flow relation, as previously identified by transfer function analysis (Zhang et al. 1998a, 2000, 2002). Coherence functions were calculated to assess the linear relation between the two variables and the reliability of the transfer function gain and phase. Phase estimates were calculated to determine the temporal relation between these two variables. Transfer function gain estimates were calculated to quantify the ability of the cerebral vascular bed to buffer changes in cerebral blood flow velocity induced by transient arterial pressure changes at different frequencies. For example, if coherence is low (< 0.50), the signals tend to be independent of each other, suggesting effective autoregulation. When coherence is high, however, pressure and velocity vary together closely, and the gain can be used to evaluate the effectiveness of cerebral autoregulation. If the gain is small, large changes in blood pressure lead to only small changes in cerebral blood flow velocity, implying effective autoregulation. Conversely, if the gain is large, large changes in pressure lead to similarly large changes in flow velocity, implying impaired autoregulation.
Statistical analysis
Data are given as means ±s.e.m. Data across conditions (pre-, in- and postflight) were compared with a repeated measures analysis of variance (ANOVA) with Dunnet's post hoc test for multiple comparisons, with measurements made ∼23 days preflight as the baseline condition. Changes in response to upright tilt between preflight and landing day sessions, or among responses to lower body suction across conditions (pre-, in- and postflight) were examined with a repeated measures, two-way analysis of variance. If a significant interaction was observed, a Student–Newman–Keuls post hoc test was used to determine the source of the difference. All statistical analyses were performed with a personal computer-based analysis program (Abstat, Anderson-Bell, Denver, CO, USA). A P value of 0.05 was considered significant.
Results
Baseline measurements
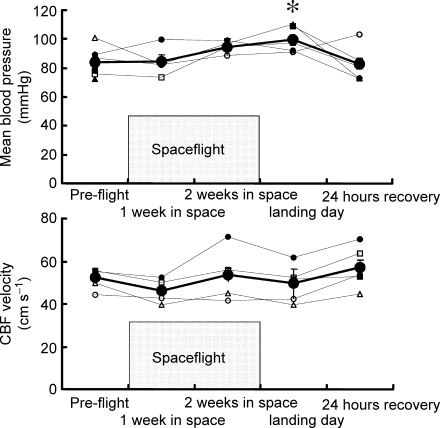
Table 1 and Fig. 1 indicate haemodynamic measurements across sessions and interventions. Most baseline measurements were similar before, during and after spaceflight. However, mean arterial pressure was significantly higher on landing day than during the preflight session. Mean baseline cerebral blood flow velocities and end-tidal carbon dioxide partial pressures did not vary significantly across sessions. End-tidal carbon dioxide partial pressures averaged 39.6 ± 0.7, 38.6 ± 1.8 and 42.5 ± 1.8 mmHg during preflight, landing day and 24 h after landing day sessions (n= 6, n.s.).
Table 1.
Haemodynamic data
| In-flight | Post-flight | ||||
|---|---|---|---|---|---|
| Pre-flight | 1 week in space | 2 weeks in space | Landing day | 24 hours recovery | |
| Supine rest | |||||
| n | 6 | 4 | 4 | 6 | 6 |
| MBP (mmHg) | 84 ± 4 | 85 ± 6 | 95 ± 2 | 99 ± 3* | 83 ± 5 |
| CBFvel (cm s−1) | 52 ± 2 | 46 ± 3 | 54 ± 7 | 50 ± 3 | 57 ± 4 |
| Lower body suction | |||||
| n | 6 | 4 | 4 | — | 6 |
| MBP (mmHg) | |||||
| 0 mmHg | 84 ± 4 | 85 ± 6 | 95 ± 2 | — | 83 ± 5 |
| −15 mmHg | 84 ± 5 | 87 ± 4 | 99 ± 3 | — | 86 ± 7 |
| −30 mmHg | 85 ± 5 | 90 ± 3 | 98 ± 3 | — | 89 ± 7† |
| CBFvel (cm s−1) | |||||
| 0 mmHg | 52 ± 2 | 46 ± 3 | 54 ± 5 | — | 57 ± 4 |
| −15 mmHg | 52 ± 3 | 45 ± 2 | 53 ± 6 | — | 55 ± 5 |
| −30 mmHg | 47 ± 2† | 45 ± 3 | 51 ± 6 | — | 54 ± 5 |
| Upright tilt | |||||
| n | 6 | — | — | 6 | — |
| MBP (mmHg) | |||||
| Supine | 89 ± 1 | — | — | 91 ± 3 | — |
| Tilt 5 | 95 ± 2 | — | — | 89 ± 2 | — |
| Tilt 10 | 93 ± 2 | — | — | 90 ± 3 | — |
| CBFvel (cm s−1) | |||||
| Supine | 55 ± 3 | — | — | 50 ± 3 | — |
| Tilt 5 | 45 ± 2 | — | — | 48 ± 3 | — |
| Tilt 10 | 40 ± 2† | — | — | 46 ± 3 | — |
Average (±s.e.m.) mean blood pressure (MBP) and cerebral blood flow velocity (CBFvel), preflight, in-flight (1 week and 2 weeks in space), on landing day, and postflight (24 h recovery), at supine rest, during lower body suction, or after 5 and 10 min 60 deg upright tilt (Tilt 5 and Tilt 10).
P < 0.05 compared with preflight
P < 0.05 compared with baseline (0 mmHg, or supine).
Figure 1.
Mean arterial pressure and cerebral blood flow velocity Average (±s.e.m.) and individual mean blood pressure and cerebral blood flow (CBF) velocities, preflight, in-flight (1 and 2 weeks in space), on landing day and postflight (24 h recovery). In this and subsequent figure, standard error bars appear to be missing for some points because of the tight data and small standard errors. Each astronaut is represented by the same symbols in all figures, in this and other Neurolab articles (Levine et al. 2002; Fu et al. 2002). Astronauts 1–6 are represented by ^, ▵, □, •, ▪ and ▴, respectively. There were no statistically significant differences compared with preflight in cerebral blood flow velocity. *P < 0.05 compared with preflight.
Responses to simulated and real orthostatic stress
Lower body suction
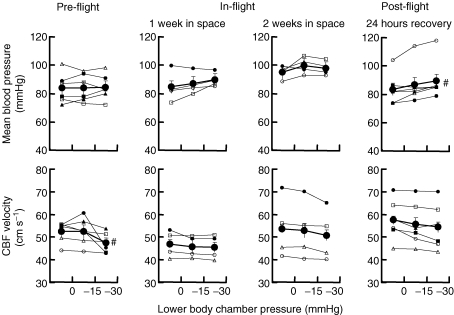
Figure 2 shows mean arterial pressure and cerebral blood flow velocity responses to lower body suction. Lower body suction tended to raise mean arterial pressure and lower mean cerebral artery blood flow velocity. However, the increase of pressure was significant only 24 h after landing, and the reduction of cerebral blood flow velocity was significant only during the preflight session.
Figure 2.
Responses to lower body suction Average (±s.e.m.) and individual mean blood pressure and cerebral blood flow (CBF) velocities during lower body suction. #P < 0.05 compared with baseline (0 mmHg).
Upright tilt
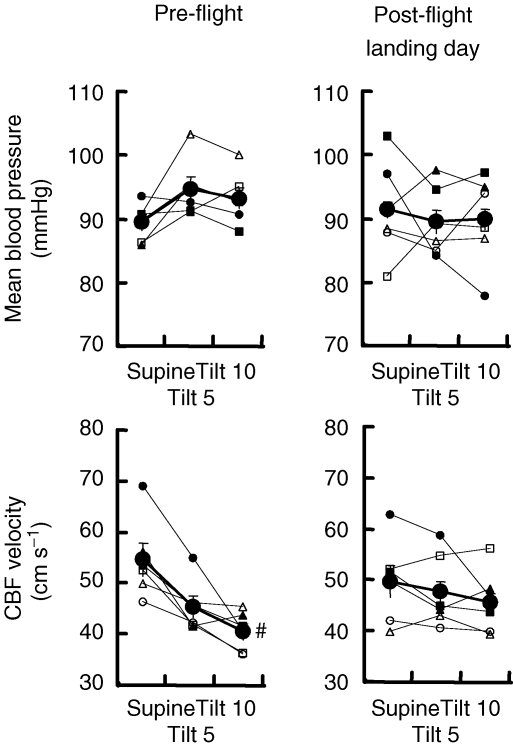
Figure 3 shows pre- and postflight responses to upright tilt. Mean arterial pressure was similar before and during tilt, and changes occurring during tilt were similar before and after spaceflight. Cerebral blood flow velocity before spaceflight decreased significantly by 10 min of tilt. Reductions of cerebral blood flow velocity during tilt after spaceflight were not significant, but the absolute and percentage changes were significantly less than those recorded before spaceflight (absolute, −4 ± 3 cm s−1 after versus−14 ± 3 cm s−1 before, P= 0.001 for the interaction statistic, two-way ANOVA, n= 6; and −8.0 ± 4.8% after versus−24.8 ± 4.4% before, P < 0.05)
Figure 3.
Responses to 60 deg upright tilt Average (±s.e.m.) and individual mean blood pressure and cerebral blood flow (CBF) velocities in the supine position and after 5 and 10 min 60 deg upright tilt (Tilt 5 and Tilt 10), preflight and postflight on landing day. #P < 0.05 compared with baseline (supine).
Dynamic cerebral autoregulation
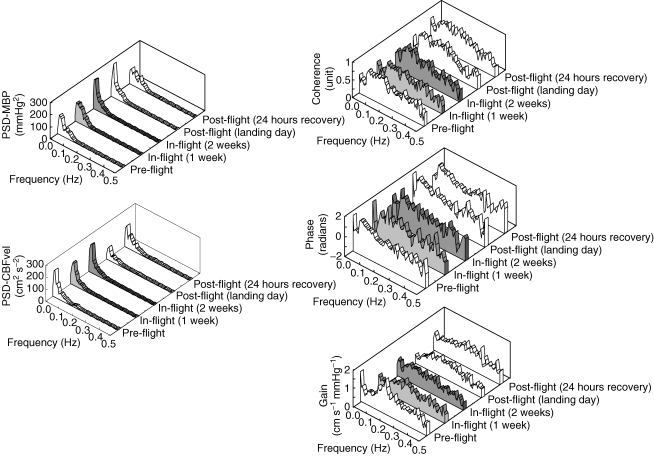
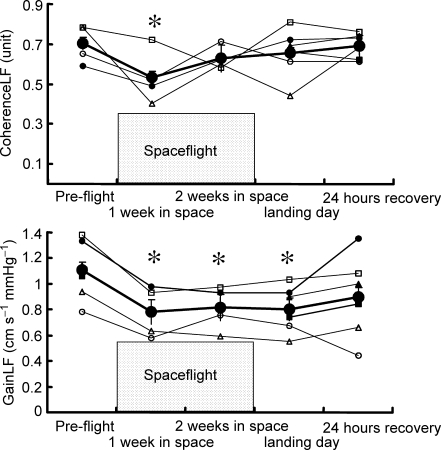
Group average frequency domain data are given in Table 2 and shown in Fig. 4. Mean arterial pressure spectral power did not change significantly during any session. Cerebral blood flow velocity spectral powers at very low and high frequencies also were similar before, during and after spaceflight. However, cerebral blood flow velocity spectral power at low frequencies was significantly less after 1 week in space and on landing day than before spaceflight (Table 2). Coherence between arterial pressure and cerebral blood flow velocity fluctuations, which was above 0.5 in all but one subject during all sessions, was unchanged at very low and high frequencies, but was significantly lower at low frequencies after 1 week in space. Similarly, transfer function gain between mean arterial pressure and cerebral blood flow velocity was unchanged at very low and high frequencies, but was significantly reduced at low frequencies: responses were decreased by 26% after 1 week in space, by 23% after 2 weeks in space and by 27% on landing day, compared with preflight measurements (all P < 0.05, Figs 4 and 5). These changes of transfer function gain are more than twice the typical error calculated from the two preflight sessions (12%).
Table 2.
Spectral analyses of mean arterial pressure and cerebral blood flow velocities
| In-flight | Post-flight | ||||
|---|---|---|---|---|---|
| Preflight (n = 6) | 1 week (n = 4) | 2 weeks (n = 4) | Landing day (n = 6) | 24 h recovery (n = 6) | |
| VLFMBP (mmHg2) | 3.50 ± 1.00 | 4.52 ± 0.89 | 3.17 ± 0.58 | 3.13 ± 0.32 | 3.63 ± 1.22 |
| VLFCBFvel (cm2 s−2) | 3.12 ± 1.36 | 3.07 ± 0.34 | 3.26 ± 0.28 | 1.58 ± 0.19 | 3.60 ± 0.88 |
| CohVLF (a.u.) | 0.44 ± 0.08 | 0.33 ± 0.05 | 0.44 ± 0.07 | 0.47 ± 0.04 | 0.50 ± 0.03 |
| GainVLF (cm s−1 mmHg−1) | 0.61 ± 0.11 | 0.47 ± 0.09 | 0.62 ± 0.03 | 0.47 ± 0.03 | 0.74 ± 0.05 |
| PhaseVLF (rad) | 0.77 ± 0.27 | −0.05 ± 0.39 | 0.16 ± 0.21 | 1.07 ± 0.21 | 0.80 ± 0.18 |
| LFMBP (mmHg2) | 1.88 ± 0.33 | 1.36 ± 0.21 | 1.43 ± 0.22 | 1.43 ± 0.12 | 2.89 ± 0.97 |
| LFCBFvel (cm2 s−2) | 1.98 ± 0.34 | 1.13 ± 0.21* | 1.44 ± 0.20 | 1.07 ± 0.15* | 2.01 ± 0.29 |
| CohLF (a.u.) | 0.71 ± 0.03 | 0.53 ± 0.07* | 0.63 ± 0.03 | 0.66 ± 0.05 | 0.69 ± 0.03 |
| GainLF (cm s−1 mmHg−1) | 1.11 ± 0.09 | 0.78 ± 0.10* | 0.81 ± 0.09* | 0.80 ± 0.07* | 0.90 ± 0.13 |
| PhaseLF (rad) | 0.66 ± 0.15 | 0.61 ± 0.09 | 0.58 ± 0.05 | 0.74 ± 0.08 | 0.56 ± 0.07 |
| HFMBP (mmHg2) | 0.11 ± 0.03 | 0.12 ± 0.04 | 0.13 ± 0.02 | 0.26 ± 0.08 | 0.21 ± 0.08 |
| HFCBFvel (cm2 s−2) | 0.18 ± 0.04 | 0.16 ± 0.06 | 0.15 ± 0.06 | 0.49 ± 0.21 | 0.37 ± 0.17 |
| CohHF (a.u.) | 0.51 ± 0.05 | 0.46 ± 0.08 | 0.44 ± 0.02 | 0.58 ± 0.07 | 0.61 ± 0.07 |
| GainHF (cm s−1 mmHg−1) | 0.94 ± 0.11 | 0.73 ± 0.07 | 0.69 ± 0.09 | 0.79 ± 0.10 | 1.07 ± 0.15 |
| PhaseHF (rad) | 0.27 ± 0.11 | 0.07 ± 0.15 | 0.14 ± 0.07 | 0.19 ± 0.05 | 0.27 ± 0.11 |
Values are means ±s.e.m. VLFMBP, very low-frequency component of the mean blood pressure variability; VLFCBFvel, very low-frequency component of cerebral blood flow velocity variability; CohVLF, coherence in the very low-frequency range; GainVLF, transfer function gain in the very low-frequency range; PhaseVLF, phase in the very low-frequency range; LFMBP, low-frequency component of the mean blood pressure variability; LFCBFvel, low-frequency component of the cerebral blood flow velocity variability; CohLF, coherence in the low-frequency range; GainLF, transfer function gain in the low-frequency range; PhaseLF, phase in the low-frequency range; HFMBP, high-frequency component of the mean blood pressure variability; HFCBFvel, high-frequency component of the cerebral blood flow velocity variability; CohHF, coherence in the high-frequency range; GainHF, transfer function gain in the high-frequency range; PhaseHF, phase in the high-frequency range.
P < 0.05 compared with preflight.
Figure 4.
Group-averaged frequency-domain analysis of changes in mean blood pressure and cerebral blood flow velocity before, during and after spaceflight Power spectral density of mean blood pressure (PSD-MBP) and cerebral blood flow velocity (PSD-CBFvel), and coherence, phase and transfer function gain (Gain) between mean blood pressure and cerebral blood low velocity. (Note that because of variable spontaneous breathing rates, group-averaged data are smoothed, and that because of enormous peak at very low frequency, the graph scale makes a spectral peak at high frequency indistinct.)
Figure 5.
Changes in coherence and transfer function gain between mean blood pressure and cerebral blood flow velocity variabilities at low frequency Coherence at low frequency (CoherenceLF), transfer function gain between mean blood pressure and cerebral blood flow velocity variabilities at low frequency (GainLF). *P < 0.05 compared with preflight.
Discussion
We studied static and dynamic cerebral vascular control in astronauts before, during and after space travel, because terrestrial analogues of weightlessness suggest that disordered cerebral autoregulation may contribute to postspaceflight orthostatic intolerance. Our results indicate that: (1) reductions of cerebral blood flow velocity during lower body suction are similar before, during and after spaceflight; (2) reductions of cerebral blood flow velocity during upright tilt are smaller after than before space travel; and (3) dynamic fluctuations of cerebral blood flow velocity in response to changes of arterial pressure, as reflected by transfer function gain, phase and coherence, are significantly less in the low-frequency range during and after than before spaceflight. These results reject the hypothesis that exposure to microgravity impairs cerebral autoregulation and suggest rather that microgravity improves it.
Orthostatic intolerance and cerebral autoregulation
In most subjects studied during upright tilt, autonomic reflexes and cerebral autoregulatory changes of vascular resistance work in concert to maintain constant levels of cerebral perfusion, and thereby prevent presyncope and syncope (Schondorf et al. 1997). In some subjects, however, arterial pressure falls; in this circumstance, cerebral autoregulation may nonetheless maintain normal levels of cerebral blood flow or it may fail, leading to reductions of cerebral blood flow and presyncope (Novak et al. 1998). A third, seemingly paradoxical group of subjects, maintains systemic arterial pressure at normal levels during upright tilt but becomes presyncopal because cerebral autoregulatory mechanisms fail (Jacob et al. 1999).
Thus, cerebral autoregulation and reflex mechanisms that maintain systemic arterial pressure may change independently, and disordered cerebral autoregulation by itself may contribute to orthostatic intolerance. Bondar et al. (1995) and Zhang et al. (1997, 1998b) showed that in healthy volunteers, mean cerebral blood flow velocity may fall during lower body suction, even though systemic arterial pressure remains constant. As mentioned in the introduction, the occurrence of presyncope during standing in some astronauts who do not have hypotension (Buckey et al. 1996) raises the possibility that changes of cerebral autoregulation during spaceflight adversely affect astronauts' orthostatic tolerance when they return to Earth.
Static cerebral autoregulation in microgravity
Published evidence supports the possibility that microgravity impairs cerebral autoregulation. In rats, microgravity, as simulated by tail-suspension, causes a persistent cephalad fluid shift and provokes hypertrophy of the medial layer of cerebral arteries (Wilkerson et al. 1999). The situation in humans is probably more complex, such that actual spaceflight probably leads to sequential increases followed by decreases of cerebral artery dimensions (Watenpaugh & Hargens, 1996). In either case, sustained changes of cerebral artery dimensions may remodel cerebral vessels and alter either intrinsic myogenic mechanisms or responsiveness of cerebral vessels to changes of neural input or the metabolic milieu, and impair steady-state cerebral autoregulation. Indeed, in humans, spaceflight or simulated microgravity alters several factors involved in cerebral autoregulation: blood volume and presumably vascular dimensions decline (Alfrey et al. 1996; Iwasaki et al. 2000, 2004; Pawelczyk et al. 2001; Levine et al. 2002); parasympathetic heart rate modulation changes (Iwasaki et al. 2000, 2004); muscle sympathetic nerve activity, plasma noradrenaline levels and noradrenaline spillover increase (Ertl et al. 2002); and plasma nitrite/nitrate concentrations, indices of endothelial nitric oxide release, decline (Kamiya et al. 2000).
Simulated microgravity studies in humans show no major changes of cerebral blood flow velocity during supine rest (Pavy-Le Traon et al. 1995, 2002; Zhang et al. 1997). However, mean cerebral blood flow velocity falls significantly more during graded lower body suction after than before prolonged head-down bed rest, before the occurrence of hypotension (Zhang et al. 1997). This raises the possibility that spaceflight impairs static cerebral autoregulation, leading to orthostatic reductions of cerebral blood flow and presyncope, despite adequate autonomic neural control of systemic arterial pressure (Levine et al. 2002).
There are several reports of cerebral blood flow velocity in humans exposed to microgravity. Bagian & Hackett (1991) evaluated four astronauts with transcranial Doppler ultrasound measurements in space, and observed no clear change between pre- and in-flight measurements. Arbeille et al. (2001) also reported stable cerebral blood flow velocities during space missions lasting 6 days to 6 months. Herault et al. (2000) observed that the ratio of cerebral to femoral blood flow velocities increased less during lower body suction in space, and on Earth on the day of return from space, than it did on Earth in preflight experiments. Increases of this ratio (which includes femoral arterial blood flow velocity changes secondary to hypovolaemia) were significantly correlated with orthostatic tolerance. However, blood pressure during these manoeuvres was not reported, and the interpretation of this indirect index of blood flow distribution is unclear. Fritsch-Yelle et al. (1996) studied astronauts' cerebral blood flow velocity with transcranial Doppler ultrasound during standing, before and after spaceflight. Although some subjects experienced orthostatic presyncope after space travel, there were no clear differences in Doppler measurements in presyncopal and non-syncopal groups. Moreover, these measurements were made after inhalation of carbon monoxide, which increases cerebral blood flow velocity (Rucker et al. 2002), and carbon dioxide concentrations were not reported. Serrador et al. (2000b) measured cerebral blood flow velocity in subjects during repeated parabolic flights, interventions that provoke brief (tens of seconds) microgravity, alternating with hypergravity. Middle cerebral artery blood flow velocity decreased to a significantly greater extent in seven of 13 subjects who were orthostatically intolerant after parabolic flights than in the remaining six subjects who were orthostatically tolerant. Importantly, reductions of cerebral blood flow velocity were not associated with reductions of blood pressure in either group.
In the present study, static cerebral blood flow velocity did not change at rest and was well maintained during orthostatic stress simulated by lower body suction in-flight. Moreover, decreases of cerebral blood flow velocity during upright tilt were significantly smaller after than before spaceflight; indeed, none of the six astronauts had a greater reduction during upright tilt on landing day than before spaceflight. Thus, spaceflight does not seem to impair, and may even enhance, static cerebral autoregulation. By extension of this argument, impaired cerebral autoregulation cannot explain the inability to remain standing for 10 min after spaceflight despite preserved arterial pressure in some astronauts (Buckey et al. 1996). We speculate that other mechanisms, such as kinesthetic or vestibular discordance (Young et al. 1996), may be responsible for this orthostatic intolerance with a normal blood pressure. For example, postural instability from changes in somatic sensory mechanisms or vestibular dysfunction could make standing difficult after space flight, even with normal cerebral blood flow and autoregulation.
Dynamic cerebral autoregulation in microgravity
Transcranial ultrasonography yields beat-by-beat measurements of large cerebral artery flow velocities (Åaslid et al. 1982), which fluctuate with and buffer dynamic changes of arterial pressure. Transfer function analysis quantifies the ability of cerebral vessels to respond to beat-by-beat arterial pressure changes, and thus yields insights into dynamic, frequency-dependent properties of cerebral autoregulation (Zhang et al. 1998a, 2000, 2002). For example, short-term fluctuations in cerebral blood flow velocity at high frequencies (> 0.20 Hz, or faster than every 5 s), closely match those observed in arterial pressure, and probably reflect a simple mechanical response to systemic arterial pressure changes, without effective cerebral vasomotor buffering. In contrast, slow fluctuations (below about 0.07 Hz, or slower than every 14 s; Blaber et al. 1997; Zhang et al. 1998a, 2000, 2002; Panerai et al. 1999; Lipsitz et al. 2000; Ogoh et al. 2005; Ogawa et al. 2006) in cerebral blood flow velocity are more independent of changes in arterial pressure (low coherence or low gain), indicating that dynamic autoregulation is more effective at lower frequency ranges. Thus, the buffering capacity of the cerebral vascular bed is dependent on the frequency of fluctuations in perfusion pressure.
In the present study, we tested the hypothesis that dynamic cerebral autoregulation is impaired by changes occurring in microgravity, including, especially, increases in sympathetic nerve activity (Ertl et al. 2002), which may shift the autoregulatory relation (Giller, 1990) rightwards and thereby increase the dependency of cerebral blood flow on changes of blood pressure (Levine et al. 1994; Zhang et al. 1997). We found that the gain between arterial pressure and cerebral blood flow velocity variability at low frequencies decreases significantly during and immediately after spaceflight. In other words, smaller oscillations in cerebral blood flow velocity occur for given changes in blood pressure, which is suggestive of improved, rather than impaired, dynamic cerebral autoregulation. Thus, reductions of cerebral blood flow velocity at low frequencies were less during spaceflight and on landing day than before spaceflight. Reasons for the discrepancy between the results from a simulated microgravity study (Zhang et al. 1997) and the present spaceflight study are not apparent. Head-down tilt-bed rest leads to a persistently increased headward hydrostatic pressure gradient, which is not observed in space (Watenpaugh & Hargens, 1996).
Mechanisms underlying improved dynamic cerebral autoregulation are unclear. One potential explanation is that spaceflight increases the responsiveness of cerebral vascular smooth muscle to changes of transmural pressure. This possibility is supported by a simulated microgravity study (Pavy-Le Traon et al. 2002). In this study, net cerebral vascular responses to abrupt thigh cuff release were not impaired. However, the time to maximum vasodilatation was longer in subjects who experienced orthostatic intolerance after 7 days of bed rest than in subjects who did not. In our study, no astronaut developed orthostatic intolerance.
Limitations
There are several potential limitations in the present study. First, transcranial Doppler ultrasound signals may not be reproducible if the angle of insonation or the position of the insonation window is not constant during serial experiments. However, in our experiments, we precisely positioned and fixed the Doppler probe with custom-made holders molded to each astronaut's facial bone structure and ear (Giller & Giller, 1997). This resulted in relatively small repeated measurement errors in two preflight sessions.
Second, cerebral blood flow velocity is not necessarily equivalent to cerebral blood flow, since the relationship between velocity and flow depends on the area of the insonated vessel. However, if middle cerebral artery diameter remains relatively constant, changes in cerebral blood flow velocity are proportional to changes in cerebral blood flow. Angiographic studies (Huber & Handa, 1967), magnetic resonance imaging with transcranial Doppler ultrasound measurements (Serrador et al. 2000a) and direct visualization of the middle cerebral artery during surgery (Giller et al. 1993) indicate that during a variety of stimuli known to affect cerebral blood flow, the diameter of the middle cerebral artery changes only minimally (less than 4%). Thus, it is likely that the changes of blood flow velocity that we measured with Doppler ultrasound are directly proportional to changes in global cerebral blood flow (Larsen et al. 1994; Newell et al. 1994).
Third, our study suffers from a major limitation shared by most research conducted in astronauts, namely that the number of subjects is small. Conceivably, some of our results might have been different with a larger study population, though by every known criterion, these subjects were indistinguishable from and representative of the broader astronaut population. As a consequence, it is possible that our inability to identify significant changes reflects type II statistical errors owing to the small number of subjects. We studied six astronauts preflight and postflight and only four astronauts in-flight. To partly mitigate this problem, we present individual data as much as possible.
Fourth, in our study, all astronauts were able to remain upright at 60 deg for 10 min both pre- and postflight. Conceivably, orthostatic intolerance might have developed had more intense or more prolonged orthostatic challenges been mounted. Zhang et al. (1997) reported impaired dynamic cerebral autoregulation (increases in low-frequency gain) with intense, 50 mmHg, lower body suction after bed rest. Bondar et al. (1995) and Zhang et al. (1997) showed that decreases of cerebral blood flow velocity may not be apparent until just before presyncope. This was shown also by Carey et al. (2001), who found that autoregulatory responses to tilt are initially preserved in patients with recurrent vasovagal syncope, but deteriorate at the onset of vasovagal syncope. It should be noted that the absence of presyncope during upright posture in this study was considerably different from the incidence of orthostatic intolerance reported in previous spaceflight studies (e.g. Buckey et al. 1996). This apparent difference has been discussed at length in other reports from the Neurolab experiments (e.g. Levine et al. 2002). We cannot exclude the possibility that the nature of the research conducted in this complex spaceflight experiment might have been different from other studies on astronauts, and the previous protocols in this study might have influenced our results. For example, some of these subjects were exposed to periodic centrifugation as part of another experiment. However, one-third of our subjects (2 of 6) were never exposed to centrifugation, yet all their haemodynamic and autonomic responses were identical to those that were. Moreover, there is no evidence that interactions between the neurovestibular system and other circulatory control mechanisms are anything but short lived, lasting seconds to minutes (Shortt & Ray, 1997). Furthermore, the same experimental order was followed during all experiments, thereby minimizing the impact of any order effect on pre-, in- or postflight comparisons.
Fifth, arterial carbon dioxide exerts a significant influence on cerebral blood flow and cerebral autoregulation (Giller et al. 1993; Zhang et al. 1998a). This factor probably did not affect our results, since in our study, there were no significant changes of end-tidal carbon dioxide partial pressures during pre-, in- and postflight sessions.
Conclusion
In conclusion, we report that decreases in static cerebral blood flow velocity during orthostatic stress tended to be smaller during and after spaceflight than before, suggesting that static cerebral autoregulation was preserved in this group of astronauts. Moreover, fluctuations of cerebral blood flow in response to dynamic changes of arterial pressure were smaller in the low-frequency range during and after spaceflight than before, pointing towards enhanced, rather than impaired, dynamic cerebral autoregulation. Thus, this Neurolab study rejects the hypotheses that abnormalities of static or dynamic cerebral autoregulation develop during spaceflight and contribute to impaired ability of cerebral mechanisms to maintain cerebral blood flow constant in the upright position on Earth.
Acknowledgments
In a complex spaceflight experiment such as this one, there are so many individuals who contribute substantively to the success of the project that it is extremely difficult to acknowledge them all. At an absolute minimum, the crew of the Neurolab mission must be acknowledged for an outstanding effort as experimentalists and subjects in-flight. Special acknowledgement must also be made of the efforts of Mike Grande and Stuart Johnston from NASA/Lockheed Martin, and Matt Morrow, Troy Todd and Dak Quarles from the Presbyterian Hospital of Dallas, who contributed in major ways to the success of the project.
References
- Åaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. J Appl Physiol. 1996;81:98–104. doi: 10.1152/jappl.1996.81.1.98. [DOI] [PubMed] [Google Scholar]
- Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol. 2001;86:157–168. doi: 10.1007/s004210100473. [DOI] [PubMed] [Google Scholar]
- Bagian JP, Hackett P. Cerebral blood flow: comparison of ground-based and spaceflight data and correlation with space adaptation syndrome. J Clin Pharmacol. 1991;31:1036–1040. doi: 10.1002/j.1552-4604.1991.tb03668.x. [DOI] [PubMed] [Google Scholar]
- Blaber AP, Bondar RL, Stein F, Dunphy PT, Moradshahi P, et al. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 1997;28:1686–1692. doi: 10.1161/01.str.28.9.1686. [DOI] [PubMed] [Google Scholar]
- Blomqvist CG, Stone HL. Cardiovascular adjustments to gravitational stress. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III, Peripheral Circulation and Organ Blood Flow. Bethesda, MD, USA: American Physiological Society; 1983. pp. 1025–1063. [Google Scholar]
- Bondar RL, Kassam MS, Stein F, Dunphy PT, Fortney S, Riedesel ML. Simultaneous cerebrovascular and cardiovascular responses during presyncope. Stroke. 1995;26:1794–1800. doi: 10.1161/01.str.26.10.1794. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, et al. Orthostatic intolerance after spaceflight. J Appl Physiol. 1996;81:7–18. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- Bungo MW, Charles JB, Johnson PC., Jr Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med. 1985;56:985–990. [PubMed] [Google Scholar]
- Carey BJ, Manktelow BN, Panerai RB, Potter JF. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation. 2001;104:898–902. doi: 10.1161/hc3301.094908. [DOI] [PubMed] [Google Scholar]
- Cox JF, Tahvanainen KU, Kuusela TA, Levine BD, Cooke WH, Mano T, et al. Influence of microgravity on astronauts' sympathetic and vagal responses to Valsalva's manoeuvre. J Physiol. 2002;538:309–320. doi: 10.1113/jphysiol.2001.012574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, et al. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J Physiol. 2002;538:321–329. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldager N, Blomqvist CG. Repeated plasma volume determination with the Evans Blue dye dilution technique: the method and a computer program. Comput Biol Med. 1991;21:35–41. doi: 10.1016/0010-4825(91)90033-6. [DOI] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol. 1994;77:1776–1783. doi: 10.1152/jappl.1994.77.4.1776. [DOI] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. J Appl Physiol. 1996;81:2134–2141. doi: 10.1152/jappl.1996.81.5.2134. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD, Pawelczyk JA, Ertl AC, Diedrich A, Cox JF, et al. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. J Physiol. 2002;544:653–664. doi: 10.1113/jphysiol.2002.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller CA. The frequency-dependent behavior of cerebral autoregulation. Neurosurgery. 1990;27:363–368. doi: 10.1097/00006123-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–742. [PubMed] [Google Scholar]
- Giller CA, Giller AM. A new method for fixation of probes for transcranial Doppler ultrasound. J Neuroimaging. 1997;7:103–115. doi: 10.1111/jon199772103. [DOI] [PubMed] [Google Scholar]
- Herault S, Fomina G, Alferova I, Kotovskaya A, Poliakov V, Arbeille P. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets) Eur J Appl Physiol. 2000;81:384–390. doi: 10.1007/s004210050058. [DOI] [PubMed] [Google Scholar]
- Hoffler GW, Wolthuis RA, Johnson PL. Apollo space crew cardiovascular evaluations. Aerospace Med. 1974;45:807–820. [PubMed] [Google Scholar]
- Hopkins WG. A New View of Statistics. Internet Society for Sport Science; 2000. Reliability from consecutive pairs of trials. sportsci.org http://sportsci.org/resource/stats/xrely.xls. [Google Scholar]
- Huber P, Handa J. Effect of contrast material, hypercapnia, hyperventilation, hypertonic glucose and papaverine on the diameter of the cerebral arteries. Angiographic determination in man. Invest Radiol. 1967;2:17–32. doi: 10.1097/00004424-196701000-00016. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Zhang R, Perhonen MA, Zuckerman JH, Levine BD. Reduced baroreflex control of heart period after bed rest is normalized by acute plasma volume restoration. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1256–R1262. doi: 10.1152/ajpregu.00613.2002. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effect of head-down-tilt bed rest and hypovolemia on dynamic regulation of heart rate and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2189–R2199. doi: 10.1152/ajpregu.2000.279.6.R2189. [DOI] [PubMed] [Google Scholar]
- Jacob G, Atkinson D, Jordan J, Shannon JR, Furlan R, et al. Effects of standing on cerebrovascular resistance in patients with idiopathic orthostatic intolerance. Am J Med. 1999;106:59–64. doi: 10.1016/s0002-9343(98)00364-7. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Iwase S, Michikami D, Fu Q, Mano T, et al. Increased vasomotor sympathetic nerve activity and decreased plasma nitric oxide release after head-down bed rest in humans: disappearance of correlation between vasoconstrictor and vasodilator. Neurosci Lett. 2000;281:21–24. doi: 10.1016/s0304-3940(00)00804-1. [DOI] [PubMed] [Google Scholar]
- Larsen FS, Olsen KS, Hansen BA, Paulson OB, Knudsen GM. Transcranial Doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke. 1994;25:1985–1988. doi: 10.1161/01.str.25.10.1985. [DOI] [PubMed] [Google Scholar]
- Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation. 1994;90:298–306. doi: 10.1161/01.cir.90.1.298. [DOI] [PubMed] [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, et al. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke. 2000;31:1897–1903. doi: 10.1161/01.str.31.8.1897. [DOI] [PubMed] [Google Scholar]
- Newell DW, Åaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke. 1998;29:104–111. doi: 10.1161/01.str.29.1.104. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Iwasaki K, Shibata S, Kato J, Ogawa S, Oi Y. The effect of sevoflurane on dynamic cerebral blood flow autoregulation assessed by spectral and transfer function analysis. Anesth Analg. 2006;102:552–559. doi: 10.1213/01.ane.0000189056.96273.48. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, et al. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1461–H1467. doi: 10.1152/ajpheart.00948.2004. [DOI] [PubMed] [Google Scholar]
- Panerai RB, Dawson SL, Potter JF. Linear and nonlinear analysis of human dynamic cerebral autoregulation. Am J Physiol Heart Circ Physiol. 1999;277:H1089–H1099. doi: 10.1152/ajpheart.1999.277.3.H1089. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Pavy-Le Traon A, Costes-Salon MC, Vasseur-Clausen P, Bareille MP, Maillet A, Parant M. Changes in kinetics of cerebral auto-regulation with head-down bed rest. Clin Physiol Func Im. 2002;22:108–114. doi: 10.1046/j.1365-2281.2002.00403.x. [DOI] [PubMed] [Google Scholar]
- Pavy-Le Traon A, Vasseur P, Arbeille P, Guell A, Bes A, Gharib C. Effects of 28-day head-down tilt with and without countermeasures on lower body negative pressure responses. Aviat Space Environ Med. 1995;66:982–991. [PubMed] [Google Scholar]
- Pawelczyk JA, Zuckerman JH, Blomqvist CG, Levine BD. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H2230–H2239. doi: 10.1152/ajpheart.2001.280.5.H2230. [DOI] [PubMed] [Google Scholar]
- Rucker J, Tester J, Fedorko L, Takeuchi A, Mascia L, Vesely A, et al. Normocapnia improves cerebral oxygen delivery during conventional oxygen therapy in carbon monoxide-exposed research subjects. Ann Emerg Med. 2002;40:611–618. doi: 10.1067/mem.2002.129723. [DOI] [PubMed] [Google Scholar]
- Schondorf R, Benoit J, Wein T. Cerebrovascular and cardiovascular measurements during neurally mediated syncope induced by head-up tilt. Stroke. 1997;28:1564–1568. doi: 10.1161/01.str.28.8.1564. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000a;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Shoemaker JK, Brown TE, Kassam MS, Bondar RL, Schlegel TT. Cerebral vasoconstriction precedes orthostatic intolerance after parabolic flight. Brain Res Bull. 2000b;53:113–120. doi: 10.1016/s0361-9230(00)00315-4. [DOI] [PubMed] [Google Scholar]
- Shortt TL, Ray CA. Sympathetic and vascular responses to head-down neck flexion in humans. Am J Physiol Heart Circ Physiol. 1997;272:H1780–H1784. doi: 10.1152/ajpheart.1997.272.4.H1780. [DOI] [PubMed] [Google Scholar]
- Töyry JP, Kuikka JT, Lansimies EA. Regional cerebral perfusion in cardiovascular reflex syncope. Eur J Nucl Med. 1997;24:215–218. doi: 10.1007/BF02439557. [DOI] [PubMed] [Google Scholar]
- Triebwasser JH, Johnson RL, Jr, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- Watenpaugh DE, Hargens AR. The cardiovascular system in microgravity. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology. Bethesda, MD, USA: American Physiological Society; 1996. pp. 631–674. [Google Scholar]
- Wilkerson MK, Muller-Delp J, Colleran PN, Delp MD. Effects of hindlimb unloading on rat cerebral, splenic, and mesenteric resistance artery morphology. J Appl Physiol. 1999;87:2115–2121. doi: 10.1152/jappl.1999.87.6.2115. [DOI] [PubMed] [Google Scholar]
- Young LR, Mendoza JC, Groleau N, Wojcik PW. Tactile influences on astronaut visual spatial orientation: human neurovestibular studies on SLS-2. J Appl Physiol. 1996;81:44–49. doi: 10.1152/jappl.1996.81.1.44. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol. 1998a;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol. 1998b;85:1113–1122. doi: 10.1152/jappl.1998.85.3.1113. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Levine BD. Spontaneous fluctuations in cerebral blood flow: insights from extended-duration recordings in humans. Am J Physiol Heart Circ Physiol. 2000;278:H1848–H1855. doi: 10.1152/ajpheart.2000.278.6.H1848. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effects of head-down-tilt bed rest on cerebral hemodynamics during orthostatic stress. J Appl Physiol. 1997;83:2139–2145. doi: 10.1152/jappl.1997.83.6.2139. [DOI] [PubMed] [Google Scholar]