Abstract
Voltage-gated potassium channels effectively regulate dendritic excitability in neurones. It has been suggested that in the distal apical dendrite of layer 5B (L5B) neocortical pyramidal neurones, K+ conductances participate in active dendritic synaptic integration and control regenerative dendritic potentials. The ionic mechanism for triggering these regenerative potentials has yet to be elucidated. Here we used two-electrode voltage clamp (TEVC) to quantitatively record K+ conductance densities of a sustained K+ conductance in the soma and apical dendrite of L5B neurones of adult rats. We report that the somatic and proximal dendritic sustained voltage-gated K+ conductance density is more than 10-fold larger than previous estimates. The results obtained using TEVC were corroborated using current-clamp experiments in combination with compartmental modelling. Possible error sources, including inaccurate measurement of the passive membrane parameters and unknown axonal and basal dendritic conductance distributions, were shown not to distort the density estimation considerably. The sustained voltage-gated K+ conductance density was found to decrease steeply along the apical dendrite. The steep negative K+ conductance density gradient along the apical dendrite may help to define a distal, low-threshold region for amplification of distal synaptic input in L5B pyramidal neurones.
It has been shown that the apical dendrite of layer 5B (L5B) pyramidal neurones performs several non-linear transformations of synaptic input, most clearly exemplified by large, regenerative Ca2+ potentials that have been readily recorded from the apical dendrite of L5B neocortical pyramidal neurones (Amitai et al. 1993; Kim & Connors, 1993; Reuveni et al. 1993; Schiller et al. 1997; Larkum et al. 1999b, 2001; Zhu, 2000). In these neurones, when a back-propagating action potential (AP) coincides with distal synaptic input, a regenerative dendritic Ca2+ potential is generated, triggering a burst of several APs at the soma (Larkum et al. 1999b) thus inducing a large change in the informational content of the neuronal output (Lisman, 1997). It has been hypothesized that the initiation of these regenerative Ca2+ potentials may require an increase in the density of voltage-gated Ca2+ conductance along the apical dendrite (Reuveni et al. 1993; Schaefer et al. 2003b). Alternatively, because of the intricate interaction of ionic currents in the generation of regenerative Ca2+ potentials (Larkum et al. 1999a, 2001), a substantial decrease in the relative density of voltage-gated K+ conductances in the distal dendrite might render the distal dendrite sufficiently excitable to allow the generation of regenerative dendritic Ca2+ potentials.
Such complex neuronal mechanisms should be explained by extracting the kinetics of the various voltage-gated conductances in these neurones and constructing a detailed compartmental model. This approach requires quantitative information on the density and kinetics of voltage-gated conductances in various compartments of the neurone to be obtained. Dendritic distributions of K+ currents have been measured in dendrites from various type of neurones (Bischofberger & Jonas, 1997; Hoffman et al. 1997; Korngreen & Sakmann, 2000; Bekkers, 2000b; Martina et al. 2003). However, there are technical problems inherent in the frequently used cell-attached preparation – the estimation of patch membrane area introduces a significant error to the determination of conductance densities. Moreover, the high variability in measurements due to the random sampling of the membrane with each patch containing only very few channels requires a large number of recordings.
Recently, we introduced a technique that enables the measurement of the density of voltage-gated K+ conductances from the soma and apical dendrites of neurones by recording ionic currents using two-electrode voltage clamp (TEVC) combined with measurement of passive membrane parameters and reconstruction of neuronal morphology (Schaefer et al. 2003a). Here we applied this technique to measure the density of voltage-gated K+ conductances in the soma and along the apical dendrite of L5B neurones in the somatosensory cortex.
Methods
Slice preparation
Acute brain slices (sagittal, 300 μm thick) were prepared from the somatosensory cortex of 13- to 45-day-old Wistar rats killed by rapid decapitation following shallow anaesthesia with isoflurane or halothane, in accordance with the guidelines of the Max-Planck-Society and Bar-Ilan University animal welfare committees, using previously described techniques (Stuart et al. 1993). Slices were superfused throughout the experiment with an oxygenated artificial cerebrospinal fluid containing (mm): NaCl 125, NaHCO3 15, KCl 2.5, NaH2PO4 1.25, MgCl2 1, CaCl2 2 and glucose 25; pH adjusted to 7.4 with 5% CO2 and 95% O2, 310 mosmol kg−1 at room temperature (20–22°C). Pyramidal neurones from L5B in the somatosensory cortex were visually identified using infrared differential interference contrast (IR-DIC) videomicroscopy (Stuart et al. 1993).
Solutions and drugs
The standard pipette solution contained (mm): potassium gluconate 125, KCl 20, Hepes 10, MgATP 4, sodium phosphocreatine 10, EGTA 0.5, GTP 0.3, and 0.2% biocytin; pH adjusted to 7.2 with KOH, 312 mosmol kg−1. The bath solution for TEVC experiments contained (mm): NaCl 125, NaCO3 15, KCl 2.5, MgCl2 1, CoCl2 2, glucose 25, ZD7288 0.03 and TTX 0.1; pH adjusted to 7.4 with 5% CO2 and 95% O2, 308 mosmol kg−1. A liquid junction potential of 10 mV was corrected for a posteriori. For cell-attached recordings the pipette solution contained (mm): NaCl 135, KCl 5.4, MgCl2 1, CaCl2 1.8, Hepes 5 and TTX 0.1; pH adjusted to 7.2 with NaOH, 290 mosmol kg−1. TTX (Tocris, Bristol, UK) and ZD7288 (Tocris, Bristol, UK) were stored as stock solutions in doubly distilled water (at −20°C and +4°C, respectively) and added directly to the bath solution.
Electrophysiology
TEVC recordings were made with two HS-2Ax0.1M head-stages and an Axoclamp-2B amplifier (Axon Instruments, Union City, CA, USA). Whole-cell recordings were obtained with two patch pipettes, the tips of which were separated on average by 40 μm. Simulations have shown that the accuracy and stability of the conductance calculation were not affected by the interelectrode distance (Fig. 3 in Supplemental material). No series resistance compensation was used. It is important to note that series resistance has a different manifestation in a TEVC circuit than in a single-electrode voltage clamp. In the single-electrode voltage clamp the same electrode is used for current injecting and voltage recording. Therefore, part of the injected current results in a voltage drop across the electrode series resistance, greatly impairing the accurate clamping of the membrane potential. The ideal TEVC circuit does not suffer from the electrode series resistance because one electrode is used for current injection and one for voltage recording. As no current flows across the voltage recording electrode, the electrode series resistance does not affect the accurate measurement of the membrane potential. In the real TEVC circuit, the series resistance is mostly stray resistance from the bath and reference electrode. In the experiments reported in this manuscript, the bath series resistance was very small (a few kilohms) compared to the relevant membrane resistances and did not distort the recordings. Capacitive coupling between the electrodes was minimized by placing a grounded shield that extended almost to the bath fluid level between the two electrodes. To increase clamp gain, the feedback current injected via the current-injecting electrode was phase shifted (phase-lag control of the Axoclamp-2B) with a time constant of 1–5 ms leading to filtering of the initial rise in the recorded current. The phase shift had to be introduced in the TEVC circuit to allow for stability with maximal gain (Finkel & Gage, 1985). Achieving maximal gain is desirable as both the clamp fidelity and the actual bandwidth depend on the gain (Finkel & Gage, 1985). In this study we only report properties of the slow conductance activated 50 ms after the onset of the depolarizing voltage-clamp command. Hence, our results are unaffected by the limited bandwidth. Furthermore, we have shown previously (Schaefer et al. 2003a), that systematic errors introduced by misestimation of channel kinetics become important only for very rapidly changing conductances (with current rise time smaller than 0.5 ms). Voltage and current were filtered at 10 kHz, sampled at 20 or 50 kHz using the program ‘Pulse’ (Version 8.1, Heka Electronic, Lambrecht, Germany), digitized by an ITC-16 interface (Instrutech, Greatneck, NY, USA) and stored on the hard disk of a computer. Capacitive and leak currents were subtracted off-line by scaling of pulses taken at hyperpolarized potentials. Patch pipettes (5–10 MΩ) were pulled from thick-walled borosilicate glass capillaries (outer diameter, 2.0 mm; wall thickness, 0.5 mm; Hilgenberg, Malsfeld, Germany) and were coated with Sylgard 184 (Dow Corning) prior to the experiment. The distance of the dendritic recording from the soma and the distance between the tips of the current-injecting and voltage-recording electrodes were measured from video pictures taken by a frame grabber. At the end of each experiment, slices were fixed in cold (4°C) 100 mm phosphate buffer (PBS, pH 7.4) containing 4% paraformaldehyde. After fixation, the slices were incubated for 2 h in avidin–biotinylated horseradish peroxidase (ABC-Elite, Vector Laboratories, Peterborough, UK) and the stain was developed using 0.015% diaminobenzidine. The stained neurones were digitally traced using a Neurolucida system (MicroBrightField, Colchester, VT, USA). Passive membrane parameters [membrane resistance (Rm), axial resistance (Ri) and membrane capacitance (Cm)] were determined as previously described (Stuart & Spruston, 1998; Roth & Häusser, 2001). Briefly, prior to switching on the TEVC both electrodes were in bridge mode of the Axoclamp-2B. In this configuration, a current pulse was injected via one of the electrodes and the voltage deflection was monitored by both electrodes. The passive membrane properties were determined simultaneously by fitting a passive membrane model using the reconstructed morphology of the soma and dendrites to the average of 30 such membrane potential traces measured in the same cell (Clements & Redman, 1989; Stuart & Spruston, 1998; Roth & Häusser, 2001). The fitting was carried out using NEURON routines kindly provided by A. Roth (University College London, UK).
TEVC currents were analysed as previously described (Schaefer et al. 2003a). In brief, reconstructed morphology of the soma, axon and dendrites and measured passive membrane parameters were modelled in NEURON. TEVC experiments were simulated at the positions of the current-injecting and voltage-recording electrodes. Simulated clamp currents were fitted to the experimentally recorded ones with the K+ conductance densities as the only free parameters yielding conductances densities for −90 to +30 mV in steps of 10 mV (see Figs 1 and 2). A one-gate Boltzmann curve was fitted to the median of the conductance densities from 45 to 55 ms for the voltages from −90 to +20 mV, where in all cases a stable voltage clamp was achieved. To assess the influence of axonal and basal dendritic hotspots of voltage-gated K+ conductances, clamp currents were fitted with the additional constraint of high axonal or basal dendritic K+ conductance densities. The channel kinetics used for this purpose were derived from nucleated patch measurements (Korngreen & Sakmann, 2000) and can be obtained from the authors. All data analysis was performed using custom-written routines in NEURON 5.4 (Hines & Carnevale, 1997) and Igor Pro 4.09 (Wavemetrics, Lake Oswego, OR, USA).
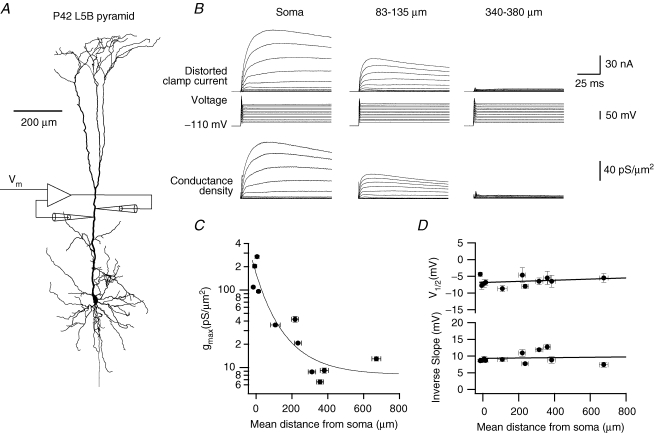
Figure 1.
Somato-dendritic distribution of voltage-gated K+ conductance densities in L5B pyramidal neurones A, reconstruction of an L5B pyramidal neurone from a P42 rat used for the recording of dendritic currents 340–380 μm distal to the soma. Schematic illustration of the dendritic two-electrode voltage clamp (TEVC) recording configuration has been added and the command potential (Vm) has been indicated. B, TEVC recordings of voltage-gated K+ currents from the soma (left traces) and apical dendrites of three L5B pyramidal neurones as indicated. The voltage-clamp command potential is shown below the current records. The K+ conductance density determined using reconstructed morphology and passive membrane parameters is displayed below the voltage traces. C, peak K+ conductance density calculated by fitting a one-gate Boltzmann function to the measured K+ conductance at the 50 ms time point. The vertical error bars display the error estimated by the curve fitting routine. The horizontal error bars indicate the positions of the voltage-recording and current-injection electrodes of the TEVC circuit (see Methods). The continuous line is a shifted exponential fit: gmax = 8 + 164 × exp(–x/80) where gmax is the K+ conductance density in pS μm−2 and x is the distance from the soma along the apical dendrite in micrometers. D, the voltage of half activation (V½) and inverse slope (k) of the activation curve, obtained by fitting a one-gate Boltzmann function to the conductance 50 ms after the onset of depolarization, are independent of the distance from the soma. Vertical error bars in C and D reflect the error of the Boltzmann fit.
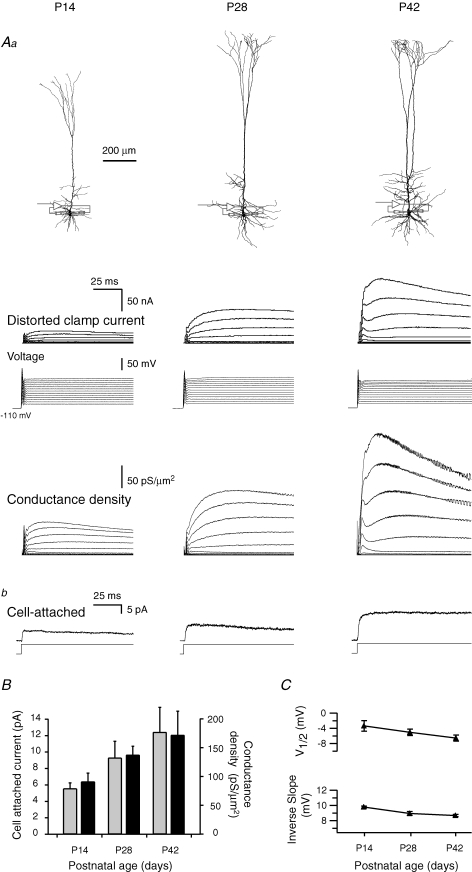
Figure 2.
Somatic K+ conductance densities increase throughout development Aa, reconstructions of neurones, from left to right, were from P14, P28 and P42 animals. Below, voltage-clamp recordings of voltage-gated K+ currents from the soma of the three L5B pyramidal neurones are shown above the voltage traces. Note distortions to the onset of the clamp potential because of incomplete voltage control. The voltage-clamp potential and the conductance density for each record are shown below the current recordings. Ab, recordings of voltage-gated K+ currents recorded using the cell-attached configuration from the soma of three different neurones (from P14, P28 and P42 animals). Responses were evoked by a voltage step to +140 mV relative to the resting membrane potential. B, summary of the developmental changes in the average maximal current from cell-attached recordings (grey bars; P14, n = 40; P28, n = 9; P42, n = 9) and the average maximal conductance density measured by the two-electrode voltage clamp technique (black bars; P14, n = 6; P28, n = 4; P42 n = 4). Error bars are s.e.m.C, summary of the developmental changes to the voltage of half activation (V½) and inverse slope (k) of the activation curve, obtained by fitting a one-gate Boltzmann function to the conductance 50 ms after the onset of depolarization. Error bars are s.e.m. between cells within each age group.
Cell-attached recordings were carried out using an Axopatch-200B amplifier (Axon Instruments). All pipettes were coated with Sylgard 184 and fire-polished before use. A constant experimental procedure was used in an effort to obtain patches with similar area. Positive pressure (20–40 mbar) was applied to the pipette as it was advanced to the cell. The tip of the pipette was gently pressed against the membrane and a negative pressure that did not exceed 10 mbar was applied. The experiments on soma displayed in Fig. 2 were carried out with electrodes that had a resistance of 8–10 MΩ. Higher resistance electrodes (13–17 MΩ) were used to record K+ currents from the initial segment of the axon. To allow comparison, several recordings were carried out at the soma with electrodes of identical properties. Linear leak and capacitance currents were subtracted either on-line with a leak substration protocol (P/6 protocol) from a hyperpolarized holding potential of −40 mV, or off-line from empty traces. The whole-cell recordings from the soma and apical dendrite in the current-clamp mode presented in Figs 3 and 4 were carried out using a Multiclamp-700B amplifier (Axon Instruments) with the same pipette and bath solutions as used in the voltage-clamp experiments.
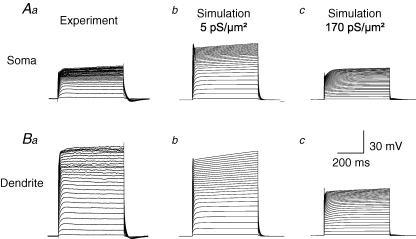
Figure 3.
Recorded and simulated membrane rectification due to the activation of voltage-gated K+ conductances Aa, membrane potential traces recorded in the current-clamp mode following injection of a series of increasing current steps (0.05–3.95 nA in steps of 0.15 nA) via a whole-cell electrode at the soma of an L5B neocortical pyramidal neurone from a P42 animal. The neurone was filled with biocytin, stained, reconstructed using a Neurolucida system, and converted to a computer code readable by the simulation environment NEURON. Ab, membrane potential traces simulated using the same morphology of the neurone from which the experimental traces displayed in Aa were recorded. In this simulation, a conductance density of 5 pS μm−2 for both Kfast and Kslow was homogenously inserted into the somato-dendritic tree. The passive parameters were set to Ri= 150 Ω cm, Rm= 15000 Ω cm2 and Cm= 0.75 μF cm−2 for all the simulations displayed in this figure. Ac, membrane potential traces simulated using the same morphology of the neurone from which the experimental traces displayed in Aa were recorded. In this simulation, a conductance density of 170 pS μm−2 for both Kfast and for Kslow was homogenously inserted into the somato-dendritic tree. Ba, membrane potential traces recorded in the current-clamp mode following injection of a series of increasing current steps (0.05–3.95 nA in steps of 0.15 nA) via a whole-cell electrode at the apical dendrite of an L5B neocortical pyramidal neurone 440 μm away from the soma. The neurone was filled with biocytin, stained, reconstructed using a Neurolucida system, and converted to a computer code readable by the simulation environment NEURON. Bb, membrane potential traces simulated using the same morphology of the neurone from which the experimental traces displayed in Ba were recorded. In this simulation, a conductance density of 5 pS μm−2 for both Kfast and for Kslow was homogenously inserted into the somato-dendritic tree. Bc, membrane potential traces simulated using the same morphology of the neurone from which the experimental traces displayed in Ba were recorded. In this simulation a conductance density of 170 pS μm−2 for both Kfast and for Kslow was homogenously inserted into the somato-dendritic tree.
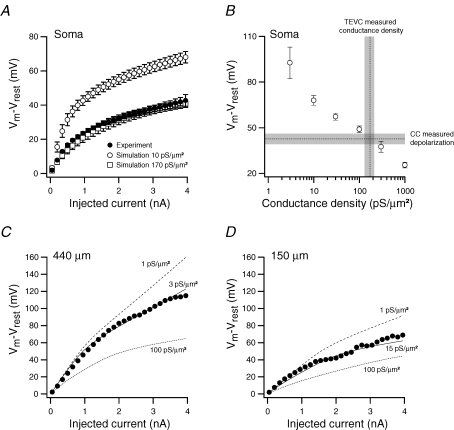
Figure 4.
Analysis of experimental and simulated current clamp experiments A, current–voltage (I–V) relationship of the somatic experiments displayed in Fig. 3A. The average deflection of the membrane potential measured 50 ms after the onset of the depolarization is displayed as a function of the injected current (•, n = 7). The same I–V relationships were also calculated for simulations using the morphologies of the same cells with a homogenous conductance density of Kslow and Kfast set to 10 (^, n = 4) and 170 pS μm−2 (□, n = 4), respectively. Error bars show s.e.m. The passive parameters were set to Ri= 150 Ω cm, Rm= 15000 Ω cm2 and Cm= 0.75 μF cm−2 for all the simulations displayed in this figure. B, the average simulated deflection of the membrane potential 50 ms after the onset of the depolarization due to the injection of 3.95 nA into the models that contained homogenous distributions of Kslow and Kfast at several values (^, n = 5). The conductance density we report in Fig. 1 (172 ± 42 pS μm−2) is displayed as a vertical line and the recorded membrane potential deflection after 3.95 nA current injection from A as a horizontal line. The grey bands show the s.e.m. for both of these values. C, an I–V relationship calculated from the dendritic recording displayed in Fig. 3Ba from 440 μm along the dendrite (•). The continuous lines are simulated I–V relationships of 1 pS μm−2 (dashed line) 3 pS μm−2 (continuous line) and 100 pS μm−2 (dotted line). D, an I–V relationship calculated from another dendritic recording from 150 μm along the dendrite of another neurone (•). The continuous lines are simulated I–V relationships (using the same morphology) of 1 pS μm−2 (dashed line) 15 pS μm−2 (continuous line) and 100 pS μm−2 (dotted line).
Results
Somato-dendritic K+ conductance densities measured with TEVC recordings
Figure 1B shows three TEVC experiments, one from the soma and two from the apical dendrite of three different L5B pyramidal neurones in acute brain slices prepared from the neocortex of 6-week-old (postnatal day (P) 42) rats. The K+ currents were recorded in response to voltage-clamp commands ranging from −90 to +20 mV shown below the current traces. Voltage-gated Ca2+ currents were eliminated by complete replacement of bath Ca2+ by Co2+. Consequently, currents from Ca2+-dependent K+ conductances were attenuated. The non-selective cation current Ih was blocked by bath application of 30 μm ZD7288, and the voltage-gated Na+ current was blocked by 100 nm TTX. Passive membrane properties were measured before the voltage-clamp protocol (see Methods). The neuronal morphology was reconstructed after biocytin filling (see Methods and Fig. 1A). Combined, these measurements provided for the quantitative calculation of K+ conductance densities and kinetics (Schaefer et al. 2003a). The onset of the current recording was distorted as a result of the overshoot of the clamp voltage during voltage commands produced by the phase lag imposed on the feedback signal of the TEVC (Fig. 1B). Therefore, we did not analyse the kinetics of the initial rise of the conductance, including the activation of the A-type K+ conductance (Korngreen & Sakmann, 2000; Bekkers, 2000a). Activation curves were calculated from the corrected conductance density traces using the values recorded 50 ms after the start of the depolarizing voltage pulse. It has been reported that at this time point the A-type K+ conductance is fully inactivated (Korngreen & Sakmann, 2000; Bekkers, 2000a). Thus, hereafter the Results and Discussion relate only to the slowly inactivating K+ conductance.
Our initial observation was that the maximal K+ current decreased as a function of the distance along the apical dendrite (Fig. 1B top traces). The application of the space-clamp correction algorithm to these current recordings revealed that the K+ conductance density decreased along the apical dendrite as a function of the distance from the soma, from 172 ± 41 (mean ±s.e.m., n = 4) at the soma to 9.6 ± 1.4 pS μm−2 (n = 4) further than 300 μm along the dendrite (Fig. 1B and C). The voltage of half activation (V½) and the inverse slope (k) of the steady-state activation curve were not dependent on the distance along the dendrite (R2 < 0.1, P > 0.4, Fig. 1D) suggesting that the K+ conductance density gradient was unlikely to be due to changes in channel types along the dendrite.
K+ conductance densities during development
The somatic K+ conductance density was more than 10-fold larger than previous estimates from nucleated patches made from 2-week-old (P14) animals (Korngreen & Sakmann, 2000; Bekkers, 2000a) but comparable to densities assumed in compartmental simulations of L5B pyramidal neurones (Mainen et al. 1995; Rhodes & Llinás, 2001; Antic, 2003; Schaefer et al. 2003b). To allow comparison of age groups and to assess whether the high somatic K+ conductance densities were also present in younger animals we conducted somatic TEVC experiments on L5B pyramidal neurones from animals that were 2, 4 and 6 weeks old (Fig. 2). K+ conductance densities displayed an apparent linear increase from 91 ± 15 (n = 6) to 172 ± 41 pS μm−2 (n = 4) between 2 and 6 weeks of age that was highly correlated (Fig 2A and B, r = 0.6, P < 0.03). The steady-state activation curve of the K+ conductance (described by V½ and k) displayed only a slight developmental change (Fig. 2C). Voltage-clamp experiments in the cell-attached configuration, although failing to provide accurate density estimates, reproduced this relative developmental increase (Fig. 2Ab). The average current in cell-attached patches increased significantly from 5.5 ± 0.7 (n = 40, P14) to 12.3 ± 3.0 pA (n = 9, P42; P < 0.05, ANOVA).
Estimation of K+-conductance densities from current-clamp recordings
The K+-conductance densities reported in Figs 1 and 2 were calculated using the previously published numerical algorithm for space-clamp correction (Schaefer et al. 2003a). To verify these results, we measured membrane rectification in response to current stimuli at the soma and apical dendrite of L5B pyramidal neurones. As first reported by Cole & Curtis (1941), the response of the neuronal membrane potential is not linear when large depolarizing currents are injected into the cell. This is caused by the activation of voltage-gated K+ channels that draws the membrane potential towards the K+ Nernstian equilibrium potential. Here we used this non-linearity to provide a rough estimate of the voltage-gated K+ conductance density at the site of current injection. The kinetics of two voltage-gated K+ conductances, an A-type fast-inactivating voltage-gated K+ conductance (Kfast) and a delayed rectifier slow-inactivating voltage-gated K+ conductance (Kslow), have been previously characterized in L5B pyramidal neurones (Korngreen & Sakmann, 2000; Bekkers, 2000a). Furthermore, Hodgkin-Huxley-type models for these conductances have been previously provided (Keren et al. 2005). These models were used to calculate the degree of membrane potential rectification at several levels of current injection and conductance densities (see Fig. 2 in Supplemental material for examples of the effect of Kslow and Kfast on the simulated membrane potential).
Based on this rationale, we carried out current-clamp experiments using the whole-cell configuration of the patch-clamp technique at the soma and apical dendrite of L5B pyramidal neurones. Voltage-gated Ca2+ currents were eliminated by complete replacement of bath Ca2+ by Co2+. Consequently, currents from Ca2+-dependent K+ conductances attenuated considerably; concurrently, the voltage-gated Na+ current was blocked by 100 nm TTX. Depolarizing current pulses were injected via the whole-cell recording pipette after balancing of the bridge. The changes to the somatic membrane potential following the injection of current steps ranging from 0.05 to 3.95 nA with 0.15 nA increments are displayed in Fig. 3Aa. After the experiment, the neuronal morphology was reconstructed after biocytin filling and converted to a code readable by the simulation environment NEURON (Hines & Carnevale, 1997, 2000). The kinetic models of Kfast and Kslow were inserted with a homogenous conductance density throughout the somato-dendritic tree, a current-clamp electrode was simulated at the soma and current pulses, identical to those used in the experiment, were injected via this electrode. When the densities of Kfast and Kslow were both set to 5 pS μm−2, similar to the values reported using nucleated patches, the simulated depolarization of the somatic membrane potential (Fig. 3Ab) was substantially larger than the experimentally recorded depolarization (Fig. 3Aa). Next we simulated the same experiment again only with a homogenous density of 170 pS μm−2 that is similar to the density we obtained for the soma using TEVC recordings and the space-clamp correction algorithm. These simulations produced membrane potential depolarization (Fig. 3Ac) of similar magnitude to those recorded experimentally (Fig. 3Aa). In the second stage of the experiment, a whole-cell pipette was attached to the apical dendrite 440 μm away from the soma (Fig. 3B). Injecting identical current pulses to the dendrite (Fig. 3Ba) as those applied at the soma (Fig. 3Aa) produced much larger changes to the membrane potential. As with the somatic experiment, the dendritic experiment was simulated using two values of the conductance density of Kfast and Kslow, 5 (Fig. 3Bb) and 170 pS μm−2 (Fig. 3Bc), respectively. In contrast to the simulations of the somatic membrane potential, the simulated changes of the dendritic membrane potential resembled more closely the experimentally recorded traces when a conductance density of 5 pS μm−2 was used than when a conductance density of 170 pS μm−2 was inserted into the model.
The experiment and simulations displayed in Fig. 3 were performed on several neurones and the analysis of the results obtained is displayed in Fig. 4. Figure 4A shows a graph of the average current–voltage relationship recorded from seven neurones compared to the average current–voltage relationships simulated in four reconstructed morphologies using conductance densities of 10 or 170 pS μm−2 for both Kfast and Kslow. The simulated current–voltage relationship obtained using 170 pS μm−2 was in close agreement with the experimentally recorded relationship whereas that simulated with a conductance density of 10 pS μm−2 deviated considerably from the experimental one (Fig. 4A). The average simulated depolarization obtained following 3.95 nA current injection via the somatic electrode is plotted in Fig. 4B as a function of several conductance densities. The average experimental depolarization obtained following injection of the same amount of current was also plotted as a horizontal line on the same graph and the conductance density value calculated from the voltage-clamp experiments with the space-clamp correction algorithm values as a vertical line with their respective error ranges indicated as grey bands. The intercept of these two experimental results was in good agreement with the simulated values (Fig. 4B). Finally, the current–voltage relationship was measured for two dendritic experiments at 440 (Fig. 4C) and 154 μm (Fig. 4D) along the apical dendrite of two different L5B pyramidal neurones. The simulated current–voltage relationships, assuming different conductance density values, are shown as dashed lines. In the experiment carried out with the whole-cell electrode connected at 440 μm along the apical dendrite, the simulated curve that overlapped the experimental curve was calculated using a 3 pS μm−2 homogenous conductance density for both Kslow and Kfast (Fig. 4C). In the experiment carried out with the whole-cell electrode connected 154 μm along the apical dendrite, the simulated curve that overlapped the experimental curve was calculated using a 15 pS μm−2 homogenous conductance density for both Kslow and Kfast (Fig. 4D). Taken together, the somatic and dendritic recordings and simulations described in Figs 3 and 4 were consistent with the results obtained using voltage-clamp experiments and the space-clamp correction algorithm (Figs 1 and 2).
Impact of passive parameter measurements
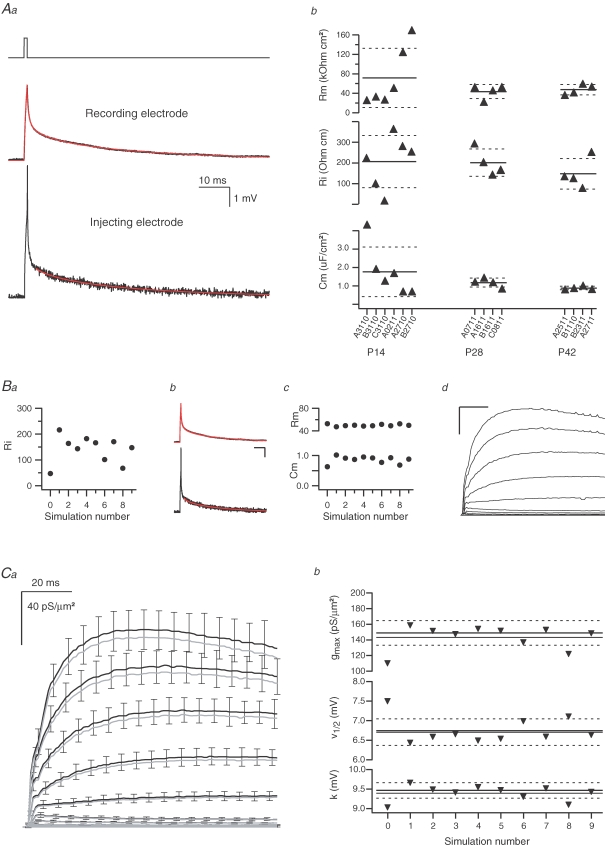
Measurement of K+ conductance densities using TEVC recordings and morphological reconstruction requires estimation of the passive membrane parameters for each experiment (Schaefer et al. 2003a). Could possible errors in the estimation of these parameters account for the difference in the densities measured by the TEVC technique compared to the densities estimated from nucleated and cell-attached membrane patch measurements? As detailed in the Methods, for each neurone the passive membrane parameters in the vicinity of the recording site were estimated by injecting a current pulse prior to the voltage-clamp experiment. The reconstructed soma and dendrite geometry was used in combination with this voltage recording to calculate the passive membrane parameters (Fig. 5Aa). The passive membrane parameters obtained from 14 somatic recordings are displayed in Fig. 5Ab. The scatter in the parameters decreased as a function of postnatal age. This is clearest for Cm and Rm (Fig. 5Ab). Although the scatter in the values of Ri also decreased as a function of postnatal age, it still displayed variation even at P42 and is generally least constrained by local current-injection protocols (Major et al. 1994; Stuart & Spruston, 1998). We then applied Monte Carlo simulations to investigate whether the scatter in Ri could cause large errors in the estimation of the conductance density. For each of the 14 somatic recordings, Ri was randomly changed within the range given by the age group distribution (Fig. 5Ba). The two additional passive membrane parameters (Rm and Cm) were then re-estimated by fitting the original voltage traces (Fig. 5Bb) and the new set of passive membrane parameters (Fig. 5Bc) was used in the calculation of the conductance densities (Fig. 5Bd). This procedure was repeated 10 times and the error caused by the scatter of the passive membrane parameters was estimated from the standard deviation of the conductance densities (Fig. 5Ca). The measured variability in the values of Ri (Fig. 5Ab) could not account for more than a 10% error in the measurement of the conductance density (Fig. 5Cb, and Fig. 1 Table 1 in Supplemental material). It has to be emphasized that this procedure is likely to strongly overestimate the influence of erroneous estimates of Ri as it assumes that all variability between recordings is due to measurement errors rather than reflecting true cell-to-cell heterogeneity.
Figure 5.
The effect of erroneous estimates of passive membrane parameters on the calculation of the K+ conductance density A, illustration of the procedure used to estimate the passive membrane parameters in each somatic and dendritic experiment. A brief current (0.6 nA, 1 ms) was injected via one electrode of the AxoClamp-2B in bridge mode. The voltage deflection was filtered at 10 kHz and measured with a 50 kHz sampling rate with both electrodes (Aa). To reduce experimental noise, 30 sweeps were averaged. The current pulse was always injected via ME2 because this electrode has larger intrinsic voltage recording noise in the AxoClamp-2B amplifier. Following reconstruction of the cells using Neurolucida, the passive membrane parameters were estimated by custom-written routines in NEURON. The result of this fit is shown as red lines. Ab, the passive membrane parameters (membrane resistance (Rm), axial resistance (Ri) and membrane capacitance (Cm)) are displayed against the experiment identifier of each somatic experiment that was analysed (six experiments from P14 rats, and four experiments from P28 and P42 rats each). The solid line in each group indicates the mean for the age group and the dashed lines indicate ±s.d. from this mean. B, Monte Carlo simulation used to investigate the sensitivity of the fit to Ri measurement. For each of 10 runs of the estimation fit of the passive membrane parameters (shown in Aa), the value of Ri was fixed to a random number drawn from a normal distribution based on the originally determined Ri as a mean and the age population standard deviation displayed in Ab as s.d. (Ba). The fit was repeated 10 times with the 10 fixed Ri values from Ba (one example shown in Bb, data, black lines; fit, red lines; scale bar 10 ms and 1 mV) and the resulting new values of Rm and Cm (Bc) were used to calculate K+ conductance densities in the same neurone (one example shown in Bd, scale bar 20 ms and 40 pS μm−2). C, K+ conductance density calculated using the originally measured passive membrane parameters (A) and using those obtained from the Monte Carlo simulation (B). The results of 10 repetitions of the conductance measurement for the different passive membrane parameters were averaged and the s.d. was calculated for each data point. The mean value of each data point obtained by this Monte Carlo simulation (black lines in Ca) did not differ considerably from the conductance density calculation using the original set of passive membrane parameters (grey lines in Cb). The s.d. determined by the Monte Carlo simulation is shown only for some of the points to avoid visual cluttering. Each mean conductance density (the result of 10 repetitions) was analysed by fitting a Boltzmann function to the 50 ms time point. The results of this curve fitting are shown in Cb for each of the 10 repetitions. The value obtained from the original parameters is indicated by a thick line. The mean from the Monte Carlo simulation is shown as a thin line (dashed lines indicate ±s.d).
As we have detailed previously (Schaefer et al. 2003a), the spatial resolution of the space-clamp correction algorithm is of the order of a few tens of micrometers. Thus, even if the passive parameters are different distal to the recording electrode it is predicted to have little impact on the correction of the conductance density. To further explore this point, we also repeated the determination of passive parameters assuming a dendritic gradient of Rm (defined by four parameters: Cm, Ri and two parameters specifying a linear somato-dendritic gradient in Rm). However, using these inhomogeneous passive parameters as a basis for the analysis of conductance densities resulted in only a small deviation of 1.6% in the conductance density estimate.
To further assess the contribution of inhomogeneous passive parameters, we analysed the passive data in a way similar to that presented in Fig. 5. We introduced a somato-dendritic gradient of Ri (somatic value is 0.5 times the original, homogeneous, Ri value linearly increasing with distance to twice its original value at 1000 μm), refitted the passive traces and re-analysed the conductance measurements. This was repeated for a decreasing 2- to 0.5-fold gradient of Ri, and an increasing 0.5- to 2-fold as well as a decreasing 2- to 0.5-fold gradient for Rm and Cm. Although in virtually all cases the resulting passive model was clearly a poor description of the cell, conductance measurements were robust with respect to these distortions displaying on average only 14% deviation (Fig. 1 in Supplemental material). In summary, taking into consideration the results of Fig. 5 and those of our previous paper (Schaefer et al. 2003a), we conclude that the passive model only marginally influences the conductance density estimation by the space-clamp correction algorithm. This is likely to be due to two reasons. First, the fitting procedure to obtain the passive model ensures that critical parameters such as input resistance and time constants are comparatively accurately described by the passive parameters. Second, as soon as voltage-gated conductances are activated, the membrane properties (space constant and time constant) are dominated by the voltage-gated conductances, marginalizing the influence of misestimating Ri, Rm and Cm.
Impact of K+ conductances in the axon and basal dendrites on somatic K+ conductance density measurements
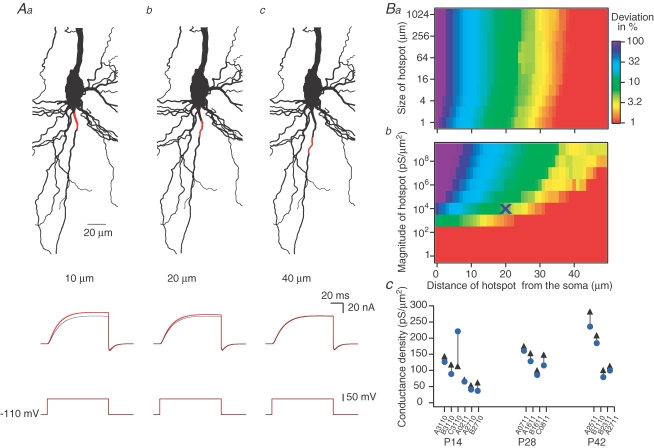
It has been suggested that the density of voltage-gated K+ channels in the axon hillock is several-fold higher than at the soma (Mainen et al. 1995). Such a K+ conductance ‘hot-spot’ may be activated by the somatic voltage-clamp command and contribute current to the somatic recording. Although this is unlikely to affect distal dendritic recordings, it might cause an overestimation of the somatic K+ conductance density. To assess the error in the conductance density measurement we simulated experiments with an axonal hot-spot of voltage-gated K+ conductances. A high conductance density (104 pS μm−2) of a delayed rectifier K+ conductance model (Kslow, as in Figs 3 and 4 and Fig. 2 in Supplemental material) was placed in the axonal initial segment 10 μm away from the soma, while the density of K+ conductances was homogeneous and low in the remainder of the cell (Fig. 6A), and a voltage-clamp experiment was simulated. Following a voltage-clamp step from –110 to 0 mV, the recorded current was larger than the current recorded when all the neurone expressed a low and homogenous conductance density (Fig. 6A). When the hot spot was moved to a position 20 μm away from the soma, only a small fraction of the current recorded at the soma was due to activation of K+ conductances in the hot spot (Fig. 6Ab; and for 40 μm, Fig. 6Ac). The fraction of the somatic current due to activation of conductances in the hot spot was dependent on the distance of the hot spot from the soma (Fig. 6Ba) and the density of the K+ conductance at the site of the hot spot (Fig. 6Bb) but only weakly on its spatial extent (Fig. 6Ba). Currently, there are no direct recordings of the conductance density of voltage-gate K+ conductances from the initial segment of the axon of L5B neurones. However, it has been reported that the density of the voltage-gated Na+ conductance in the initial 30 μm of the axon in L5B pyramidal neurones is similar to that recorded at the soma (Colbert & Pan, 2002). Assuming that the density of the voltage-gated Na+ conductances is counterbalanced by similar densities of voltage-gated K+ conductances it is possible to hypothesize that the initial segment of the axon of L5B neurones does not contain high densities of voltage-gated K+ conductances. To verify this hypothesis, we made cell-attached recordings from the initial segment of the axon of L5B pyramidal neurones. The mean maximal voltage-gated K+ current recorded following depolarization of the patches from −20 mV relative to the resting membrane potential to +140 mV relative to the resting membrane potential was 7.4 ± 3.8 (n = 10) for somatic patches and 3.8 ± 1.8 pA (n = 9) for patches made at distances of 6–21 μm along the initial segment (data not shown). These results support the hypothesis that in the first 20 μm of the axon initial segment the voltage-gated K+ conductance density is not higher than the corresponding somatic density.
Figure 6.
Axonal conductance hot spot does not result in substantial overestimation of the K+ conductance A, in this simulation a high density (104 pS μm−2) of the slow delayed rectifier K+ conductance (Kslow) was inserted, at 10 (Aa), 20 (Ab) and at 40 μm (Ac) away from the soma for 20 μm along the axon (shown in red). The soma was subjected to a voltage step from −110 to 0 mV. The current traces simulated without an axonal hot spot (black lines) and with axonal hot spot (red lines) are shown after leak subtraction. B, the dependence of the deviation (expressed in percentage and displayed in colour coding) of the current simulated at the soma in the presence of an axonal hot spot on the current simulated at the soma when no hot spot was incorporated into the axon. Ba, the dependence of the deviation on the size and distance of the hot spot from the soma. Bb, the dependence of the deviation on the magnitude and distance of the hot spot from the soma. The X marks the values used for the simulations shown in C. C, the deviation in the maximal conductance density for each of the somatic simulations when a hot spot of 104 pS μm−2 was positioned starting at 20 μm along the axon of each neurone. The conductance values in the absence of a hot spot are shown ( ) together with those in the presence of an axonal hot spot (▴). Simulations in A and B are from neurone B1110.
) together with those in the presence of an axonal hot spot (▴). Simulations in A and B are from neurone B1110.
To obtain an upper limit of the error that a hot spot of K+ conductance more distal than 20 μm along the axon would produce on the K+ conductance density measurements, we re-corrected the 14 somatic experiments using the space-clamp correction algorithm assuming a drastically increased K+ conductance density of 104 pS μm−2 stretching from 20 μm from the soma along the entire axon. This assumption indeed led to a smaller estimate of the somatic conductance density (Fig. 6C). However, the error introduced by an axonal hot spot was on average 7% (P28, P42) or 14% (P14) and thus did not increase significantly the estimate of the somatic conductance density (Fig. 6C). As predicted by cable theory, dendritic measurements were essentially unaffected (Fig. 1 and Table 1 in Supplemental material). Errors introduced by incomplete knowledge of passive membrane parameters and by potential axonal K+ conductance hot-spots are summarized in Fig. 1 and Table 1 in Supplemental material.
Another possible source of error for the somatic K+ conductance density measurements are K+ conductance densities in the basal dendrites that deviate substantially from the somatic measurements. It has been reported recently (Antic, 2003), based on voltage-sensitive dye recordings from basal dendrites of L5B neurones, that the conductance density in these dendrites should be ∼200 pS μm−2. This agrees with our estimates of the somatic conductance density and should not contribute additional current to the soma. Nevertheless, we repeated our conductance density analysis assuming basal dendritic channel densities of between 0.3- and 3-fold of the somatic value. As expected, only small deviations in the conductance measurements were observed for the dendritic measurements (−3.6% to 3.1%); however, somatic deviations were larger (−20.1% to 14.3%). This error analysis was incorporated in the summary Fig. 1 in Supplemental material.
Discussion
Using a newly developed numerical technique (Schaefer et al. 2003a), we corrected space-clamp distorted voltage-gated K+ currents recorded using TEVC from the soma and apical dendrite of L5B pyramidal neurones. We show that in 6-week-old rats the decrease in the density of voltage-gated K+ conductances along the apical dendrite is considerably steeper than the gradient we previously reported using cell-attached recordings from 2-week-old rats (Korngreen & Sakmann, 2000). Furthermore, we show that the somatic conductance density increased approximately 2-fold from P14 to P42. In addition, we used an alternative approach, extracting an estimate of the conductance density from membrane rectification to corroborate the results obtained using TEVC and the space-clamp correction algorithm. Finally, the somatic density we measured was substantially larger than estimates previously obtained from nucleated patches (Korngreen & Sakmann, 2000; Bekkers, 2000a) and similar to the values postulated in several simulation studies (Mainen et al. 1995; Rhodes & Llinás, 2001; Antic, 2003; Schaefer et al. 2003b). This discrepancy is probably not due to variability in passive parameters or axonal hot-spots of voltage-gated K+ conductance.
Estimating voltage-gated K+ conductance density in non-spherical cells
To summarise the principles of the space-clamp correction algorithm let us first consider the simplest case of a cylindrical neurone with a delayed rectifier-like voltage-gated K+ conductance and assume that the leak reversal potential is –60 mV and that the K+ reversal potential is −90 mV, then at rest the voltage-gated K+ conductance is low. Therefore, the leak conductance will draw the resting membrane potential towards the leak reversal potential (−60 mV). Depolarizing the membrane potential of the cylinder with a small voltage-clamp command will cause a local depolarization that decays along the cylinder according to passive cable theory. Now deflect the membrane potential using a larger voltage command that will activate the voltage-gated K+ conductance. Once activated, the K+ current will draw the membrane potential on both sides of the voltage-clamp electrode (which are not clamped by the voltage-clamp circuit as the non-spherical morphology of the cylindrical neurone renders it non-isopotential) towards the K+ reversal potential (−90 mV in this case). In other words, voltage-gated K+ channel activation increases membrane conductance which effectively decreases the passive space constant. Thus, distal to the recording pipette the membrane potential will be lower than the threshold for K+ channel activation. Consequently, the spatial activation of the K+ conductance is limited to a relatively small region around the voltage-clamp electrode as shown graphically in Fig. 1 of the review Häusser (2003). This local activation allows the use of a relatively simple, stepwise linear approximation for the construction of the kinetics of the K+ conductance (Schaefer et al. 2003a). Of more importance, the detailed morphology and passive properties of the dendritic tree distal to the voltage-clamp electrode are not important for the accuracy of the correction because the relevant membrane area is close to the voltage-clamp electrode.
We can consider the same cylindrical neurone with a current-clamp electrode inserted at its centre instead of the voltage-clamp electrode. The only difference is that the voltage-clamp circuit uses the recorded potential as a feedback signal to increase the injected current until the recorded membrane potential matches the command potential. Assuming, in addition, that the kinetics of the K+ conductance are known, the membrane potential recorded in the current-clamp experiment can be used to calculate the conductance density of the K+ conductance around the recording electrode.
As we have previously characterized the kinetics of the voltage-gated K+ conductances in L5B pyramidal neurones (Korngreen & Sakmann, 2000), we were able to analyse current-clamp experiments for these neurones. The results illustrated in Figs 3 and 4 were similar to the results we obtained using the space-clamp correction algorithm predicting a higher density of K+ conductance at the soma and lower density along the apical dendrite. This corroborates the numerical validity of the space-clamp correction algorithm and the adequate use of the TEVC recording mode.
Relation to previous work
It has been reported that in order to perform realistic simulations, the effective somatic conductance density of voltage-gated K+ channels had to be several-fold higher than that measured from nucleated patches (Mainen et al. 1995; Rhodes & Llinás, 2001; Antic, 2003; Schaefer et al. 2003b). Therefore, it is possible that although recording from nucleated outside-out patches provides an adequate measurement of channel kinetics it underestimates the value of conductance density. This could be due to altered function of channels in excised membranes (Fenwick et al. 1982) or to an overestimation of the apparent size of the patch that might be a consequence of applied suction. Furthermore, channels recorded from outside-out patches are sensitive to the selection of the pipette solution. Distinct differences were observed between voltage-gated K+ currents recorded in nucleated patches from L5B neurones that were made with a gluconate-based pipette solution (Korngreen & Sakmann, 2000) to those recorded when the pipette solution was based on methyl-sulphonate (Bekkers, 2000a). Thus, it is possible that ‘rundown’ of the K+ current in nucleated patches may be responsible for the large difference between somatic conductance densities obtained by nucleated patches and by TEVC recording (Fig. 2).
The combination of whole-cell voltage-clamp recordings from the soma and dendrite in brain slices with measurement of morphology and passive parameters merges the advantages of the whole-cell recording technique with the visual identification of neuronal subtypes. It allows not only estimation of channel kinetics but also a quantitative measurement of the conductance density (Schaefer et al. 2003a).
In this work we investigated two major sources of possible error in the conductance density estimate provided by the space-clamp correction method. First, Monte Carlo simulations were used to investigate whether the inaccuracy in the estimation of Ri may cause over estimation of the conductance density (Fig. 5). This procedure revealed that the observed scatter in the values of Ri (Fig. 5Ab) could not account for more than a 10% error in the measurement of the conductance density. Second, simulating a hypothetical increase in the voltage-gated K+ conductance density in the axon revealed that the error introduced by an axonal hot-spot was on average 7% (P28 and P42) or 14% (P14), and as such did not significantly increase the estimate of the somatic conductance density (Fig. 6C). Hence, error sources such as incomplete knowledge about passive membrane parameters or current contributions from unknown sources did not bring about a significant uncertainty in the conductance density measurements. Statistical errors, on the other hand, are very small compared to those of cell-attached recordings as the whole-cell currents measured are large and consist of contributions from many channels distributed in the membrane around the recording pipette. Application of specific channel antagonists could aid the space-clamp correction method. In this study, our approach was to investigate the compound K+ conductance density and in particular focus on the slowly inactivating component, as the transient phase is less readily reconstructed, and so obtain a map of the somato-dendritic K+ conductance distribution.
Possible functional significance
We have not attempted to present a complete mechanism for the electrophysiology of the apical dendrite of L5B neurones. This would require at the very least a detailed characterization of dendritic Ca2+ and Na+ channels in adult animals combined with construction of a detailed compartmental model. However, it is tempting to speculate what might be the function of the K+ conductance gradient in the physiology of L5B neurones. The steep decrease of the K+ conductance density along the apical dendrite of L5B pyramidal neurones of the neocortex differentiates them from other neuronal cell types studied so far. In Purkinje neurones of the cerebellum, the density of the sustained voltage-gated K+ conductance was observed to gradually decrease as a function of distance from the soma (Martina et al. 2003). In these neurones, in contrast to L5B neurones, dendritic Ca2+ potentials do not propagate to the soma (Llinás & Sugimori, 1979, 1980) and axonally generated APs do not back-propagate into the dendritic tree (Stuart & Häusser, 1994) – in part due to the high degree of branching and low Na+ channel densities (Vetter et al. 2001). It has, however, been observed that in proximal dendrites, voltage-gated K+ conductances are activated by passive membrane responses and help to further attenuate the amplitude of the back-propagating AP (Martina et al. 2003). In more distal dendrites, voltage-gated K+ conductances have been observed to inactivate only at depolarized levels (Martina et al. 2003). Thus, as a result of their large conductance, effectively shaping dendritic Ca2+ spikes without completely abolishing them.
In CA1 pyramidal neurones of the hippocampus the density of an A-type voltage-gated K+ conductance along the apical dendrite has been observed to increase steeply with the distance from the soma (Hoffman et al. 1997). This K+ conductance gradient has been proposed to act as a ‘dendritic shock absorber’ in modulating the amplitude of the back-propagating AP (Hoffman et al. 1997). As a result, the dendritic tree in CA1 pyramidal neurones receives highly variable feedback information from the soma based on the level of inactivation of the A-type voltage-gated K+ conductance that controls the amplitude of the back-propagating AP (Hoffman et al. 1997).
It is clear that the kinetic properties and distribution of the voltage-gated K+ conductances in L5B are different from those observed for dendritic voltage-gated K+ conductances in Purkinje and CA1 pyramidal neurones and may reflect differences in their function. In L5B pyramidal neurones, the AP readily back-propagates into the dendritic tree (Amitai et al. 1993; Kim & Connors, 1993; Reuveni et al. 1993; Schiller et al. 1997; Larkum et al. 1999b, 2001; Zhu, 2000). When a back-propagating AP coincides with distal synaptic input in these neurones, a regenerative dendritic Ca2+ potential is generated, triggering a burst of several APs at the soma (Larkum et al. 1999b). This coincidence mechanism has been shown to be regulated by membrane potential changes in the proximal apical dendrite (Larkum et al. 2001). Thus as in Purkinje neurones, the steep ∼13-fold decrease of the voltage-gated K+ conductance density from the soma along the apical dendrite of L5B pyramidal neurones may be responsible for segmenting the apical dendrite into several functional regions including a distal low-threshold region for the initiation of dendritic regenerative Ca2+ potentials and a proximal region that may modulate the propagation of dendritic potentials to the soma. Nevertheless, it is important to note that, due to the restrictions in the temporal resolution of the TEVC, we could not resolve the gradient of the A-type voltage-gated K+ conductance along the apical dendrite. The gradient of the A-type K+ conductance may differ from that of the delayed K+ conductance and may confer additional function to the apical dendrite of L5B neurones. Keeping this constraint in mind, it may be that the dendritic ‘shock absorbers’ are loosened in the distal apical dendrite of L5B pyramidal neurones. This would allow the synaptic input to the apical dendrite to generate dendritic regenerative Ca2+ potentials, to boost distal synaptic input, and to modulate the AP output of neurones.
Acknowledgments
The authors would like to thank Drs Dan Johnston, Arnd Roth, Dana Cohen, Avy Susswein, Thomas Kuner, Kamilla Angelo and Michele Migliore for commenting on the manuscript at various stages and Dr Troy Margrie for continuous support and encouragement. This research was supported in part by the Israel Science Foundation (grant no. 345/04) and the Leopoldina Akademie der Naturforscher.
Supplementary material
The online version of this paper can be accessed at:
Somato-dendritic K+ conductance density in P42 pyramidal neurons
The effect of Kfast or Kslow on the simulated membrane potential
The effect of inter-electrode distance on the accuracy of the space-clamp correction algorithm
Parameters of each individual experiment
DOI: 10.1113/jphysiol.2006.123836
http://jp.physoc.org/cgi/content/full/jphysiol.2006.123836/DC1 and contains supplemental material consisting of three figures and a table.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Amitai Y, Friedman A, Connors BW, Gutnick MJ. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex. 1993;3:26–38. doi: 10.1093/cercor/3.1.26. [DOI] [PubMed] [Google Scholar]
- Antic SD. Action potentials in basal and oblique dendrites of rat neocortical pyramidal neurons. J Physiol. 2003;550:35–50. doi: 10.1113/jphysiol.2002.033746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM. Properties of voltage-gated potassium currents in nucleated patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol. 2000a;523:593–609. doi: 10.1111/j.1469-7793.2000.t01-1-00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM. Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol. 2000b;523:611–620. doi: 10.1111/j.1469-7793.2000.t01-2-00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Jonas P. Action potential propagation into the presynaptic dendrites of rat mitral cells. J Physiol. 1997;504:359–365. doi: 10.1111/j.1469-7793.1997.359be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Redman SJ. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J Physiol. 1989;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–538. doi: 10.1038/nn0602-857. [DOI] [PubMed] [Google Scholar]
- Cole KS, Curtis HJ. Membrane potential of the squid giant axon during current flow. J Gen Physiol. 1941;24:551–563. doi: 10.1085/jgp.24.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel AS, Gage PW. Conventional voltage clamping with two intracellular microelectrodes. In: Smith TG, Lecar H, Redman SR, Gage PW, editors. Voltage and Patch Clamping with Microelectrodes. Bethesda: American Physiological Society; 1985. [Google Scholar]
- Häusser M. Revealing the properties of dendritic voltage-gated channels: a new approach to the space clamp problem. Biophys J. 2003;84:3497–3498. doi: 10.1016/s0006-3495(03)75083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. Expanding NEURON's repertoire of mechanisms with NMODL. Neural Comput. 2000;12:995–1007. doi: 10.1162/089976600300015475. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Keren N, Peled N, Korngreen A. Constraining compartmental models using multiple voltage recordings and genetic algorithms. J Neurophysiol. 2005;94:3730–3742. doi: 10.1152/jn.00408.2005. [DOI] [PubMed] [Google Scholar]
- Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993;13:5301–5311. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol. 2000;523:621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Kaiser KM, Sakmann B. Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proc Natl Acad Sci U S A. 1999a;96:14600–14604. doi: 10.1073/pnas.96.25.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999b;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J Physiol. 2001;533:447–466. doi: 10.1111/j.1469-7793.2001.0447a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Calcium conductances in Purkinje cell dendrites: their role in development and integration. Prog Brain Res. 1979;51:323–334. doi: 10.1016/S0079-6123(08)61312-6. [DOI] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Joerges J, Huguenard JR, Sejnowski TJ. A model of spike initiation in neocortical pyramidal neurons. Neuron. 1995;15:1427–1439. doi: 10.1016/0896-6273(95)90020-9. [DOI] [PubMed] [Google Scholar]
- Major G, Larkman AU, Jonas P, Sakmann B, Jack JJ. Detailed passive cable models of whole-cell recorded CA3 pyramidal neurons in rat hippocampal slices. J Neurosci. 1994;14:4613–4638. doi: 10.1523/JNEUROSCI.14-08-04613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Yao GL, Bean BP. Properties and functional role of voltage-dependent potassium channels in dendrites of rat cerebellar Purkinje neurons. J Neurosci. 2003;23:5698–5707. doi: 10.1523/JNEUROSCI.23-13-05698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni I, Friedman A, Amitai Y, Gutnick MJ. Stepwise repolarization from Ca2+ plateaus in neocortical pyramidal cells: evidence for nonhomogeneous distribution of HVA Ca2+ channels in dendrites. J Neurosci. 1993;13:4609–4621. doi: 10.1523/JNEUROSCI.13-11-04609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes PA, Llinás RR. Apical tuft input efficacy in layer 5 pyramidal cells from rat visual cortex. J Physiol. 2001;536:167–187. doi: 10.1111/j.1469-7793.2001.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Häusser M. Compartmental models of rat cerebellar Purkinje cells based on simultaneous somatic and dendritic patch-clamp recordings. J Physiol. 2001;535:445–472. doi: 10.1111/j.1469-7793.2001.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AT, Helmstaedter M, Sakmann B, Korngreen A. Correction of conductance measurements in non-space-clamped structures: 1. Voltage-gated K+ channels. Biophys J. 2003a;84:3508–3528. doi: 10.1016/S0006-3495(03)75086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AT, Larkum ME, Sakmann B, Roth A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol. 2003b;89:3143–3154. doi: 10.1152/jn.00046.2003. [DOI] [PubMed] [Google Scholar]
- Schiller J, Schiller Y, Stuart G, Sakmann B. Calcium action potentials restricted to distal apical dendrites of rat neocortical pyramidal neurons. J Physiol. 1997;505:605–616. doi: 10.1111/j.1469-7793.1997.605ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Stuart G, Häusser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron. 1994;13:703–712. doi: 10.1016/0896-6273(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci. 1998;18:3501–3510. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Roth A, Häusser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–937. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Zhu JJ. Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol. 2000;526:571–587. doi: 10.1111/j.1469-7793.2000.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Somato-dendritic K+ conductance density in P42 pyramidal neurons
The effect of Kfast or Kslow on the simulated membrane potential
The effect of inter-electrode distance on the accuracy of the space-clamp correction algorithm
Parameters of each individual experiment