Abstract
Polycystin-2 (PC2), encoded by PKD2, which is one of the genes whose mutations cause polycystic kidney disease, is abundantly produced in the apical domain of the syncytiotrophoblast (hST) of term human placenta. PC2, a TRP-type (TRPP2) non-selective cation channel, is present in primary cilia of renal epithelial cells, a microtubule-based ancillary structure with sensory function. The hST has abundant cytoskeletal structures, and actin filament dynamics regulate PC2 channel function in this epithelium. However, it is expected that the apical hST excludes microtubular structures. Here, we demonstrated by Western blot and immunocytochemical analyses that hST apical vesicles indeed contain microtubule structural components, including tubulin isoforms, acetylated α-tubulin, and the kinesin motor proteins KIF3A and KIF3B. PC2 and tubulin were substantially colocalized in hST vesicles. Treatment of hST vesicles with either the microtubular disrupter colchicine (15 μm) or the microtubular stabilizer paclitaxel (taxol, 15 μm) resulted in distinct patterns of microtubular re-organization and PC2 redistribution. We also observed that changes in microtubular dynamics regulate PC2 channel function. Addition of colchicine rapidly inhibited PC2 channel activity in lipid-bilayer reconstituted hST membranes. Addition of either tubulin and GTP, or taxol, however, stimulated PC2 channel activity in control hST membranes. Interestingly, we found that the kinesin motor protein KIF3A was capable of increasing PC2 channel activity in hST. We believe that the data are the first to provide a direct demonstration of a microtubular interaction with PC2 in the hST. This interaction thus plays an important regulatory role in the control of ion transport in the human placenta.
The chorionic villous tree of the human placenta is an intricate structure that grows continuously by branching and invading the uterine mucosa during gestation (Demir et al. 1997; Ockleford et al. 1981; Kingdom et al. 2000). Various cytoskeletal components (Truman & Ford, 1986), including microtubules (Smith et al. 1977; Douglas & King, 1993) and actin filaments (Beham et al. 1988; Parast & Otey, 2000), are present in the developing placenta. Apical hST membrane preparations present prominent actin-based microfilamental structures (Smith et al. 1977). However, apical microvilli are expected to exclude microtubules (Ockleford et al. 1981). Recent studies indicate that actin cytoskeletal structures interact with (Li et al. 2003a, b,) and modulate PC2 channel function (Li et al. 2005; Montalbetti et al. 2005b) in the human placenta. We determined that in hST apical membranes, PC2 anchors the actin-bundling protein, α-actinin (Li et al. 2005), which is implicated in cell adherence and proliferation (Gluck & Ben Ze'ev, 1994). This interaction is functional, in that α-actinin is able to modulate PC2 channel activity in this syncytial epithelium. We further determined that the actin-severing protein gelsolin also regulates PC2 channel function in hST, by re-modelling actin structures in a Ca2+-dependent manner (Montalbetti et al. 2005b).
Microtubules are long cylindrical polymers composed of hetero-dimeric units of α and β tubulins. Microtubules are implicated in vesicular trafficking, the regulation of cell cycle, cell motility and morphology. Microtubular organization is essential for the assembly and organization of mitotic spindles, centriole and organelles emerging from such structures, including cilia and flagella. Previous evidence has suggested a complementary regulatory role of microtubules and actin filaments in channel function. Johnson and Byerly originally determined that cytoskeletal modifying agents alter Ca2+ channel activity in Lymnaea (Johnson & Byerly, 1993) and rat hippocampal pyramidal neurons (Johnson & Byerly, 1994). In those studies it was concluded that drugs which stabilize (taxol and phalloidin) and destabilize (colchicine and cytochalasin B) the actin and microtubular cytoskeletons altered the development of and recovery from Ca2+-dependent inactivation of Ca2+ currents. More recent studies have shown a direct interaction between TRP channels, such as the vanilloid receptor (TRPV1), and β-tubulin, which is one of the key elements in microtubular formation (Goswami et al. 2004).
A microtubular connection to PC2 function in hST is, heretofore, unknown. However, recent evidence suggests potential links between PC2 and microtubular structures. Various forms of cystic kidney disease not directly linked to mutations in the PKD2 gene have been associated with microtubular-associated ciliary proteins (Morgan et al. 1998; Murcia et al. 2000; Pazour et al. 2002; Yoder et al. 2002b; Hou et al. 2002). For example, the cpk gene, whose mutations cause renal cystic disease, encodes cystin (Hou et al. 2002), a novel protein which is expressed and colocalizes with polaris in primary cilia of renal epithelial cells. Treatment of homozygous cpk/cpk mice with taxol (Woo et al. 1997) dramatically moderated progression of the disease. The Tg737 gene mutated in the orpk PKD mice encodes another ciliary protein, polaris, which localizes to the ciliary basal body and the microtubular axoneme (Yoder et al. 2002b). orpk mice display shortened cilia, left-right symmetry defects (Murcia et al. 2000), and increased ciliary PC2 expression (Pazour et al. 2002). A closer microtubular connection to PC2 was provided by Rundle et al. (2004) who found that mDial/Drf1 interacts with PC2 in a cell-cycle-dependent manner. mDia1/Drf1 is a member of the RhoA GTPase-binding formin homology protein families, which participate in cytoskeletal organization, cytokinesis and signal transduction. Interestingly, PC2 is functional in primary cilia of renal epithelial cells (Raychowdhury et al. 2005). More recently, we determined that microtubular dynamics indeed regulate PC2 channel function in primary cilia. We observed that acute addition of the microtubular disrupter colchicine rapidly abolished, while addition of the microtubular stabilizer paclitaxel increased, ciliary PC2 channel activity in isolated ciliary membranes from LLC-PK1 epithelial cells reconstituted in a lipid bilayer system (Li et al. 2006). We further observed that PC2 colocalizes, structurally associates, and functionally interacts with the microtubule-dependent motor kinesin-2 subunit KIF3A, a protein involved in anterograde cargo transport in cilia and flagella (Marszalek et al. 1999; Takeda et al. 1999; Lin et al. 2003). Thus, microtubular organization regulates PC2 function in microtubular-containing organelles.
Therefore, herein, we explored whether microtubules are present in hST apical membranes, and further hypothesized that colocalization of PC2 with microtubules may be potentially important in regulating PC2 function in the human placenta. We determined that the apical domain of hST contains various tubulin isoforms, structured microtubules, and kinesin motor protein subunits. We further determined that PC2 colocalizes with microtubules in this preparation, and observed that microtubular formation activates, while disruption of microtubules inhibits, PC2 channel activity in hST. Interestingly, the kinesin-2 motor subunits KIF3A and KIF3B were also found in hST vesicles, and addition of exogenous KIF3A activated the PC2 channel in this membrane preparation. Thus, a microtubule–PC2 interaction acts as a novel regulatory mechanism of hST PC2 channel function.
Methods
Human placenta membrane preparation
Human placenta syncytiotrophoblast (hST) apical membrane vesicles were obtained as previously reported (González-Perrett et al. 2001; Montalbetti et al. 2005b). Term human placentas were obtained within 20 min of normal vaginal delivery under strict institutional guidelines, and immediately processed (González-Perrett et al. 2001). The placentas were obtained from the Instituto Médico de Obstetricia SA, TTE General J. D. Perón 2247, Buenos Aires, Argentina, C1040AAI. Briefly, villous tissue was fragmented, washed with non-buffered NaCl saline (150 mm), and minced into small pieces. The fragmented tissue was stirred for 1 h in 1.5 vols of a solution containing 10 mm Hepes, adjusted to pH 7.4 with KOH, and also containing 0.1 mm EGTA, a protease inhibitor cocktail (González-Perrett et al. 2001), and 250 mm sucrose. The tissue preparation was filtered and centrifuged for 10 min at 1000 g. The supernatant was again centrifuged for 10 min at 14 500 g and for 90 min at 23 400 g. The final pellet was resuspended in a buffer solution containing 10 mm Hepes-KOH, pH 7.4, 250 mm sucrose, and 20 mm KCl. The hST apical membrane preparation was aliquoted and stored at –20°C until the time of the experiment. At least six different hST membrane preparations (i.e. individual donors) were used for the electrophysiological studies in this report.
Immunochemistry and reagents
Specific PC2 labelling in hST apical membranes was conducted with anti-PC2 mouse monoclonal (1A11) and goat polyclonal G-20 antibodies, as reported (Li et al. 2005). Other primary antibodies included rabbit anti-KIF3A polyclonal and mouse antiacetylated α-tubulin monoclonal (Sigma-Aldrich, Oakville, ON, Canada) antibodies. Secondary antibodies used for immunofluorescence included goat antimouse IgG fluorescein isothiocyanate and goat antirabbit IgG-rhodamine (Chemicon International, Temecula, CA, USA). Goat antimouse and -rabbit IgG-horseradish peroxidase (HRP; Chemicon International), were used for Western blot analysis (WB). Tubulin (Cytoskeleton, Denver, CO, USA) was prepared in buffer containing (mm): 2.0 Tris-HCl, 0.5 ATP, 0.2 CaCl2 and 0.5 β-mercaptoethanol, pH 8.0. At the time of the experiment, GTP (0.5 mm) was added to the solution from a stock solution (100 mm). Colchicine (Sigma-Aldrich) was dissolved in water and used at concentrations ranging from 1 to 15 μm in ‘trans’ buffer solution (see below). Paclitaxel (taxol, 1 mg ml−1; Sigma-Aldrich) was used at a 15 μm final concentration. The hST vesicles were placed in Eppendorf tubes (1.5 ml total volume), diluted in 10% mouse and/or rabbit sera in PBS, and incubated for 30 min to prevent non-specific binding of antibodies. A PBS buffer containing 2% BSA was used for antibody dilution and washing. Vesicles were treated with primary antibodies for 1 h, rinsed (×3) by centrifugation, and incubated with the secondary antibody for 45 min. This procedure was followed by another wash (×3) with PBS. Pelleted vesicles were transferred to glass slides, mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA), and visualized with a Zeiss 510 confocal laser scanning microscopy with argon 488 nm and helium–neon 543 nm lasers (Zeiss, Weesp, the Netherlands). Composite images were created using Adobe Photoshop 5.5.
KIF3A constructs and protein preparation
PolyHis-tagged KIF3A C terminus (aa 403–702) was purified from Escherichia coli cultures, and the full-length KIF3A (aa 1–702) was obtained from MDCK cells, as described (Li et al. 2006).
Protein immunoblotting
The hST apical membranes were subjected to 8% SDS-PAGE electrophoresis and transferred to a nitrocellulose membrane (Amersham, Baie d'Urfe, Canada). The membrane was then blocked with 3% skim milk powder in PBS supplemented with 0.1% Tween-20, which was incubated with the primary antibody and an HRP-coupled secondary antibody, and visualized with enhanced chemiluminescence (Amersham).
Ion channel reconstitution
hST vesicles were used in the lipid bilayer reconstitution experiments, as previously reported (González-Perrett et al. 2001). Briefly, lipid bilayers were formed with a mixture of synthetic phospholipids (Avanti Polar Lipids, Birmingham, AL, USA) in n-decane (Sigma-Aldrich). The lipid mixture contained 1-palmitoyl-2-oleoyl phosphatidylcholine and phosphatidyl-ethanolamine in a 7:3 ratio. The lipid solution (∼20–25 mg ml−1) in n-decane was spread in the aperture of a polystyrene cuvette (CP13-150) of a bilayer chamber (model BCH-13; Warner Instruments, Hamden, CT, USA). Both sides of the lipid bilayer were bathed with a solution containing 10 mm MOPS-KOH, and 10 mm MES-KOH, pH 7.40, and 10–15 μm Ca2+. The final K+ concentration in the solution was approximately 15 mm. KCl was further added to the cis compartment, to reach a final concentration of 150 K+.
Data acquisition and analysis
Electrical signals were obtained with a PC501A patch-clamp amplifier (Warner Instruments) with a 10 GΩ feedback resistor. Output (voltage) signals were low-pass filtered at 700 Hz (−3 dB) with an eight-pole Bessel-type filter (Frequency Devices, Haverhill, MA, USA). Single-channel current tracings were further filtered for display purposes only. Unless otherwise stated, pCLAMP version 5.5.1 (Axon Instruments, Union City, CA, USA) was used for data acquisition and analysis, and Sigmaplot Version 2.0 (Jandel Scientific, Corte Madera, CA, USA) for statistical analysis and graphics. Multiple single-channel currents were expressed as mean currents (I) where I =NiPo was obtained as previously reported (Montalbetti et al. 2005b). In this equation, N is the total number of active channels, i is the average single channel current for the channel species (i.e. PC2), and Po is the open probability of the open channel, at a given holding potential. Statistical significance was obtained by an unpaired Student's test comparison of sample groups of similar size. Averaged data are expressed as the mean ±s.e.m. (n) under each condition, where n represents the total number of experiments analysed and s.e.m. is the standard error of the mean. Statistical significance was accepted at P < 0.05 as calculated by a paired Student's test (Snedecor & Cochran, 1973).
Results
Presence of microtubular components in hST membrane vesicles
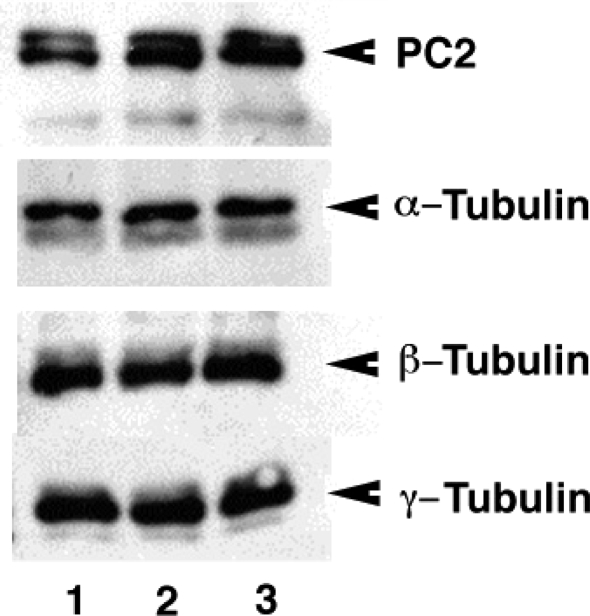
To assess whether microtubular organization affects PC2 channel function, we first determined the presence of tubulin isoforms and endogenous microtubular structures in hST vesicles by Western blot and immunofluorescence analyses (Figs. 1 and 2). Indeed, Western blot analysis (Fig. 1) confirmed the presence of PC2, as reported (Li et al. 2005), and several tubulin isoforms in the preparation, including α-, β- and γ-tubulins, as well as the post-translationally modified acetylated tubulin heavily localized in such organelles as primary cilia. Immunofluorescence analysis of control hST vesicles (Fig. 2A) showed strong colocalization of PC2 and microtubules, although not all microtubules were decorated (Fig. 2B) with the channel protein. Some membrane-apposed microtubules distinctly displayed a complete absence of PC2 (Fig. 2A). A comparison between control and colchicine-treated vesicles (15 μm, 24 h, at 4°C) indicated that despite the fact that protein content remained largely the same, the drug affected tubulin–PC2 colocalization in the vesicles. Interestingly, however, microtubular stabilization with paclitaxel (15 μm), clearly displayed longer and PC2-decorated microtubules (Fig. 2B).
Figure 1.
Presence of PC2 and tubulin isoforms in hST vesicles Western blot analysis of human syncytiotrophoblast (hST) apical membrane vesicles. The presence of the polycystin-2 (PC2) channel was confirmed as reported (Montalbetti et al. 2005b) by labelling with specific anti-PC2 monoclonal antibodies. The hST vesicles also contained various tubulin isoforms, including α, β, γ and acetylated α-tubulin. Data are representative of samples from two donor placentas. The total amount of tubulin in the hST vesicle preparation (lane 1) did not significantly change after treatment with either colchicine (15 μm, 24 h, lane 2) or paclitaxel (taxol, 15 μm, 24 h, lane 3). PC2 channel labelling was also unaffected by either treatment.
Figure 2.
Presence of microtubular components in the hST vesicle preparation A, hST apical membrane vesicles were labelled with anti-PC2 and antitubulin antibodies (Ctr) to determine the colocalization of microtubules with the channel protein in the preparation. The presence of tubulin was confirmed in all vesicles, with specific spots showing higher concentration of tubulin in membrane apposition. Co-labelling of PC2 and tubulin (Ctr, Merge), showed discrete areas of interaction, particularly in the intravesicular compartment. Data are representative of duplicate samples from two donor placentas. Treatment of hST membrane vesicles with either the microtubular depolymerizing agent colchicine (15 μm, 24 h, +Col) or the microtubule stabilizer paclitaxel (15 μm, 24 h, +Tax) had a significant effect on tubulin redistribution in the vesicle preparation. B, intravesicular colabelling of PC2 (anti-PC2 antibody, green) and β-tubulin (red) indicated strong colocalization after paclitaxel treatment (15 μm, 24 h, yellow). Interestingly, PC2 decorated specific intravesicular microtubules, suggesting their ability to transport and relocate the channel protein (arrowheads). The colabelled image also showed microtubules devoid of decorated PC2 (arrows).
Effect of colchicine on PC2 channel activity
To assess a regulatory role of endogenous microtubules on PC2 channel function in hST, apical vesicles were first reconstituted in a lipid bilayer system. Experiments were conducted in the presence of a K+ chemical gradient, with 150 mm and 15 mm in the cis and trans compartments, respectively. Data were chosen for analysis from tracings where spontaneous single channel activity was observed at the beginning of the experiment (Fig. 3A). Currents were highly cation selective (Fig. 3B) and further characterized as those previously observed as mediated by PC2 (González-Perrett et al. 2001). In 17 out of 19 experiments, addition of colchicine (15 μm) to the cis compartment completely inhibited PC2-mediated K+ currents (Fig. 3A–C). Colchicine decreased channel activity from 6.0 ± 3.3 to 0.40 ± 0.30 pA (n = 5, P < 0.01) within 30 s after exposure to the drug (Fig. 3C). In the presence of colchicine, further addition of GTP and tubulin had no effect on channel re-activation (from 0.40 ± 0.30–0.30 ± 0.10, n = 3, P > 0.4). In contrast to the inhibitory effect of the microtubule depolymerizing agent colchicine, addition of the microtubular stabilizer paclitaxel (taxol, 15 μm) to the cis chamber stimulated channel activity (Fig. 4). As expected, PC2 channel activity was completely inhibited by the addition of amiloride to the trans chamber (Fig. 4).
Figure 3.
Effect of colchicine on PC2 channel activity in hST A, representative single-channel tracings of hST apical membranes. Membranes showing spontaneous K+-permeable channel activity were exposed to colchicine (15 μm) in the cis chamber. Addition of the drug completely inhibited PC2-mediated K+ currents. The hST apical vesicles were reconstituted in the presence of a KCl chemical gradient, with 150 mm KCl in cis, and 15 mm KCl in trans compartments, respectively. All-point histograms, showing the current amplitude of the K+ permeable channels, are shown on the right. Data are representative of 19 experiments. B, the single-channel conductance of the K+-permeable channels was highly cation selective, with an average conductance of 135 pS, as reported (González-Perrett et al. 2001). Experimental data (•) are indicated as means ±s.e.m. (n = 3). The continuous line is the fitting of data with the Goldman-Hodgkin-Katz (GHK) equation (González-Perrett et al. 2001). C, time dependence of colchicine effect. The drug completely blocked PC2 channel activity within 1 min after addition. Data are the means ±s.e.m. of four experiments. D, addition of tubulin plus GTP was without effect in restoring K+ channel activity in the presence of colchicine. E, average data, expressed as NPo, are shown as the means ±s.e.m. of K+ currents before (n = 4), 5 min after colchicine addition, and after further addition of tubulin plus GTP (n = 3) with the drug (P > 0.4).
Figure 4.
Effect of taxol on PC2 channel activity in hST A, representative single-channel tracings of hST apical membranes. Membranes showing spontaneous K+-permeable channel activity were exposed to taxol (15 μm) in the cis chamber. Addition of the drug increased PC2-mediated K+ currents. The hST apical vesicles were reconstituted in the presence of a KCl chemical gradient, with 150 mm KCl in cis, and 15 mm KCl in trans compartments, respectively. B, the highly cation-selective single-channel conductance of the K+-permeable channels was 135 pS conductance, as reported (González-Perrett et al. 2001). Experimental data (•) are indicated as means ±s.e.m. (n = 3). The continuous line is the fitting of data with the GHK equation (González-Perrett et al. 2001). C, changes in K+ channel activity after addition of taxol. Data are expressed as NPo, which increased (P < 0.05, n = 4) after addition of the drug. The channel activity (both control and increased by taxol) was completely inhibited by addition of amiloride (100 μm).
Effect of tubulin and GTP on PC2
To further assess the nature of the microtubular regulation of PC2 channel activity, K+ currents were monitored in control hST vesicles. Contrary to the inhibitory effect of colchicine (Fig. 3) and similar to the stimulatory effect of taxol (Fig. 4), addition of polymerizing concentrations of tubulin (5 mg ml−1) and GTP (500 μm) to the cis compartment increased cation channel activity of spontaneously inactivated PC2 in eight out of nine experiments (Fig. 5A). The mean channel current increased by fivefold, from 1.5 ± 1.2 to 9.1 ± 5.2 pA (n = 6, P < 0.03). It is interesting to note, however, that addition of tubulin plus GTP to the purified PC2 channel protein had no stimulatory effect (reported elsewhere, Li et al. 2006), suggestive of the presence of regulatory proteins mediating this interaction.
Figure 5.
Effect of tubulin on PC2 channel activity in hST A, top, representative single-channel tracings of hST apical membranes before and after addition of tubulin (15 μm) plus GTP (500 μm). The hST apical vesicles were reconstituted in the presence of a KCl chemical gradient, with 150 mm KCl in cis, and 15 mm KCI in trans compartments, respectively. The drugs were added to the cis compartment. Membranes with little spontaneous ion channel activity were first obtained (left). Addition of GTP (500 μm) to the cis chamber had no effect, while further addition of tubulin increased cation-selective ion channel activity (right). Data are representative of nine experiments. B, single-channel currents observed under control conditions, before and after self-inhibition, and after addition of both GTP and tubulin (n = 3) in hST vesicles. All-point histograms indicate the change in channel activity and the conductance of the channel (right panels).
Presence of kinesin-2 motor subunits in hST and their regulatory role in PC2 channel function
The above findings indicate that while a structural/functional interaction between microtubules and PC2 exists in apical hST vesicles, intermediate, perhaps microtubule-associated, proteins are likely to mediate this linkage. Indeed a number of proteins have been found associated with the microtubule-containing axoneme of primary cilia, and possibly colocalize with PC2 (Yoder et al. 2002a). The kinesin-2 complex, composed of motor subunits KIF3A and KIF3B and the non-motor subunit KAP3, is involved in ATP-dependent anterograde-directed vesicular transport along axonemal microtubules. Interestingly, KIF3A colocalized with PC2 in primary cilia of renal epithelial cells (Li et al. 2006), and directly associated with the carboxy terminus of PC2, which provides a molecular link between microtubular structures and the channel protein. To confirm this structural interaction, we conducted a Western blot analysis of hST apical vesicles and found that both KIF3A and KIF3B are present in the hST apical vesicles (Fig. 6A). Addition of KIF3A chimera (GST-gusion protein) or purified KIF3A either from bacteria or MDCK cells (data not shown) activated (Fig. 6B) or increased PC2 channel in reconstituted hST apical vesicles (n = 5).
Figure 6.
Presence and effect of kinesin 2 motor proteins in hST A, the presence of the kinesin motor proteins KIF3A and KIF3B was confirmed in hST by Western blot analysis. Data are representative of two donor placentas (control, Ctr), and after treatment with colchicine (Col) and paclitaxel (taxol, Tax). B, the regulatory effect of KIF3A on PC2 channel activity in hST was determined by addition of the KIF3A C-terminus (403–702 aa) purified from E. coli, which was added (10 μg ml−1) to the cis compartment of the lipid bilayer chamber. KIF3A increased spontaneous PC2 channel activity in reconstituted hST apical vesicles. Data are representative of three experiments.
Discussion
In the present study, we demonstrated that PC2 colocalizes with, and is regulated by, microtubular structures in the human syncytiotrophoblast. PC2 is a TRP channel that is abundantly expressed in the hST, and is likely to represent the most relevant component of apical transport of Ca2+ into the fetus. To date, several Ca2+-permeable cation channels have been identified in the term placenta (Belkacemi et al. 2005), including L-type Ca2+ channels (Robidoux et al. 2000; Moreau et al. 2003; Bernucci et al. 2006), several TRP channels, including TRPP2 (polycystin-2) (González-Perrett et al. 2001), TRPC1–6 (Clarson et al. 2003; Niger et al. 2004), TRPV5–6 (ECaC, CaT1) (Bernucci et al. 2006), and the purinergic receptors P2X4, P2X7, P2Y2 and P2Y6 (Roberts et al. 2006a, b). Clarson et al. (2003) detected the presence of message and protein encoding the canonical TRP channels TRPC3 and TRPC4 in both apical and basal membranes of the hST. However, no electrophysiological information was available to confirm channel phenotypes. More recently, Riquelme's group addressed the issue of the presence of various Ca2+ permeable cation channels, including L-type Ca2+ channels, in the basal membrane of term hST (Bernucci et al. 2006). This exhaustive study confirmed the presence of various Ca2+-permeable channels by using a number of techniques, including electrophysiology (Bernucci et al. 2006). As L-type Ca2+ channels are regulated by voltage changes, namely dynamic shifts in the resting potential of the syncytial epithelium, it is likely that this pathway relates to Ca2+ signalling by placental epithelial cells (Cronier et al. 1999).
We originally observed that the gene product of PKD2, implicated in autosomal dominant polycystic kidney disease (ADPKD), is the most abundant Ca2+-permeable non-selective cation channel in the apical domain of term hST (González-Perrett et al. 2001). PC2 belongs to the superfamily of TRP channel proteins (Montell et al. 2002a, b,), has a 1:1 cation perm-selectivity ratio for monovalent cations, and a slight preference for divalent cations, ranging from ∼1.5–5:1. This is likely to depend on the experimental conditions under which PC2 was studied (González-Perrett et al. 2001; Vassilev et al. 2001; Koulen et al. 2002; Luo et al. 2003; see Cantiello, 2004 for discussion). Based on its abundance in the apical hST, its single-channel conductance (130–150 pS), and weak self-inhibition by cytoplasmic Ca2+, PC2 can be considered an important contributor to the apical entry step in the transepithelial movement of Ca2+. Ca2+-transporting TRP channels, quintessentially considered as Ca2+-transporting elements in absorption epithelia, include ECaC1 and CaT1, now known as TRPV5 and TRPV6, respectively (Peng et al. 2003a, b). TRPV5 and 6, originally identified as the Ca2+-transporting mechanism in intestine (Peng et al. 1999), and the kidney (Hoenderop et al. 2000), respectively, have been identified in both apical and basal membranes of the hST (Moreau et al. 2002, 2003). At least two parameters in the channel properties of PC2, as compared with ECaC1 and CaT1, make it most relevant in terms of its potential capabilities as a Ca2+-entry pathway in the human placenta. First, PC2 has a much larger monovalent cation single-channel conductance (Nilius et al. 2000). Second, compared with other TRP channels, PC2 presents a much weaker self-inhibition by Ca2+. Thus, PC2 is an important electrodiffusional pathway for Ca2+ transport from the maternal side of the human placenta in late gestation. PC2 is also implicated in signalling mechanisms, in particular those associated with volume flow in epithelial cells (Nauli et al. 2003), and osmotic signals in the hST (Montalbetti et al. 2005a).
How PC2-mediated Ca2+ transport relates to placentation, and the functional properties of the chorionic place, remains to be defined. Concerning its potential role in cell signalling, PC2 has been implicated in cell division and replication (Aguiari et al. 2004). PC2 is also involved in the developmental pattern of branching in epithelial cells (Grimm et al. 2006). Thus, PC2 may play an important role in placental development. The chorionic villous tree, deriving from the chorionic plate, is an intricate structure, which continuously grows by branching during gestation in a process requiring a dynamic cytoskeleton (Demir et al. 1997). All major cytoskeletal components (Truman & Ford, 1986), including actin filaments (Beham et al. 1988; Parast & Otey, 2000), intermediate filaments (Clark & Damjanov, 1985; Hesse et al. 2000; de Souza & Katz, 2001), and microtubules (Smith et al. 1977; Douglas & King, 1993) are present in the developing placenta. Both the basal and microvillous hST plasma membranes exhibit structural and functional differences, reflecting specific transport and regulatory mechanisms controlled by the maternal and fetal sides of the placental interface, respectively. These differences are not only reflected in the specific segregation of particular Ca2+-transport mechanisms (Moreau et al. 2002, 2003; Clarson et al. 2003; Bernucci et al. 2006), but also depend on the selective expression and location of specific cytoskeletal components. The apical hST cytoskeleton, containing the ‘syncytioskeletal layer’ (Ockleford et al. 1981), expresses the actin bundling protein α-actinin (Booth & Vanderpuye, 1983), which is excluded from the basal membrane cytoskeleton (Vanderpuye et al. 1986). The cytoskeletal anchoring protein EBP50 colocalizes with ezrin and actin only in the apical microvilli of epithelial ST (Berryman et al. 1995; Reczek et al. 1997). The actin cytoskeleton also plays a relevant role in the regulation of PC2 channel function in the term placenta, such that actin filamental dynamics control its function and interaction with actin-associated proteins (Li et al. 2005; Montalbetti et al. 2005b). In this regard, the interactions of PC2 with the actin cytoskeleton may provide a functional unit implicated in signal transduction (Cantiello et al. 2004), which plays a role in osmotic sensing, and thus probably the hydro-electrolytic homeostasis in the human placenta (Montalbetti et al. 2005a). However, apical microvilli are thought to exclude microtubules (Ockleford et al. 1981). This localization not only plays a structural role, but microtubular disruption has been implicated in chorionic differentiation (Douglas & King, 1993). In that study, addition of the microtubular destabilizer colchicine prevented human cytotrophoblast cell differentiation and the process of syncytiation. Microtubular stability has also been implicated in normal fertilization (Wu et al. 1996), and transport in trophoblast-derived cells (Ellinger et al. 1999).
F-actin and microtubular cytoskeletal networks are thought to fulfil separate and independent cellular roles. Highly dynamic actin networks are implicated in cell spreading, motility, and contraction, while the more stable microtubule cytoskeleton is best known for its importance in cell division and organelle trafficking. In the present report, we speculated and confirmed that a functional role(s) of the microtubular cytoskeleton exists in the control of PC2 function, which may have important implications for the control of Ca2+ transport in the human placenta. Our findings indicate that manoeuvres that stabilize microtubules, such as addition of taxol, increase, while addition of colchicine to disassemble microtubules inhibit, endogenous PC2 channel activity in hST membranes. These data suggest a dynamic role of microtubular formation in the activation and regulation of PC2. This was confirmed by addition of exogenous tubulin and GTP, which was also stimulatory. Recent findings from our laboratory indicated, however, that this interaction is not a direct binding of tubulin to the PC2 channel (Li et al. 2006), as the same experiment conducted with purified PC2 channel was unresponsive to the addition of tubulin and GTP (Li et al. 2006). This information suggested the necessity of intermediate protein(s) to mediate the microtubular regulation of PC2. Our data also indicate that, in similar fashion to our findings in ciliary membranes (Li et al. 2006), the kinesin motor protein KIF3A is present in hST, and acts as a mediator protein linking PC2 and the microtubular cytoskeleton. This evidence further suggests a regulatory mechanism that requires microtubule-associated proteins, such as the kinesin-2 in the regulation of PC2. This is particularly relevant in the context of cell trafficking of PC2 by microtubular structures. The data in Fig. 2 indicate that PC2 decorates specific microtubules within the hST vesicles. This is consistent with the possibility that the microtubular connection helps target the channel protein for delivery to specific cell domains. However, a microtubular interaction with PC2 may also be indicative of a novel more universal targeting and signalling mechanism. This hypothesis stems from recent advances in renal and other cystic diseases. Renal polycystic kidney diseases have recently been linked to ciliary dysfunction and microtubular proteins (Morgan et al. 1998; Murcia et al. 2000; Hou et al. 2002; Pazour et al. 2002; Yoder et al. 2002b), including genetic disorders such as autosomal dominant and recessive forms of cystic disease. Dysfunctional ciliary proteins, namely proteins, which preferentially segregate to the primary cilia of epithelial cells, include cystin (Hou et al. 2002) and polaris. These proteins localize to the ciliary basal body and the microtubular axoneme (Yoder et al. 2002b), and produce genetically recessive forms of polycystic kidney disease. PC1 and PC2, also present in primary cilia (Nauli et al. 2003), conform the two main contributors to autosomal dominant polycystic kidney disease. An early observation for a microtubular connection with cystic disease arose by the finding that treatment of homozygous cpk/cpk mice with the microtubular stabilizer paclitaxel (Woo et al. 1997) moderates progression of renal cystic disease, suggesting that the ability to promote microtubule assembly controls cyst formation in mouse kidney. orpk mice, with a dysfunctional polaris, display shortened cilia and left–right asymmetry defects (Murcia et al. 2000) and increased PC2 in cilia (Pazour et al. 2002), in addition to PKD. Primary cilia are clearly implicated in the transduction of signals associated with capacitative Ca2+ entry (Praetorius & Spring, 2001; Praetorius et al. 2003) in renal epithelial cells. Thus, the fact that the PC2 channel interacts with cystin, polaris and ciliary tubulin in primary cilia of renal epithelial cells, strongly suggests that microtubular organization is likely to be a regulator of PC2 function.
In summary, the data from this report and our previous studies (Li et al. 2005; Montalbetti et al. 2005b) suggest a strong complementarity in the cytoskeletal regulation of PC2. The cytoskeletal regulation of PC2 in hST may play two roles: first, as a potential key element in the control of branching and invasion in the developing placenta; and second, fulfilling the fetal needs for Ca2+ intake of late gestational placenta.
Acknowledgments
H.F.C. was funded by the Polycystic Kidney Disease Foundation. The Canadian Institutes of Health Research and the Kidney Foundation of Canada supported X.-Z.C. and his group. X.-Z.C. is an Alberta Heritage Foundation for Medical Research Scholar. Q.L. is a recipient of the Polycystic Kidney Disease Foundation.
References
- Aguiari G, Banzi M, Gessi S, Cai Y, Zeggio E, Manzati E, Piva R, Lambertini E, Ferrari L, Peters DJ, Lanza F, Harris PC, Borea PA, Somlo S, Del Senno L. Deficiency of polycystin-2 reduces Ca2+ channel activity and cell proliferation in ADPKD lymphoblastoid cells. FASEB J. 2004;18:884–886. doi: 10.1096/fj.03-0687fje. [DOI] [PubMed] [Google Scholar]
- Beham A, Denk H, Desoye G. The distribution of intermediate filament proteins, actin and desmoplakins in human placental tissue as revealed by polyclonal and monoclonal antibodies. Placenta. 1988;9:479–492. doi: 10.1016/0143-4004(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Belkacemi L, Bédard I, Simoneau L, Lafond J. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium. 2005;37:1–8. doi: 10.1016/j.ceca.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Bernucci L, Henriquez M, Diaz P, Riquelme G. Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta. 2006;27:1082–1095. doi: 10.1016/j.placenta.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Berryman M, Gary R, Bretscher A. Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J Cell Biol. 1995;131:1231–1242. doi: 10.1083/jcb.131.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AG, Vanderpuye OA. Structure of human placental microvilli. Ciba Found Symp. 1983;95:180–194. doi: 10.1002/9780470720769.ch11. [DOI] [PubMed] [Google Scholar]
- Cantiello HF. Regulation of calcium signaling by polycystin-2. Am J PhysiolRenal Physiol. 2004;286:F1012–F1029. doi: 10.1152/ajprenal.00181.2003. [DOI] [PubMed] [Google Scholar]
- Cantiello HF, Montalbetti N, Timpanaro GA, González-Perrett S. Polycystin-2 as a signal transducer. In: Lauf PK, Adragna NC, editors. Cell Volume and Signaling. Vol. 559. New York: Springer; 2004. pp. 235–244. [DOI] [PubMed] [Google Scholar]
- Clark RK, Damjanov I. Intermediate filaments of human trophoblast and choriocarcinoma cell lines. Virchows Arch A Pathol Anat Histopathol. 1985;407:203–208. doi: 10.1007/BF00737077. [DOI] [PubMed] [Google Scholar]
- Clarson LH, Roberts VH, Hamark B, Elliott AC, Powell T. Store-operated Ca2+ entry in first trimester and term human placenta. J Physiol. 2003;550:515–528. doi: 10.1113/jphysiol.2003.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronier L, Dubut A, Guibourdenche J, Malassine A. Effects of endothelin on villous trophoblast differentiation and free intracellular calcium. Trophoblast Res. 1999;13:69–86. [Google Scholar]
- Demir R, Kosanke G, Kohnen G, Kertschanska S, Kaufmann P. Classification of human placental stem villi: review of structural and functional aspects. Microsc Res Tech. 1997;38:29–41. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<29::AID-JEMT5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Douglas GC, King BF. Colchicine inhibits human trophoblast differentiation in vitro. Placenta. 1993;14:187–201. doi: 10.1016/s0143-4004(05)80260-7. [DOI] [PubMed] [Google Scholar]
- Ellinger I, Schwab M, Stefanescu A, Hunziker W, Fuchs R. IgG transport across trophoblast-derived BeWo cells: a model system to study IgG transport in the placenta. Eur J Immunol. 1999;29:733–744. doi: 10.1002/(SICI)1521-4141(199903)29:03<733::AID-IMMU733>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Gluck U, Ben Ze'ev A. Modulation of alpha-actinin levels affects cell motility and confers tumorigenicity on 3T3 cells. J Cell Sci. 1994;107:1773–1782. doi: 10.1242/jcs.107.7.1773. [DOI] [PubMed] [Google Scholar]
- González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Ntal Acad Sci U S A. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C, Hucho F. Identification and characterization of a Ca2+-sensitive interaction of the vanilloid receptor TRPV1 with tubulin. J Neurochem. 2004;91:1092–1103. doi: 10.1111/j.1471-4159.2004.02795.x. [DOI] [PubMed] [Google Scholar]
- Grimm DH, Karihaloo A, Cai Y, Somlo S, Cantley LG, Caplan MJ. Polycystin-2 regulates proliferation and branching morphogenesis in kidney epithelial cells. J Biol Chem. 2006;281:137–144. doi: 10.1074/jbc.M507845200. [DOI] [PubMed] [Google Scholar]
- Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 2000;19:5060–5070. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JGJ, Hartog A, Stuiver M, Doucet A, Willems PHGM, Bindels RJM. Localization of the epithelial Ca2+ channel in rabbit kidney and intestine. J Am Soc Nephrol. 2000;11:1171–1178. doi: 10.1681/ASN.V1171171. [DOI] [PubMed] [Google Scholar]
- Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D'Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest. 2002;109:533–540. doi: 10.1172/JCI14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+ Neuron. 1993;10:797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Byerly L. Ca2+ channel Ca2+-dependent inactivation in a mammalian central neuron involves the cytoskeleton. Pflügers Arch. 1994;429:14–21. doi: 10.1007/BF02584025. [DOI] [PubMed] [Google Scholar]
- Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Li Q, Dai Y, Guo L, Liu Y, Hao C, Wu G, Basora N, Michalak M, Chen XZ. Polycystin-2 associates with tropomyosin-1, an actin microfilament component. J Mol Biol. 2003a;325:949–962. doi: 10.1016/s0022-2836(02)01333-5. [DOI] [PubMed] [Google Scholar]
- Li Q, Montalbetti N, Shen PY, Dai X-Q, Cheeseman CI, Karpinski E, Wu G, Cantiello HF, Chen X-Z. Alpha-actinin associates with polycystin-2 and regulates its channel activity. Hum Mol Genet. 2005;14:1587–1603. doi: 10.1093/hmg/ddi167. [DOI] [PubMed] [Google Scholar]
- Li Q, Montalbetti N, Wu Y, Ramos AJ, Raychowdhury MK, Chen X-Z, Cantiello HF. Polycystin-2 cation channel function is under the control of microtubular structures in primary cilia of renal epithelial cells. J Biol Chem. 2006;281:37566–37575. doi: 10.1074/jbc.M603643200. [DOI] [PubMed] [Google Scholar]
- Li Q, Shen PY, Wu G, Chen XZ. Polycystin-2 interacts with troponin I, an angiogenesis inhibitor. Biochemistry. 2003b;42:450–457. doi: 10.1021/bi0267792. [DOI] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LSB, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Ntal Acad Sci U S A. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol. 2003;23:2600–2607. doi: 10.1128/MCB.23.7.2600-2607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbetti N, Li Q, González-Perrett S, Semprine G, Chen X-Z, Cantiello HF. Effect of hydro-osmotic pressure on polycystin-2 channel function in the human syncytiotrophoblast. Pflugers Arch. 2005a;451:294–303. doi: 10.1007/s00424-005-1458-7. [DOI] [PubMed] [Google Scholar]
- Montalbetti N, Li Q, Timpanaro GA, González-Perrett S, Dai X-Q, Chen X-Z, Cantiello HF. Cytoskeletal regulation of calcium-permeable cation channels in the human syncytiotrophoblast. Role of gelsolin. J Physiol. 2005b;566:309–325. doi: 10.1113/jphysiol.2005.087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002a;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002b;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Moreau R, Hamel A, Daoud G, Simoneau L, Lafond J. Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta. Biol Reprod. 2002;67:1473–1479. doi: 10.1095/biolreprod.102.005397. [DOI] [PubMed] [Google Scholar]
- Moreau R, Simoneau L, Lafond J. Calcium fluxes in human trophoblast (BeWo) cells: calcium channels, calcium-ATPase, amd sodium–calcium exchanger expression. Mol Reprod Dev. 2003;64:189–198. doi: 10.1002/mrd.10247. [DOI] [PubMed] [Google Scholar]
- Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, Elder FF, Penman-Splitt M, Overbeek P, Strachan T. Inversin, a novel gene in the vertebrate left–right axis pathway, is partially deleted in the inv mouse. Nat Genet. 1998;20:149–156. doi: 10.1038/2450. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge polycystic kidney (orpk) disease gene is required for left–right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Niger C, Malassine A, Cronier L. Calcium channels activated by endothelin-1 in human trophoblast. J Physiol. 2004;561:449–458. doi: 10.1113/jphysiol.2004.073023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Vennekens R, Prenen J, Hoenderop JG-J, Bindels RJM, Droogmans G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol. 2000;527:239–248. doi: 10.1111/j.1469-7793.2000.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockleford CD, Wakely J, Badley RA. Morphogenesis of human placental chorionic villi: cytoskeletal, syncytioskeletal and extracellular matrix proteins. Proc R Soc Lond B Biol Sci. 1981;212:305–316. doi: 10.1098/rspb.1981.0041. [DOI] [PubMed] [Google Scholar]
- Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–656. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Peng JB, Brown EM, Hediger MA. Apical entry channels in calcium-transporting epithelia. News Physiol Sci. 2003a;18:158–163. doi: 10.1152/nips.01440.2003. [DOI] [PubMed] [Google Scholar]
- Peng JB, Brown EM, Hediger MA. Epithelial Ca2+ entry channels: transcellular Ca2+ transport and beyond. J Physiol. 2003b;551:729–740. doi: 10.1113/jphysiol.2003.043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JB, Chen XZ, Berger UV, Vassilev M, Tsukaguchi H, Brown EM, Hediger M. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol. 2003;191:193–200. doi: 10.1007/s00232-002-1055-z. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Raychowdhury MK, McLaughlin M, Ramos A, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem. 2005;280:34718–34722. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts VH, Greenwood SL, Elliott AC, Sibley CP, Waters LH. Purinergic receptors in human placenta: evidence for functionally active P2X4, P2X7, P2Y2, and P2Y6. Am J Physiol Regul Integr Comp Physiol. 2006a;290:R1374–R1386. doi: 10.1152/ajpregu.00612.2005. [DOI] [PubMed] [Google Scholar]
- Roberts VH, Webster RP, Brockman DE, Pitzer BA, Myatt L. Post-translational modifications of the P2X(4) purinergic receptor subtype in the human placenta are altered in preeclampsia. Placenta. 2006b doi: 10.1016/j.placenta.2006.04.008. in press. [DOI] [PubMed] [Google Scholar]
- Robidoux J, Simoneau L, Masse A, Lafond J. Activation of L-type calcium channels induces CRF secretion from human placental trophoblasts. J Clin Endocrinol Metab. 2000;85:3356–3364. doi: 10.1210/jcem.85.9.6774. [DOI] [PubMed] [Google Scholar]
- Rundle DR, Gorbsky G, Tsiokas L. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells. Role of mDia1 in PKD2 localization to mitotic spindles. J Biol Chem. 2004;279:29728–29739. doi: 10.1074/jbc.M400544200. [DOI] [PubMed] [Google Scholar]
- Smith CH, Nelson DM, King BF, Donohue TM, Ruzycki S, Kelley LK. Characterization of a microvillous membrane preparation from human placental syncytiotrophoblast: a morphologic, biochemical, and physiologic, study. Am J Obstet, Gynecol. 1977;128:190–196. doi: 10.1016/0002-9378(77)90686-x. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa: The Iowa State University Press; 1973. [Google Scholar]
- de Souza PC, Katz SG. Coexpression of cytokeratin and vimentin in mice trophoblastic giant cells. Tissue Cell. 2001;33:40–45. doi: 10.1054/tice.2000.0148. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A–/– mice analysis. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman P, Ford HC. Proteins of human placental microvilli. I. Cytoskeletal proteins. Placenta. 1986;7:95–110. doi: 10.1016/s0143-4004(86)80001-7. [DOI] [PubMed] [Google Scholar]
- Vanderpuye OA, Edwards HC, Booth AG. Proteins of the human placental microvillar cytoskeleton. alpha-Actinin. Biochem J. 1986;233:351–356. doi: 10.1042/bj2330351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska MYeC, Brown EM, Hediger MA, Zhou J. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun. 2001;282:341–350. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- Woo DD, Tabancay AP, Jr, Wang CJ. Microtubule active taxanes inhibit polycystic kidney disease progression in cpk mice. Kidney Int. 1997;51:1613–1618. doi: 10.1038/ki.1997.222. [DOI] [PubMed] [Google Scholar]
- Wu GJ, Simerly C, Zoran SS, Funte LR, Schatten G. Microtubule and chromatin dynamics during fertilization and early development in rhesus monkeys, and regulation by intracellular calcium ions. Biol Reprod. 1996;55:260–270. doi: 10.1095/biolreprod55.2.260. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002a;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu J, Bugg CE, JrSchafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002b;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]