Abstract
The descending control of respiratory-related motoneurones in the thoracic spinal cord remains the subject of some debate. In this study, direct connections from expiratory bulbospinal neurones to identified motoneurones were investigated using spike-triggered averaging and the strengths of connection revealed were related to the presence and size of central respiratory drive potentials in the same motoneurones. Intracellular recordings were made from motoneurones in segments T5–T9 of the spinal cord of anaesthetized cats. Spike-triggered averaging from expiratory bulbospinal neurones in the caudal medulla revealed monosynaptic EPSPs in all groups of motoneurones, with the strongest connections to expiratory motoneurones with axons in the internal intercostal nerve. In the latter, connection strength was similar irrespective of the target muscle (e.g. external abdominal oblique or internal intercostal) and the EPSP amplitude was positively correlated with the amplitude of the central respiratory drive potential of the motoneurone. For this group, EPSPs were found in 45/83 bulbospinal neurone/motoneurone pairs, with a mean amplitude of 40.5 μV. The overall strength of the connection supports previous measurements made by cross-correlation, but is about 10 times stronger than that reported in the only previous similar survey to use spike-triggered averaging. Calculations are presented to suggest that this input alone is sufficient to account for all the expiratory depolarization seen in the recorded motoneurones. However, extra sources of input, or amplification of this one, are likely to be necessary to produce a useful motoneurone output.
There is general acceptance that the respiratory drive to spinal motoneurones is conveyed by long-axoned inspiratory or expiratory bulbospinal neurones in the caudal medulla. For phrenic motoneurones there is also general acceptance that this happens in most part via numerous direct connections between bulbospinal neurones and motoneurones, for which there is ample and consistent evidence (Monteau & Hilaire, 1991). However, even for these motoneurones, the quantitative proof that these connections can provide all of the drive is lacking. For inspiratory motoneurones at thoracic levels, the evidence is very much in favour of the inspiratory drive to external intercostal motoneurones being conveyed via interneurones, but it is possible that the drive to the parasternal group is more direct, like the phrenic (De Troyer et al. 2005). The situation for the expiratory drive is currently much less clear and there are conflicting reports as to the strength of the direct connections (Kirkwood & Sears, 1973; Cohen et al. 1985; Merrill & Lipski, 1987; Kirkwood, 1995; Kirkwood & Road, 1995).
The question is worth pursuing for two reasons. Firstly, such an investigation can provide unique insights into the integrative role of the mammalian motoneurone during the production of a naturally occurring motor output. Secondly, the possibility of direct connections between long descending expiratory axons and thoracic motoneurones provides a particularly favourable system for studying plasticity or restoration of connections following spinal cord injury. Indeed, Ford et al. (2000) have already demonstrated plasticity in this system, in that the occurrence of spike-triggered averaged focal synaptic potentials produced by expiratory bulbospinal neurones (EBSNs) in the thoracic cord above a chronic spinal hemisection was increased compared to controls. It is unknown, however, whether this increase represented an increase in existing connections or the presence of new connections to other motoneurones or interneurones.
The experiments described here were undertaken with both of these aims in mind, the intention being to survey thoracic motoneurones in general, to characterize them by their central respiratory drive potentials (CRDPs; Sears, 1964b), and to ask which of these received direct, monosynaptic inputs from EBSNs. A relationship between the connections and the CRDPs might then be established and the general survey could provide baseline data for subsequent measurements in the situation of plasticity or regeneration. It was found that the connections are widespread, being present for all groups of motoneurones, though not surprisingly they were concentrated mostly in expiratory motoneurones. Within that group there was a close relationship between the strength of the individual connections and the overall level of the expiratory drive. Although several explanations are possible for this, we suggest that a major contributory factor is likely to be a state-dependent modulation of the EBSN–motoneurone synapse.
Some of the data have appeared in preliminary reports (Saywell et al. 1999; Ford et al. 1999; Kirkwood et al. 1999; Anissimova et al. 2001).
Methods
The preparation
Experiments were conducted according to UK legislation (Animals (Scientific Procedures) Act 1986). The data come from 28 cats of either sex, weighing 2.3–4.3 kg. Animals were anaesthetized with sodium pentobarbitone (initial dose 37.5 mg kg−1i.p., then i.v. as required), neuromuscular blockade was carried out using gallamine triethiodide (subsequent to surgery, i.v. repeated doses 24mg as required) and then animals were artificially ventilated via a tracheal cannula with oxygen-enriched air, so as to bring the end-tidal CO2 fraction initially to about 4%. CO2 was then added to the gas mixture to raise the end-tidal level to a value sufficient to give a brisk respiratory discharge in the mid-thoracic intercostal nerves (typically 6–7%).
We aimed to use a (surgically adequate) level of anaesthesia in the range light to moderately deep as described by Kirkwood et al. (1982). In the animals without neuromuscular blockade a weak withdrawal reflex was elicited by noxious pinch applied to the forepaw, but not to the hindpaw. When present, pinch-evoked changes in blood pressure (measured via a femoral arterial cannula), were only small and of short duration. During neuromuscular blockade, anaesthesia was assessed by continuous observations of the patterns of the respiratory discharges and blood pressure together with responses, if any, of both of these to a noxious pinch of the forepaw. Only minimal, transient responses were allowed before supplements (5 mg kg−1) of pentobarbitone were administered. The animal was supported by vertebral clamps, a clamp on the iliac crest and a plate screwed to the skull. Rectal temperature was maintained between 37 and 38°C by a thermostatically controlled heating blanket. Systolic blood pressures were above 100 mmHg throughout, maintained in a few animals by occasional infusions of 5% dextran in saline. In 19 of the animals (63 acceptable motoneurone recordings, see below) the primary aim was intracellular recording from interneurones (Saywell et al. 1998) and the motoneurone recordings were an inevitable by-product. These animals were vagotomized and most also received a bilateral pneumothorax. Results did not differ between this group and the other experiments (9 animals, 77 acceptable motoneurone recordings), so these are considered together.
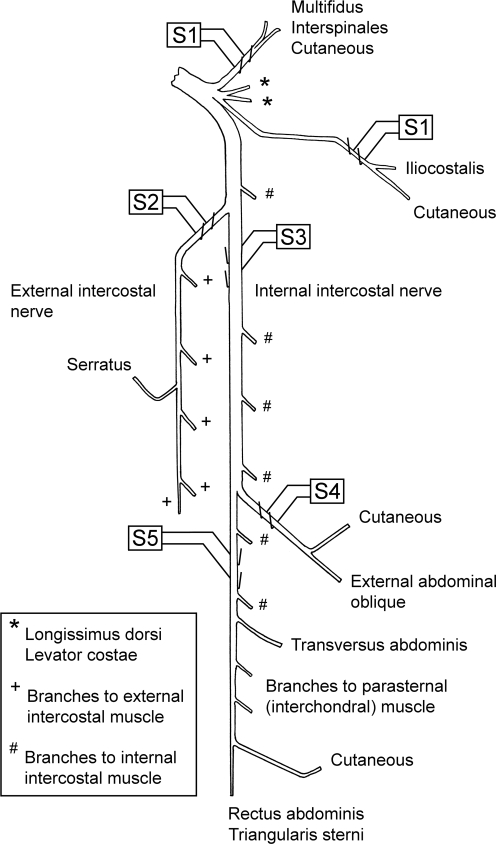
The following nerves (see Fig. 1) were prepared for stimulation via platinum wire electrodes on the left side of one segment or sometimes of two segments to be used for intracellular recording: (1) a bundle of dorsal ramus nerves (Kirkwood et al. 1988); (2) the external intercostal nerve; (3) the most proximal point on the internal intercostal nerve (in continuity, but arranged to be lifted away from the volume conductor separately from the external intercostal nerve, so as to avoid stimulus spread); (4) the lateral branch of the internal intercostal nerve; (5) the distal remainder of the internal intercostal nerve. These nerves were used for antidromic identification of motoneurones, which therefore fell into five anatomical categories. Those identified from the dorsal ramus bundle (DR motoneurones) innervated iliocostalis, multifidus or interspinales. Those identified from the external intercostal nerve (EI motoneurones) innervated external intercostal muscle, together with a few innervating serratus dorsalis cranialis (Tani et al. 1994; Meehan et al. 2004). Stimuli to the lateral branch of the internal intercostal nerve identified external abdominal oblique (EO) motoneurones (Sears, 1964a), while motoneurones excited from the proximal electrodes on the internal intercostal nerve but not from either of the more distal branches were identified as innervating the proximal part of internal intercostal or intracostalis (Sears, 1964a) muscles (IIm motoneurones). Those identified from the distal remainder (Dist motoneurones) innervated the distal part of internal intercostal muscle or transversus abdominis, rectus abdominis, parasternal intercostal, or, in the more rostral segments, triangularis sterni (see Meehan et al. 2004). In the experiments aimed primarily at recording from interneurones, the lateral and distal internal intercostal nerve branches were not prepared and the whole internal intercostal nerve (cut) was stimulated at the proximal site. In these animals, there were thus only three categories, DR, EI and internal intercostal nerve (IIn). A combined category of EO, Dist, IIm, IIn together (all internal intercostal nerve groups) was defined as IIN.
Figure 1.
Arrangement of stimulating electrodes Diagrammatic layout of the nerves for a typical mid-thoracic segment, showing the positions of the stimulating electrodes (S1–S5). Stimulating electrode S1 is shown at two positions to represent the normal anatomical layout. These two nerves were actually mounted together on one pair of electrodes. Motoneurones were defined according to their antidromic activation, as follows: DR, activated from S1; EI, activated from S2; IIm, activated from S3, but not from S4 or S5; EO, activated from S4; Dist, activated from S5; IIn, activated from S3, without testing from S4 or S5. IIN applies to all motoneurones activated from S3–S5 grouped together. See text for further explanation and abbreviations and Meehan et al. (2004) for references for the ventral ramus.
The only muscles innervated from these segments whose motoneurones were not sampled (all innervated from the dorsal ramus) were two with inconveniently short nerves, longissimus dorsi and levator costae and possibly also rotatores. The segments used for intracellular recording ranged from T5 to T9, but most motoneurones (93/141) came from T8 and only 40 motoneurones came from the more rostral segments (T5 or T6). The results from T5–T6 were very similar to those from T7–T9, so they are all considered together. The left external intercostal nerve of a rostral segment (most often T6) was prepared for recording efferent discharges, which were used to define the timing of central inspiration.
A thoracic laminectomy was made, the dura opened and small patches of pia removed from the dorsal columns of the segment(s) to be used for motoneurone recording. Stimulating electrodes were inserted into the left spinal cord 1–2 segments below the chosen segment and a shaped pressure plate lightly applied to the cord dorsum of the chosen segment, to aid mechanical stability. The laminectomy and nerves were submerged in a single paraffin oil pool constructed from skin flaps. An occipital craniotomy was made, the dura opened and a small patch of pia removed from the right side of the medulla. At the end of the experiment the animals were killed with an overdose of anaesthetic.
Recording
Either a glass microelectrode filled with 3 m NaCl (pulled on Model 753 electrode puller, Campden Instruments, UK, and broken back to a tip diameter of 3.0–3.2 μm) or a platinum-plated, epoxy-insulated glass microelectrode, typical resistance 1 MΩ (Ford et al. 1987) was introduced into the medulla through a hole in a small pressure plate so as to record, via a band-pass amplifier, the extracellular activity of single units in the right caudal medulla. Units were located in nucleus retroambiguus as indicated by the narrow column of multi-unit expiratory activity (Merrill, 1970), around 2.5 mm caudal to obex and 1.8–2.4 mm from the dorsal surface of the medulla. Expiratory bulbospinal neurones (EBSNs) with axons descending on the left side were identified by antidromic responses to stimuli (0.1 ms pulses) delivered via the spinal cord stimulating electrodes. Identification was confirmed by a collision test (at 2 × threshold) and by double stimuli to show that the minimum interval in the collision test was not due to soma refractoriness. In some animals two recording electrodes (independent manipulators) were used for simultaneous recordings of two units. Sometimes two units were discriminated by spike amplitude from a single recording. Simultaneous recordings from the EBSNs and intracellular recordings from antidromically identified thoracic motoneurones (potassium acetate electrodes) were stored on magnetic tape and/or acquired for computer analysis (Spike2, CED, Cambridge, UK). Both a low-gain DC version and a high-gain, high-pass-filtered (time constant, 50 ms) version of the motoneurone membrane potential were included. The only periods of recordings used for analysis were those where the membrane potential was below −40 mV (checked on exiting the motoneurone) and which included more than about 1000 EBSN spikes (actual minimum accepted was 957 spikes). Control recordings were made subsequently at extracellular sites close to each recorded motoneurone.
Analysis
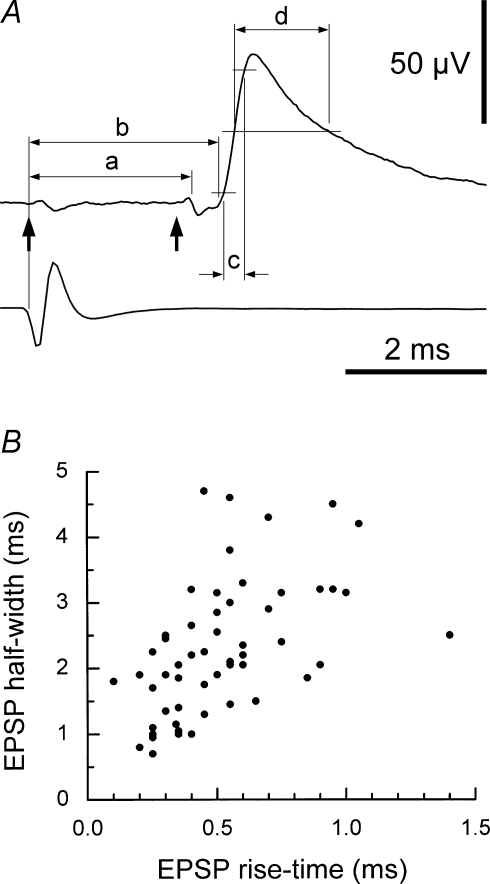
The low-gain recording was used for estimation of the central respiratory drive potential (CRDP, Sears, 1964b). Spike-triggered averaging (STA) was carried out for each EBSN/motoneurone pair, using the high-gain record. If necessary, periods of motoneurone firing were edited out of the record before analysis. An essential criterion for a waveform to be accepted as a synaptic potential was that of repeatability; the potential (independent of any judgement about synaptic linkage) had to be present with a similar time course at the same latency in each of three successive averaging epochs (Kirkwood & Sears, 1982). The single unit nature of the EBSN recording was always confirmed by an interval histogram or auto-correlation plot. Values of interval from the first peak or the shoulder of the interval histogram made from the first recording of each EBSN were used to calculate an approximate modal firing frequency of the EBSN (Kirkwood, 1995; Ford et al. 2000). Delays in the collision test and latencies in the averages (Fig. 6A) were all referred to the early rising phase of the (main) negative-going phase of the trigger spikes (Davies et al. 1985a; Kirkwood, 1995). Conventional shape indices (Rall, 1967; Jack et al. 1971) were measured for synaptic potentials (Fig. 6A): rise-times from 10% to 90% amplitude and durations at 50% amplitude (half-widths). Correction was made for a sloping baseline, if present, as in Kirkwood & Sears (1982).
Figure 6.
Diagram of temporal measurements and shape-index plot for EPSPs A, the same EPSP as Fig. 3A, together with an average of its trigger spike (lower trace), labelled to show the temporal parameters measured: arrows, as previously, the trigger time and the calculated axonal time; a, latency of the TP; b, latency of the EPSP; c, rise-time (10–90%) of the EPSP; d, the half-width of the EPSP (duration at 50% amplitude). B, the shape-index plot for all the EPSPs, which is provided for comparison with similar plots in the literature for single-fibre EPSPs in motoneurones (see text for references). The one outlier point at a rise-time of 1.4 ms was derived from the EPSP of Fig. 5D.
The rostro-caudal positions of the motoneurones were noted with respect to the rostral border of the segment, defined by the most rostral dorsal root entry on the left (Meehan et al. 2004). Motoneurone recordings were made either in a rostral part of the segment (approximately 0–1 mm caudal to the rostral border) or a caudal part (approximately 5–6 mm caudal to the rostral border). Orthodromic conduction times for the EBSNs were calculated from the collision test as the critical delay minus 0.5 ms (Davies et al. 1985a; Kirkwood, 1995) and conduction velocities were calculated from these values, together with distances from the medulla to the stimulating electrodes. These distances were calculated from a table of values related to the weight of the animal and derived from the animals used by Davies et al. (1985a) and Kirkwood (1995). For each EBSN, the conduction time to the appropriate rostral or caudal part of the segment (the ‘axonal time’) was estimated as in Ford et al. (2000).
Mean values are quoted as ±s.d. In statistical tests, P < 0.05 was taken as significant.
Results
EBSN properties
Both the conduction velocity distribution (range 28.0–136.8 m s−1, mean 60.7 ± 20 m s−1) and the modal firing frequency distribution (range 15.3–178.6 impulses s−1, mean 79.6 ± 57 impulses s−1) for the whole population of EBSNs (n = 54) were similar to those of Kirkwood (1995) or Ford et al. (2000), although the mean modal firing frequency here is a little lower than in those two studies. These previous studies involved partly overlapping populations of units with an overall mean modal firing frequency of 101.7 ± 47 impulses s−1. The difference between this combined group and the present population is significant (Mann-Whitney, P = 0.002) and probably arose because here we accepted for study a higher proportion of the slower firing EBSNs, fewer trigger spikes being required for intracellular STA than for the extracellular STA or cross-correlations used previously. The locations of the EBSNs in the medulla and the experimental conditions were very similar. Apart from the minor difference in firing frequencies, we can assume a similar population of neurones was studied.
Motoneurones
Data from 170 EBSN-motoneurone pairs were analysed, comprising 54 EBSNs and 141 motoneurones, 1–12 motoneurones per EBSN. The numbers of sweeps averaged ranged from 957 to 22 642 (median, 5223). The noise level in the averages varied considerably, both as a result of variation in the number of sweeps and because of variation in the ongoing synaptic noise. To avoid too many false negative observations we discarded averages with peak-to-peak noise levels > 10 μV (excluding slow drifts). A deflection in the average was considered to represent noise if it did not satisfy the repeatability criterion (see Methods). The noise criterion eliminated 8 of the above EBSN-motoneurone pairs (numbers of sweeps 967–3354), leaving 162 pairs, from 134 motoneurones, with 52 EBSNs.
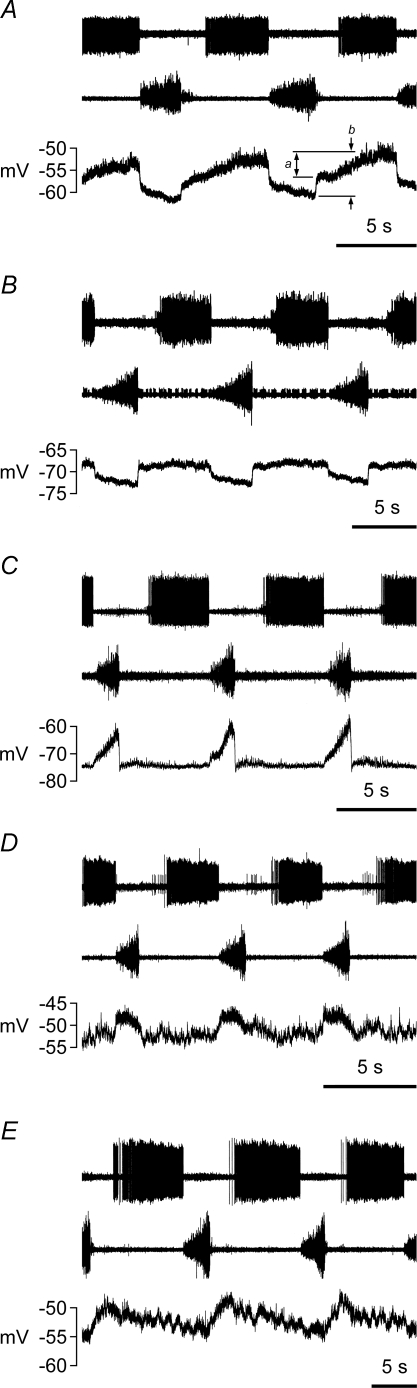
Most of the motoneurones showed a CRDP. These were classified as expiratory, inspiratory, or expiratory decrementing (Edec), according to the respiratory phase during which the motoneurone was most depolarized, plus one single example with a depolarization at the end of expiration, continuing into inspiration. Representative examples are illustrated in Fig. 2, and their distribution summarized in Table 1. The CRDP amplitudes in Table 1 are the averages of the amplitudes from several respiratory cycles. The categories of CRDP are important partly because they help indicate the muscle innervated by a given motoneurone. Here, the two motoneurone groups believed to correspond to single expiratory muscles (EO and IIm) both gave exclusively expiratory CRDPs. Inspiratory CRDPs were found in 8/34 of the IIn motoneurones. This subgroup of motoneurones may be presumed to have innervated parasternal muscle (well known to be inspiratory; Taylor, 1960), as in Kirkwood & Sears (1978) and Lipski & Martin-Body (1987), a presumption strongly supported by the observation that within the groups of motoneurones identified here from separate branches of the IIN, inspiratory CRDPs were found only in Dist motoneurones (Table 1). The CRDP classifications are also used below to classify the motoneurones, i.e. an inspiratory motoneurone is one that showed an inspiratory CRDP.
Figure 2.
Examples of central respiratory drive potentials (CRDPs) with different time courses, to illustrate the definitions of types Records in each panel, from the top: extracellular discharge from an expiratory bulbospinal neurone (EBSN); efferent discharge in an external intercostal nerve, used to define inspiration; intracellular recording from a motoneurone. A and B, expiratory CRDPs: note difference between the expiratory ramps in the two examples, substantial in A, but small in B. However, the two CRDPs have a similar inhibitory component during inspiration. CRDP measurements: a (voltage difference between upper two lines), amplitude of the ramp; b (peak-to-peak amplitude), amplitude of the CRDP. C and D, inspiratory CRDPs. C, example with a typical inspiratory ramp. D, example with an atypical inspiratory depolarization. E, an expiratory decrementing (Edec) CRDP. Motoneurone identifications: A, Dist; B, EO, C and D, EI; E, DR.
Table 1.
Distribution of CRDP types and amplitudes among the different groups of motoneurones
| Dist | EO | IIm | IIn | EI | DR | Total | ||
|---|---|---|---|---|---|---|---|---|
| CRDP type | ||||||||
| Insp | n | 7 | — | — | 8 | 15 | 11 | 41 |
| range (mV) | 1–11 | — | — | 3–15 | 1–14 | 1–5 | 1–15 | |
| mean (mV) | 4.2 | — | — | 7.0 | 6.5 | 2.3 | 5.1 | |
| s.d. | 4.0 | — | — | 4.0 | 4.0 | 1.3 | 4.0 | |
| Exp | n | 19 | 14 | 12 | 22 | — | 1 | 68 |
| range (mV) | 0.3–11.5 | 1–11.5 | 1.5–10 | 1.5–10.5 | — | 1.2 | 0.3–11.5 | |
| mean (mV) | 4.4 | 4.3 | 4.8 | 5.0 | — | — | 4.6 | |
| s.d. | 2.9 | 3.1 | 2.6 | 2.8 | — | — | 2.9 | |
| (ramps) | ||||||||
| range (mV) | 0–5.9 | 0–5.0 | 0–6.0 | 0–4.9 | — | — | 0–6.0 | |
| mean (mV) | 1.7 | 1.6 | 2.2 | 1.6 | — | — | 1.7 | |
| s.d. | 1.4 | 1.4 | 1.9 | 1.6 | — | — | 1.7 | |
| Edec | n | 2 | — | — | 2 | 4 | 4 | 12 |
| range (mV) | 1.4, 6 | — | — | 2, 3 | 0.5–4 | 1.5–3 | 0.5–6 | |
| mean (mV) | 3.7 | — | — | 2.5 | 2.1 | 2.3 | 2.5 | |
| s.d. | — | — | — | — | 1.4 | 0.6 | 1.4 | |
| Exp/Insp | n | — | — | — | — | — | 1 | 1 |
| (mV) | — | — | — | — | — | 2.5 | 2.5 | |
| No CRDP | n | 1 | — | 1 | 2 | 2 | 4 | 10 |
| Total (with CRDP) | ||||||||
| n | 28 | 14 | 12 | 32 | 19 | 17 | 122 | |
| range (mV) | 0.3–11.5 | 1–11.5 | 1.5–10 | 1.5–15 | 0.5–14 | 1.5 | 0.3–15 | |
| mean (mV) | 4.3 | 4.3 | 4.8 | 5.3 | 5.5 | 2.6 | 4.5 | |
| s.d. | 3.2 | 3.1 | 2.6 | 3.3 | 4.0 | 1.1 | 3.3 | |
Motoneurone groups: Dist, distal muscles; EO, external abdominal oblique; IIm, internal intercostal muscle; IIn, internal intercostal nerve; EI, external intercostal nerve; DR, dorsal ramus bundle. Central respiratory drive potential (CRDP) motoneurone types: Insp, inspiratory; Exp, expiratory; Edec, expiratory decrementing. The table shows the number of motoneurones, n, with the range, mean and s.d. of the CRDP amplitude in each motoneurone group, from a total of 132 motoneurones (CRDP data are missing for two DR motoneurones).
Motoneurones of the intercostal nerves, normally associated with respiration, gave the highest proportion of CRDPs (86/90 for all branches of IIN, 19/21 for EI, with 85 and 18, respectively, of these being of amplitude ≥ 1 mV). Nevertheless, in DR motoneurones CRDPs were still common (17/21), although these were generally small (maximum 5 mV, mean 2.6 mV) as compared to those in IIN motoneurones (maximum 15 mV, mean 4.8 mV) or EI (maximum 14 mV, mean 5.5 mV). The largest CRDPs were inspiratory (mean 5.1 mV, 7/41 ≥ 10 mV) with the expiratory CRDPs only a little smaller (mean 4.6 mV, 6/68 ≥ 10 mV), but the Edec CRDPs (Fig. 2E) were noticeably smaller (mean 2.5 mV) and relatively uncommon, being present in each of the four motoneurone groups which included inspiratory CRDPs but in each case about 4 times less frequently. The amplitudes of the expiratory CRDPs were similar for all branches of IIN.
One aim of this study was to relate the EBSN connections to the strength of the drive in individual motoneurones. However, although the overall amplitude of the CRDP may be taken as one indicator of the intensity of the respiratory drive, the expiratory CRDP consists of two components, an excitatory drive during expiration and an inhibitory phase during inspiration (Sears, 1964b). Independence between the two components can be seen by comparing the CRDPs of Fig. 2A and Fig. 2B, where the inspiratory hyperpolarization appears similar in the two examples, but the expiratory excitation is considerably stronger in Fig. 2A. The excitatory phase itself might have both a ‘step’ component, at the beginning of expiration, and a later ‘ramp’ component, both derived from similar components in the pattern of discharge of EBSNs (Cohen et al. 1985). The step component is impossible to separate from the rapid end of the inhibitory component, so in order to provide an index of the expiratory excitation, we measured the amplitude of the ramp, following the step of depolarization at the end of inspiration (Fig. 2A). The mean amplitude of this ramp for all the expiratory CRDPs was 1.7 mV (range 0–6 mV) and was similar for the CRDPs of each of the four groups of motoneurones from the IIN (means 1.6–2.2 mV, Table 1).
Detection of EPSPs
A high proportion of the spike-triggered averages showed repeatable deflections. However, many of these were small and some showed time courses that were more appropriate to an origin in some form of presynaptic synchronization, rather than a monosynaptic connection. Proper distinction must be made between these two possibilities in order to make an accurate estimate of the strength of connections (Davies et al. 1985a; Kirkwood, 1995). These distinctions are illustrated in Figs 3 and 4.
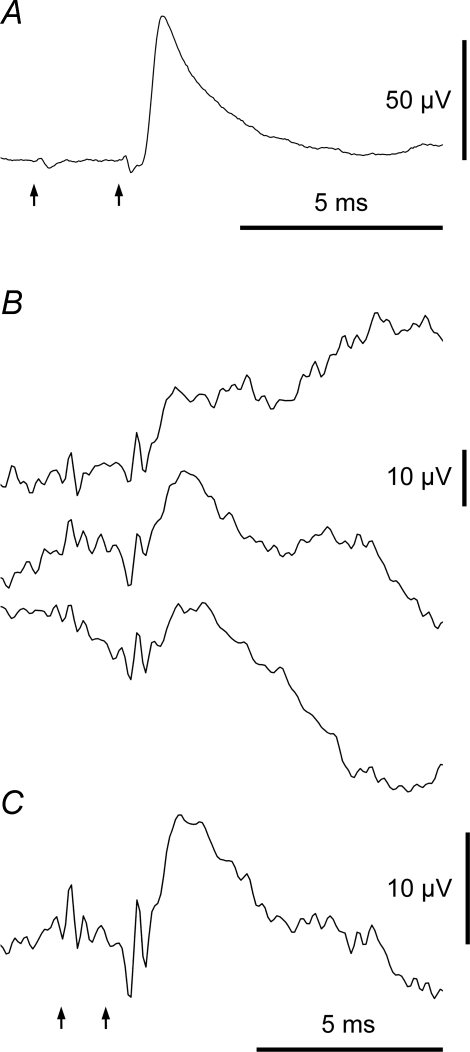
Figure 3.
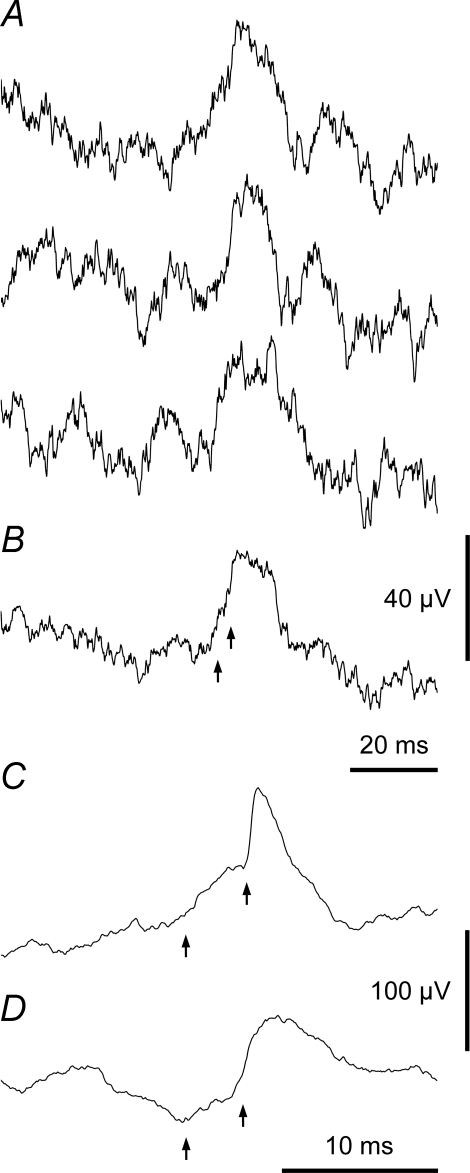
Classification of averaged waveforms: EPSPs In each example the arrows show (left) the EBSN spike time and (right) the estimated axonal time, calculated from the critical interval in the EBSN collision test. A, a typical EPSP: the deflection near the EBSN spike time is a cross-talk artefact, the deflection near the axonal time is a terminal potential (TP). Motoneurone and EBSN as in Fig. 2B. 10 826 sweeps. B and C, an example of a small EPSP: B, successive thirds of the data, 1849, 1971 and 1949 sweeps, respectively, to show repeatability; C, whole run (5769 sweeps). Note repeatable TP as well as repeatable EPSP. Inspiratory IIN motoneurone.
Figure 4.
Classification of averaged waveforms: synchrony potentials A and B, example of a synchrony potential in an IIn motoneurone: A, successive thirds of the data, 5339, 5606 and 6048 sweeps, respectively, to show repeatability; B, whole run (16 991 sweeps). Note longer time scale than the other records. C and D, averages from two different EBSNs in the same EO motoneurone: C, combination of synchrony potential with clearly separate EPSP (957 sweeps); D, an ambiguous potential (a synchrony potential which could hide an EPSP) (1101 sweeps). Arrows as Fig. 3.
A potential with a fast rising phase arising from a relatively flat baseline is shown in Fig. 3A. From all points of view, this was unequivocally characterized as an EPSP. Similarly there was little difficulty also in characterizing the waveform in Fig. 3B and C as an EPSP, although because this was one of the smallest examples, it was necessary to check that the repeatability criterion was satisfied, as illustrated. A much more slowly rising potential is illustrated in Fig. 4A and B (note the relatively long time scale). This latter potential nevertheless also satisfies the repeatability criterion (Fig. 4A) and can be seen to rise from a relatively flat baseline in the final average (Fig. 4B). Note also that the fast rising potential in Fig. 3A starts just after the estimated axonal time (right arrow), whereas in Fig. 4A and B the axonal time (right arrow) is located near the peak. A third type of average potential (Fig. 4C) included both types of waveforms, a fast rising potential superimposed on one with a slower time course. Although these components occurred together, they could nevertheless be clearly distinguished. The slow rising potentials in these two examples started too early to have arisen by synaptic excitation from the EBSN, so they must represent the actions of other neurones whose discharges were partly synchronized to the EBSN spikes, as described by Davies et al. (1985a) and Kirkwood (1995). A potential such as in Fig. 4A and B was therefore classified as a ‘synchrony potential’ and one like Fig. 4C was confidently separated into an EPSP and a synchrony potential, the synchrony potentials corresponding to the ‘medium width’ peaks in the cross-correlation analyses of those two studies.
In seven cases, there was not a clear inflexion and waveforms had to be classified as ambiguous (Fig. 4D). Such waveforms included a slow rising phase and a very short latency, thus indicating the presence of a synchrony potential but because, as here, a considerable part of the rising phase occurs after the axonal time (right arrow), the presence of an EPSP could not be ruled out. These ambiguous averages, which could have included EPSPs of amplitude 10 μV or above, had amplitudes of 20–88 μV, latencies of 2–3.5 ms and rise-times of 1.3–5.0 ms. They were all in motoneurones of the IIN (6 expiratory, 1 Edec). The EBSN-motoneurone pairs concerned were regarded as ‘not tested’ for connections and eliminated from the data set for estimating connectivity, which therefore finally comprised 155 pairs from 132 motoneurones and 52 EBSNs.
Synchrony potentials were detected in 12 of these pairs, with amplitudes of 10–44 μV (mean 20 ± 10.7 μV). Six of these potentials were seen in combination with an EPSP (e.g. Fig. 3C). Eleven showed a time course similar to those in Fig. 4, with latencies of −1.5 to 2.5 ms and rise-times of 3–8 ms (mean 4.6 ± 1.7 ms). Ten of these 11 were found in expiratory motoneurones (IIN) and one in an Edec (DR) motoneurone. The remaining synchrony potential, in an inspiratory motoneurone (EI), had an approximately symmetrical shape with a maximum near the trigger time and minima at ± 8.5 ms. All of these 12 examples satisfied the repeatability criterion (see Methods). However, many of the other averages (with or without an EPSP) showed deflections of a similar time course to the 11 mentioned above, but with amplitudes below 10 μV (Fig. 5A). For all of these, the averages from separated, shorter epochs were not convincingly repeatable, so none of these could individually be distinguished unequivocally from noise.
Figure 5.
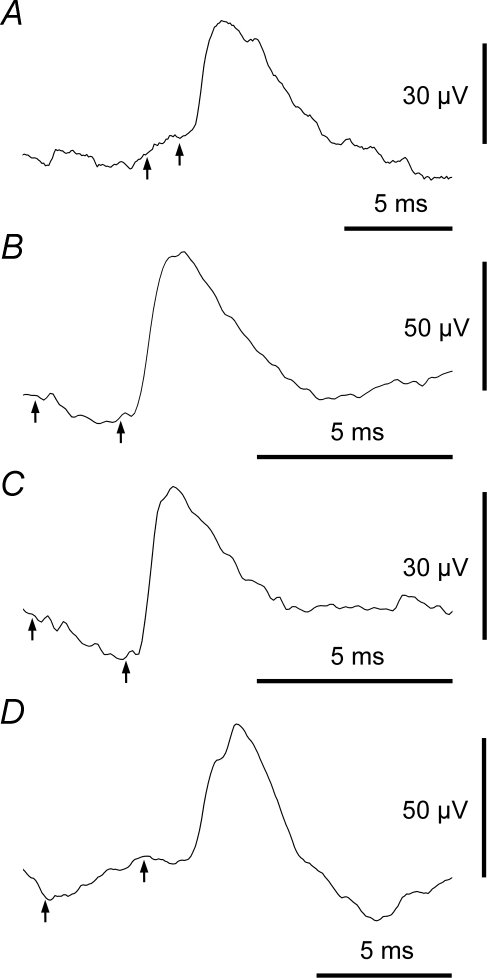
Further examples of EPSPs, including motoneurones of different groups A, motoneurone (Dist) and EBSN as Fig. 2A. Note small synchrony potential also appears to be present. B, motoneurone (EI) and EBSN as Fig. 2D. C, motoneurone (DR) and EBSN as Fig. 2E. D, Dist motoneurone: note rather long rise-time and rather long segmental delay. Numbers of sweeps: 8201, 13 545, 16 927 and 9354, respectively. Arrows as Fig. 3.
General properties of EPSPs
EPSPs, amplitudes 6–221 μV, were detected in 55/155 trials. No IPSPs were detected. Examples, drawn from the cells whose CRDPs were illustrated in Fig. 2, are shown in Fig. 5A–C. EPSP rise-times ranged from 0.1 to 1.4 ms and were therefore completely non-overlapping with the range of rise-times for the synchrony potentials. A conventional shape index plot (half-width against rise-time) (Rall, 1967; Jack et al. 1971) for all the EPSPs is shown in Fig. 6B.
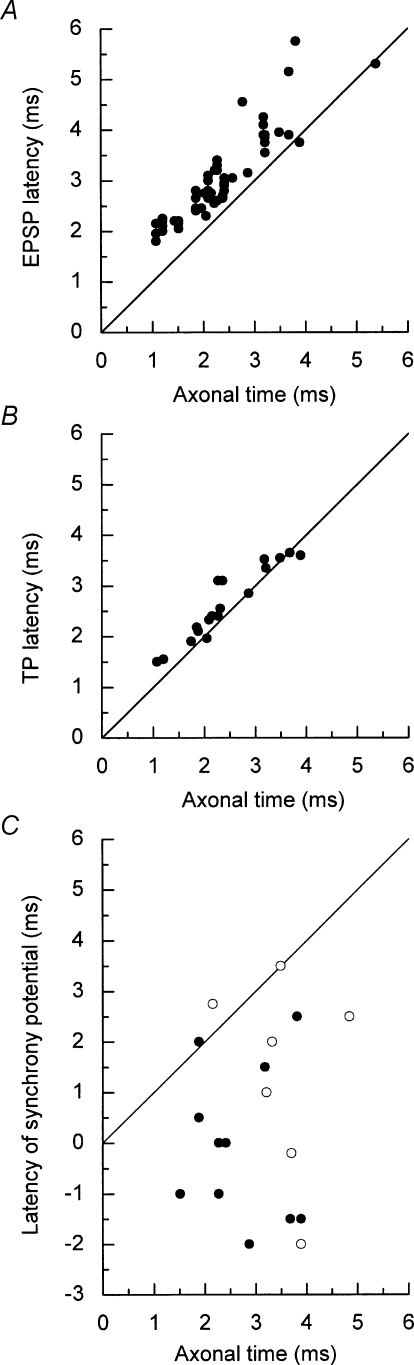
Latency measurements (Fig. 6A) confirmed the validity of the distinction between EPSPs and the synchrony potentials. Figure 7A shows the latencies of all the EPSPs plotted against the calculated conduction time (axonal time), showing a clear relationship, the points being grouped just above the line of equality. In contrast, the latencies of the synchrony potentials and the ambiguous potentials appeared to be unrelated to the axonal times (Fig. 7C) and nearly all fell below the line of equality, as for medium-width peaks in equivalent cross-correlation measurements (Kirkwood, 1995). The distribution of the difference between the EPSP latency and the axonal time (the segmental delay) was very similar to the distribution of segmental delays for extracellular focal synaptic potentials from EBSNs (Ford et al. 2000), having a mean of 0.69 ± 0.38 ms. For 18 EBSNs a terminal potential (TP), amplitude 1.5–24 μV, median 3.5 μV, n = 35, was also present in some of the intracellular (or extracellular control) averages at a slightly earlier latency than the EPSPs (e.g. Fig. 3). The latencies of these were also closely related to the axonal time (Fig. 7B) as in Ford et al. (2000). The mean segmental delay for the TPs from the 18 EBSNs (using a median value for the delay if different values were obtained for one EBSN from averages at different sites) was 0.21 ± 0.26 ms.
Figure 7.
Latencies of averaged potentials Latencies were measured with respect to the trigger spikes and plotted against the corresponding estimated axonal times. A, EPSPs: B, TPs; C, synchrony potentials (•) or ambiguous potentials (^). One outlier point (synchrony potential, axonal time 1.74 ms, latency −8.5 ms) is omitted. The line of equality is included in each graph. Note that the latencies of the points in C do not have a close relationship to the line, such as is evident in A and in B.
Two of the points (from two EBSNs) lie below the line of equality in Fig. 7A. These values are most likely to have arisen from an over-estimation of the conduction time because of slowed conduction in the EBSN axon between the sites of intracellular recording and the stimulation site one or two segments more caudal. Such slowing would be likely if the EBSN was one of the few whose axons terminate at this segmental level (≤ 3/48 in Ford et al. 2000). This presumption was confirmed for one of the EBSNs, which gave the only TP with an appreciable negative segmental delay (−0.29 ms, Fig. 7B). The other EBSN gave no TP.
One EPSP (Fig. 5D) had a rather long rise-time (1.4 ms, cf. the remainder 0.1–1.05 ms, Fig. 6B) and a rather long segmental delay (1.47 ms). These values are consistent with this EPSP being disynaptic in origin (see Discussion in Vaughan & Kirkwood, 1997). However, the EPSP here could equally well be late and slowly rising as a result of slowed conduction in one or more terminal EBSN collateral branches and/or because of dendritic location (Jack et al. 1971). Indeed, two other EPSPs had slightly longer segmental delays (Fig. 7A), but relatively short rise-times (0.6 and 0.9 ms). We have not considered any of these three separately from the rest of the data below, which are taken as representing monosynaptic connections.
Distribution of EPSPs in different categories of motoneurone
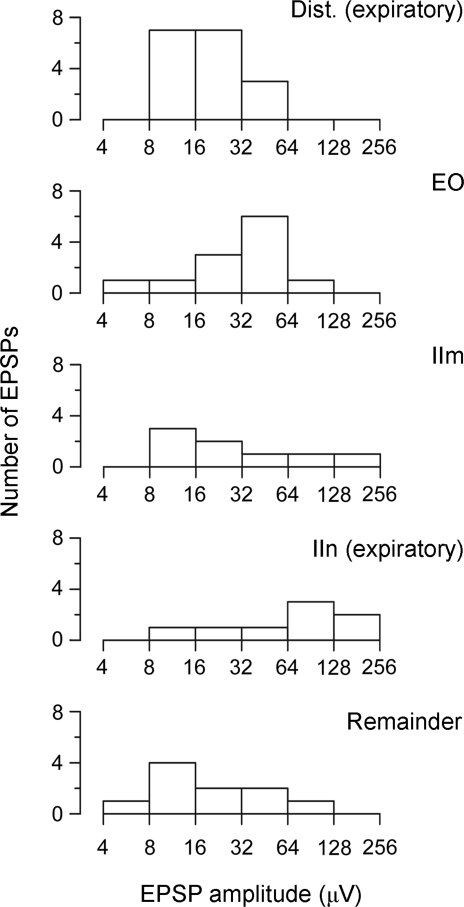
The ‘connectivity’ for any category was defined as the fraction of EBSN/motoneurone pairs showing EPSPs. The mean amplitude of the EPSPs for any group was defined using n = number of EPSPs. The ‘effective mean amplitude’ was defined as the sum of all the EPSP amplitudes (the absence of an EPSP counted as zero amplitude) divided by the number of pairs (n = number of pairs). These parameters are summarized in Table 2A–C for groups of motoneurones defined both by CRDP and by nerve identification. The distribution of EPSP amplitudes in each category is shown in Fig. 8. It can be seen that the connectivity was rather similar for three out of four groups of IIN expiratory motoneurones (17/30, 12/18, 8/13, Table 2A), but appears lower for the IIn group (8/22). The numbers in these proportions are rather low for statistical comparison. The apparently clearest difference (IIn versus the rest, 37/61) is not significant (χ2, P > 0.05). Note that a significant difference would not be expected here since the IIn group should be composed of members of the other three groups. The mean amplitude of the IIn group is actually the largest of the four, but this is also not significantly different from the other three taken together (Mann-Whitney, P = 0.078). Among the remaining three groups, representing two different muscles, plus the remaining Dist branch, the mean amplitude still varied, the mean for the Dist group being the smallest. However, note that the range of EPSP amplitudes within each group was very wide (log scales in Fig. 8). A significant difference was detected among these three groups (Kruskal-Wallis, P = 0.015), but none of the three subsequent pair-wise Mann-Whitney tests between the groups reached significance, although the two involving the Dist group (versus IIm and versus EO) nearly did (P = 0.054 for both, after Bonferroni correction).
Table 2.
Number and amplitudes of EPSPs in motoneurones of different groups
| Amplitude (μV) | |||||
|---|---|---|---|---|---|
| Connectivity | Range | Median | Mean | Effective mean | |
| A. IIN motoneurones with expiratory CRDPs | |||||
| Dist | 17/30 (57%) | 8.5–52 | 19 | 21.8 | 12.3 |
| EO | 12/18 (67%) | 7.5–94 | 43.5 | 41.9 | 27.9 |
| IIm | 8/13 (62%) | 11–143.5 | 21.5 | 39.8 | 24.5 |
| IIn | 8/22 (36%) | 8.5–221 | 68 | 79.3 | 28.8 |
| Total IIN expiratory | 45/83 (54%) | 8.5–221 | 22 | 40.5 | 22.0 |
| B. Motoneurones with inspiratory CRDPs | |||||
| Dist | 0/9 (0%) | — | — | — | — |
| IIn | 2/8 (25%) | 8.5, 10 | — | 9.3 | 2.3 |
| EI | 2/15 (13%) | 14.5, 62 | — | 38.2 | 5.1 |
| DR | 3/13 (23%) | 8–27 | 16 | 19.7 | 4.5 |
| Total inspiratory | 7/45 (16%) | 8–62 | 14.5 | 22.0 | 3.4 |
| C. Other motoneurones* | |||||
| Dist | 0/2 (0%) | — | — | — | — |
| IIm | 0/1 (0%) | — | — | — | — |
| IIn | 0/4 (0%) | — | — | — | — |
| EI | 1/8 (13%) | 65 | — | 65 | 8.1 |
| DR | 2/12 (17%) | 6, 34 | — | 20.0 | 3.3 |
| Total other | 3/27 (11%) | 6–65 | 34 | 35.0 | 3.9 |
| Total B + C | 10/72 (14%) | 6–65 | 15.3 | 25.9 | 3.6 |
| D. Rostral vs. caudal parts of the segment | |||||
| Rostral, IIN expiratory | 28/53 (53%) | 7.5–221 | — | 39.5 | 20.9 |
| Rostral, all others | 8/56 (14%) | 6–62 | — | 21.2 | 3.0 |
| Caudal, IIN expiratory | 17/30 (57%) | 12–143.5 | — | 43.3 | 24.5 |
| Caudal, all others | 2/16 (13%) | 16, 65 | — | 40.5 | 5.1 |
The category ‘Other motoneurones’ includes all those with Edec or inspiratory/expiratory CRDPs or no CRDP and one DR motoneurone with an expiratory CRDP. IIN refers to the combined category of EO, Dist, IIm and IIn (all internal intercostal nerve groups).
Figure 8.
Distributions of EPSP amplitudes for different categories of motoneurones Note the log scales for amplitude. The EPSPs in the motoneurones of the non-expiratory groups (Remainder) have significantly lower amplitudes than those in the motoneurones of all the expiratory groups (first four histograms) taken together. The significance of the differences between the amplitudes among these four groups is uncertain (see text).
The remaining (non-expiratory) groups showed generally lower connectivity. Considering all these other groups together, their connectivity was nearly four times lower (10/72 versus 45/83) than for the IIN expiratory motoneurones and was highly significantly different (χ2, P < 0.001). The mean size of EPSPs in these other groups was also smaller than for the IIN expiratory group (25.9 versus 40.5 μV) but this difference was not significant (Mann-Whitney, P = 0.31). Perhaps the most notable feature of the connections to these groups was that they were present at all, including in a reasonable number of inspiratory motoneurones. Some of these motoneurones were strongly inspiratory: the two IIn examples had CRDPs of amplitude 8 and 11 mV. However, both the two EPSPs concerned were small: 10 μV (Fig. 3B and C) and 8.5 μV, respectively. Further, one of the two external nerve motoneurones with relatively large EPSPs had no CRDP. The other (EPSP shown in Fig. 5B) had an inspiratory CRDP of amplitude 5 mV, but with an unusual time course, not showing a clear inspiratory ramp (Fig. 2D, cf. Fig. 2C).
Distribution of EPSPs rostral versus caudal in the segment
A notable feature of the functional projections of the EBSNs measured previously (Ford et al. 2000) was that the strength of the projection was considerably stronger in the rostral part of the segment than the caudal part. It was therefore of interest to compare the connections to motoneurones in these two regions, as is summarized in Table 2D. There is clearly no detectable difference between the two populations.
Relationship between EPSPs and CRDP amplitude
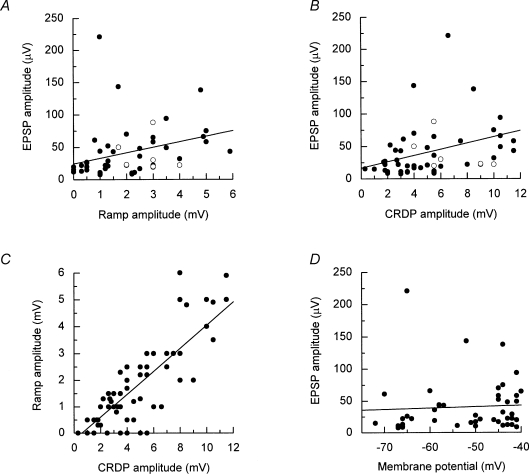
From preliminary data, a positive correlation has already been demonstrated between the EPSP amplitude and the ramp amplitude in expiratory motoneurones (Kirkwood et al. 1999). This correlation has now been performed for all the IIn motoneurones (Fig. 9A), as has a similar correlation for the total amplitude (Fig. 9B).
Figure 9.
Relationships between EPSP amplitudes and CRDP parameters for IIN expiratory motoneurones A, EPSP amplitude (•) showed a significant correlation with expiratory ramp amplitude (regression line is shown, r = 0.311, P = 0.04); B, EPSP amplitude (•) showed a significant correlation with CRDP amplitude (regression line is shown, r = 0.359, P = 0.017); ^ in A and B represents ambiguous potentials. C, ramp amplitude showed a significant correlation with CRDP amplitude (regression line is shown, r = 0.846, P < 0.001); D, EPSP amplitude did not show a significant correlation with membrane potential (regression line is shown, r = 0.085, P = 0.738).
A positive correlation was present in each case (r = 0.311, r = 0.359), the similarity between the two plots reflecting a strong correlation between the amplitudes of the CRDP and its ramp component (r = 0.846, Fig. 9C). Irrespective of this, we considered the possibility that part of the relationship between the ramp and the mean EPSP size might have arisen artefactually because both were affected by membrane potential, either of these parameters being expected to be smaller in more depolarized motoneurones on account of either a reduced driving potential or shunting from a membrane leak. However, Fig. 9D shows that there is, if anything, a positive correlation between membrane potential and EPSP amplitude (somewhat larger EPSPs in more depolarized cells), although the slope is not significant. Thus this possibility can be dismissed.
One other set of data points (open symbols) is included in Fig. 9A and B. These points indicate the amplitudes of the averaged waveforms previously excluded as being ambiguous for the presence of EPSPs or a synchrony potential. The six examples from expiratory motoneurones are plotted. Although not included in the data for the regression lines, these values clearly fit within the same scatter of points for either CRDP or ramp amplitude.
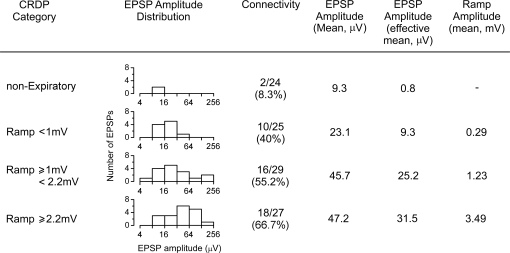
Not only was the EPSP amplitude related to the strength of the respiratory drive, but so was the connectivity. This is illustrated in Fig. 10, where connectivity and other parameters are listed for the expiratory motoneurones from IIN after division into three approximately equal-sized groups according to the ramp amplitude. A fourth group of IIN motoneurones is also included, non-expiratory, i.e. those with no CRDP or with inspiratory CRDPs or with Edec CRDPs. It can be seen that the connectivity increases steadily across the four groups, as does the mean EPSP amplitude for each group. The summary value (the effective mean) shows the clearest relationship, 9.3, 25.2 or 31.5 μV for the three expiratory groups and 0.8 μV for the non-expiratory group. A very similar set of figures was also produced when the expiratory group was divided on the basis of the CRDP amplitude (rather than the ramp amplitude) (not shown).
Figure 10.
Summarized EPSP and CRDP parameters for IIN motoneurones EPSP amplitudes are grouped according to CRDP amplitude and displayed graphically (note the log scale). For definition of effective mean amplitude see text.
Variation in connectivity of individual EBSNs
From the data where individual EBSNs were tested with several motoneurones we looked for evidence of EBSNs being specialized, either for making direct connections at all, or for connecting with particular groups of motoneurones (e.g. some EBSNs connecting only to abdominal motoneurones, or some only to intercostal motoneurones). Out of 53 EBSNs tested overall, 19 gave no EPSPs. However, only two of these were tested with more than one expiratory IIN motoneurone (indeed, 10 of these were tested with only one motoneurone). Thus there is no evidence from these data for the existence of individual EBSNs that do not give connections to motoneurones. Moreover, for EBSNs tested with more than three motoneurones (6 with 4 motoneurones, 3 with 6 motoneurones, 2 each with 7 or 8 motoneurones and one each with 11 or 12 motoneurones) the distribution of connections to motoneurones of different groups by any individual EBSN appeared to represent random samples of the population as a whole. Examples included: (1) an EBSN which yielded one 66 μV EPSP from two IIn motoneurones, one 62 μV EPSP from two EI motoneurones and one 34 μV EPSP from two DR motoneurones; (2) an EBSN which yielded one 15.5 μV EPSP from three Dist motoneurones, three EPSPs (28.5, 44, 61 μV) from three EO motoneurones, two EPSPs (12, 17 μV) from three IIm motoneurones and one 16 μV EPSP from three DR motoneurones. There was therefore no evidence from the present data of individual EBSNs being strongly specialized for connections to particular groups of motoneurones.
Discussion
This study is the first to attempt a comprehensive survey of either the respiratory drive to motoneurones of a variety of thoracic muscles or the connections of expiratory bulbospinal neurones to a similar population of motoneurones. Both the respiratory drive and the connections were found to be widespread, with new observations being made with respect to both categories. These are discussed below separately, then the relationship between the respiratory drive and the connections is considered.
Occurrence and amplitudes of CRDPs
The results here differ from the original study of Sears (1964b), not only in the range of motoneurone identifications, but by being carried out under neuromuscular blockade with a stronger respiratory drive, stimulated by CO2. Nevertheless, the bulk of the recordings showed similar patterns of inspiratory or expiratory CRDPs, albeit with a larger range of amplitudes. One difference was that inspiratory CRDPs were seen here in 7/33 of the IIn motoneurones, whereas in Sears (1964b) none were found. We presume this is related to the stronger respiratory drive here (also see Kirkwood & Sears, 1978; Lipski & Martin-Body, 1987).
The presence of CRDPs in DR motoneurones is a new observation, although the extracellular recordings of Kirkwood et al. (1988) did include three DR motoneurones, two with inspiratory firing patterns and one with an Edec pattern, consistent with the 11/17 inspiratory and 4/17 Edec CRDPs here. Despite this earlier observation, which shows that at least some DR motoneurones must receive enough excitation to reach threshold, most of the DR CRDPs here were small. The functional significance of this respiratory drive is obscure. For the inspiratory examples, it could be speculated that such an input could aid the maintenance of the stiffness of the thorax during inspiration, though without knowing exactly which muscles these motoneurones innervated, such speculation cannot be very precise. Note that the DR group of motoneurones here, as in Kirkwood et al. (1988), did not include those innervating levator costae (see Fig. 1), which is well known as having an inspiratory function (Hilaire et al. 1983).
An alternative analogy for the DR CRDPs might be the recordings from hindlimb motoneurones in a similar preparation to the one described here, in which CRDPs were common (210/233) and showed a similar range of amplitudes (mean 1.32 ± 1.1 mV) (Kirkwood et al. 2002; Ford & Kirkwood, 2006). A possible difference between these earlier reports and the present results is that most (180/210) of the CRDPs in the hindlimb motoneurones were of the Edec type. However, in subsequent recordings in decerebrate animals a higher proportion of inspiratory CRDPs have been seen (Kirkwood et al. 2005). The presence of a respiratory input to these hindlimb motoneurones itself suggests that a respiratory input need not imply a respiratory function. Such an input could represent an entirely different function, but the input could appear as respiratory simply because this is the only motor activity present under anaesthesia or in a decerebrate animal. In awake animals the input might be related to locomotion, or emesis, or even reproductive behaviour (see discussions in Kirkwood & Ford, 2004; Ford & Kirkwood, 2006), or could simply represent coordination between respiration and other motor acts. The few Edec CRDPs in EI motoneurones may also represent a non-respiratory function. It is possible that these motoneurones innervated either serratus muscle or the most lateral part of EI muscle, where, for the latter, mechanical measurements show very little or no inspiratory action (De Troyer et al. 2005).
The respiratory discharges of intercostal motor units have a very characteristic distribution across the surface of the thorax, being stronger more dorsally for the interosseus intercostals or more medially for the parasternal muscles and with more inspiratory activity rostrally, but more expiratory activity caudally (reviewed in De Troyer et al. 2005). One might expect the present measurements to throw some light on this distribution. However, the measurements in more rostral segments here could not be distinguished from those in the more caudal segments (data not given), and the distributions of expiratory CRDPs did not differ between the various expiratory groups (Table 1). In particular no difference was seen between the Dist and IIm groups. Such a lack of difference could have arisen because the Dist group included motoneurones innervating transversus abdominis or triangularis sterni, in addition to the motoneurones innervating the distal intercostal muscle that might have been expected to show the smaller input. However, the lack of difference could also have arisen because the separation of two groups for the intercostal muscle itself was too coarse to represent the proximo-distal activity gradient seen in the discharges.
Moreover, it must also be remembered that the CRDPs need not directly represent the firing patterns, in particular for the expiratory motoneurones, where none of the CRDP excitatory ramps were larger than 6 mV. Indeed, we argue below that much of the depolarization in firing expiratory motoneurones could come from non-respiratory (tonic) sources, and it is therefore possible that the spatial pattern of activity is controlled by such tonic sources.
One other study (Merrill & Lipski, 1987) has reported quantitative data on CRDP amplitudes. It is worth comparing those data with the present results in order to aid discussion of differences in connectivity between the two studies. Like us, Merrill & Lipski used a strong CO2-stimulated drive, which gave CRDPs in EI motoneurones which were similar (or larger, dependent on the CO2 level) than here (present in 31/53 motoneurones for Merrill & Lipski, range 1–15 mV, mean 7.6 mV; present in 15/21 motoneurones here, range 1–14 mV, mean 5.5 mV). However, for the IIN motoneurones, although the CRDPs covered the same range in the two studies (1–10 versus 0.3–11 mV), the mean amplitude was larger here (mean 4.8 mV, cf. Merrill & Lipski, 3.7 mV).
The clearest difference between the two studies is in the occurrence of CRDPs for the IIN motoneurones, 85/89 (or 84/89 for amplitudes ≥ 1 mV) here versus 23/52 for Merrill & Lipski (highly significant, χ2, P < 0.001). This suggests that, despite a high CO2 in both studies and a strong inspiratory drive, the expiratory drive in the motoneurones of Merrill & Lipski (1987) may have been considerably lower than here. This difference might be even greater than the simple CRDP amplitudes indicate, since there is no indication in Merrill & Lipski (1987) of how much, in general, of their expiratory CRDPs could be accounted for by the inspiratory inhibitory component. Lipski & Martin-Body (1987) quoted an intermediate value of occurrence of CRDPs (116/166) for an intermediate level of CO2, but for segments mostly different from those here or from those in Merrill & Lipski (1987).
STA: reliability of the observations and considerations of synaptic noise
Our findings support the original observations of Kirkwood & Sears (1973) in that direct connections between EBSNs and expiratory motoneurones of the internal intercostal nerve were found to be both numerous and strong, but directly contradict the only previous extensive survey (Merrill & Lipski, 1987). The latter demonstrated only 2/57 EPSPs in IIN motoneurones (amplitudes 55 and 62 μV), compared to 47/107 EPSPs here (mean amplitude 39.2 μV). Because of this discrepancy it is particularly important to consider the reliability of the present data and its possible limitations. We have taken particular care to avoid both false negatives and false positives. The false negatives were avoided by averaging a large number of sweeps (> 957) and by using a baseline noise criterion. Could we have been too zealous in the application of these criteria and thus overestimated the proportion of positive observations?Merrill & Lipski (1987) did not use a noise criterion and did not quote a formal criterion for number of sweeps, except for their illustrated examples (≥ 2048). If the examples rejected by the noise criterion are included in our data, but restriction is made to at least 2048 sweeps, the connectivity becomes 45/101, with a mean amplitude of 38.4 μV, still leaving a large discrepancy between the two studies.
False positives here were minimized by the repeatability criterion and by taking care to discriminate between EPSPs and synchrony potentials. As in Davies et al. (1985a) and Kirkwood (1995), the success of the procedures was confirmed by observations that all the EPSPs showed latencies appropriate to the conduction times for the individual EBSNs. Moreover, the distribution of shape indices for the EPSPs (Fig. 6) was very similar to those reported for other monosynaptic connections (Jack et al. 1971; Scott & Mendell, 1976; Appenteng et al. 1978; Kirkwood & Sears, 1982). The elimination of seven trials as being ambiguous for the presence of synchrony potentials, like the noise criterion, might be thought of as possibly also reducing the number of negative observations. However, if anything we think the reverse is more likely, i.e. these waveforms are most likely to contain an EPSP in addition to a large synchrony component. This can be asserted not only because of their general appearance, but because 6/7 of these were recorded in expiratory IIN motoneurones with substantial CRDPs.
In the end, it should be remembered that spike-triggered averaging is a correlation technique and no such study can eliminate all false positives or false negatives. We think we have taken sufficient care to minimize these, but in any case, if any do remain, they cannot explain the discrepancy between this study and that of Merrill & Lipski (1987). Considerations of the criteria we used may give some clues as to possible reasons for the discrepancy. Merrill & Lipski did not feel the need to use any noise criterion, nor to discriminate between EPSPs and synchrony potentials. They did not state the level of noise in their averages, but it appears low in their illustrated examples, such that an EPSP of around 10 μV could readily be discriminated. Indeed they specifically pointed out that the majority of their averages were ‘actually very flat; a consistent observation …’. This could never be claimed for the majority of the averages in our study, which is precisely why both these criteria were needed. The overall level of synaptic noise therefore could well have been different in the two studies, perhaps analogous to the different states illustrated for inspiratory motoneurones by Kirkwood et al. (1982). One could therefore hypothesize that such a different state in the experiments of Merrill & Lipski (1987) involved suppression of the monosynaptic input from EBSNs. This would concomitantly have decreased not only the CRDP ramp amplitude and the synaptic noise but also the synchrony potentials, since the most likely source for these consists of other EBSNs synchronized with the EBSN under investigation (Davies et al. 1985a; Kirkwood, 1995). Note that the extent of EBSN synchronization may itself also be state dependent, cf. decerebrate versus anaesthetized cats (Feldman et al. 1980; Graham & Duffin, 1981).
Relationship between CRDPs and EPSPs
To our knowledge, this study comprises the first demonstration that within a population of motoneurones there can be a strong positive correlation between the strength of a natural, centrally derived input and the connectivity and/or EPSP amplitude from individual neurones likely to supply that drive. An important factor in these correlations could be that these neurones (the EBSNs) supply all or most of that drive. In fact this seems quite likely, as the following calculation shows. Consider the group of expiratory motoneurones here with a ramp amplitude ≥ 2.2 mV (Fig. 10). For these, the mean ramp amplitude was 3.5 mV. For a relatively small depolarization such as this and with the EPSPs presumably being measured at the soma, we do not have to worry about dendritic non-linearities and can safely use a linear integration to calculate the mean depolarization likely to arise from the identified connections, such as Tuck's model (see Sears, 1977; Kirkwood & Sears, 1991). In that model a depolarization of 10 mV was achieved with 100 μV EPSPs (half-width 5.8 ms) at a rate of about 14 000 s−1. Here, the connectivity and mean EPSP amplitude for this group of motoneurones were, respectively, 67% and 47.2 μV (Fig. 10). For the EPSPs here, the mean half-width was 2.3 ms and the EBSN firing rate was 79.6 impulses s−1. If 400 EBSNs in the contralateral medulla are assumed, together with additional connections (about 1/3 this strength) from the ipsilateral medulla (Kirkwood, 1995), then the expected depolarization would be about 10 mV × 400 × 4/3 × 79.6/14 000 × 0.67 × 0.472 × 2.3/5.8 = 3.8 mV. This is remarkably close to the observed mean amplitude of the ramp in this group (3.5 mV).
There are several assumptions here and factors not taken into account. The most important assumption is probably the number of EBSNs, and a missing factor is that the possible presence of a step component, in addition to the ramp, has not been taken into account. However, on the basis of known factors, it seems reasonable to conclude that the observed monosynaptic connections alone are sufficient to explain the observed expiratory excitation. Note that the depolarization predicted here is considerably less than the 10 mV predicted by Kirkwood (1995) from the connections measured by cross-correlation. The main difference between the two calculations is the use of a measured value for EPSP duration here rather than an assumed, longer value.
As for all previous descriptions of motoneurone inputs, the expiratory excitation shows a great variation between individual motoneurones. At least part of this variation must represent recruitment order by motoneurone size (Henneman et al. 1965) or motor unit type (for intercostals, see discussion in De Troyer et al. 2005). For single-fibre EPSPs this has a direct analogy with the connections from individual Ia afferents to hindlimb motoneurones, where both connectivity and EPSP amplitude were observed to be related to motoneurone axon conduction velocity and to motoneurone type (Harrison & Taylor, 1981; Fleshman et al. 1981), but note that such a relationship is not present for all motoneurone inputs, being absent for the input from spindle group II afferents (Munson et al. 1982). Moreover, both the connectivity from inspiratory bulbospinal inputs and the recruitment order of phrenic motoneurones have also been reported to be independent of the size effect (Monteau et al. 1985). A second source of the variation might be an organization of the input so as to generate the spatial distribution of expiratory intercostal motoneurone activity across the surface of the thorax, already mentioned with regard to the CRDPs. This mechanism is likely to be independent of the motoneurone size effect (De Troyer et al. 2005), but, as mentioned above, the spatial distribution could be generated by non-EBSN (e.g. tonic) inputs. The only hint of an effect from this study is the observation that the average size of EPSPs (but not the connectivity) appeared smaller for Dist expiratory motoneurones than for IIm motoneurones, but even for this effect, significance could not be confirmed.
We would like to suggest a third possibility, not related to the motoneurone, but to the state, i.e. that the EBSN EPSPs are subject to a strong state-dependent modulation, and that variation in such a state with time or with the particular preparation is an important additional multiplying factor in the observed covariation of EPSP size and CRDP amplitude. The main reason why this third explanation might be the most important is that there seems to be no other explanation for the discrepancy between the present study and that of Merrill & Lipski (1987). In previous publications from this laboratory (Kirkwood, 1995; Kirkwood et al. 1999) the view was taken that the discrepancy could be explained by the uneven distribution of EBSN terminals within a spinal segment. This view is no longer tenable: the connections here were equally as strong to motoneurones in the caudal as in the rostral region. Moreover, the uneven distribution of terminals can now be explained by an uneven distribution of motoneurones (Meehan et al. 2004). Nor can we now invoke either of the first two mechanisms above: there is no reason why Merrill & Lipski should have recorded only from motoneurones innervating an inactive thoracic region, nor could one assume that their recording technique would have selected only the very largest motoneurones with the very smallest inputs, which would imply experiments that were much lower-yielding than clearly was the case. If, however, the EBSN EPSPs were very much depressed in Lipski & Merrill's experiments as compared to ours, then the apparently lower synaptic noise, the lack of synchrony potentials, the smaller expiratory CRDPs and the absence of EPSPs would all be explained.
What could the hypothesized different state represent between our experiments and those of Merrill & Lipski? One possibility is that ours were conducted at a somewhat lighter level of barbiturate anaesthesia, which would imply that the EBSN EPSPs are particularly sensitive to this anaesthetic. There is no direct evidence for this. However, as explained in Kirkwood (1995), the standard preparation in this laboratory in recent years has been one specifically chosen, as here, to be relatively lightly anaesthetized, including keeping the preparation in this state during the preparatory period. It is a common experience among those working with thoracic respiratory discharges that the expiratory discharges are much more sensitive to barbiturate than are those of the inspiratory nerves. Most often, such behaviour would be ascribed to a greater involvement of interneurones on the more sensitive pathway. It would be ironic (and instructive) if in this case, where the bulbospinal inspiratory pathway is known to transmit largely via interneurones (Davies et al. 1985b; Merrill & Lipski, 1987; Vaughan & Kirkwood, 1997), the opposite were true. Perhaps the sensitivity here could represent a particularly potent physiological modulatory mechanism (see below).
Comparison with other studies and functional implications
Apart from the major discrepancy mentioned above, there is general agreement between the present results and cross-correlation studies already mentioned (Cohen et al. 1985; Kirkwood, 1995; Kirkwood & Road, 1995), and also with anatomical studies, which showed strong direct connections from nucleus retroambiguus neurones in general (of which the EBSNs are a major part) to motoneurones of semimembranosus, cutaneous trunci and external abdominal oblique at L1 and at T8 (VanderHorst et al. 1997, 2000; Boers et al. 2006).
What are the functional implications? We have argued above that the results presented here allow for all the expiratory excitation in the CRDPs to have arisen from the direct EBSN connections; does this mean that the discharges of expiratory motoneurones are also all evoked from this one direct input. Of course we do not yet know, but one piece of evidence suggests that this is not so. The cross-correlation measurements of Kirkwood (1995) were interpreted as representing an average EPSP amplitude of about 22 μV, which is absolutely identical to the effective mean amplitude for IIN expiratory motoneurones found here (22.0 μV, Table 2). This agreement might seem gratifying were it not for Fig. 10 and the consequent need to choose a strength of connection appropriate to the necessary level of excitation. The simplest way to do this would be to extrapolate the line in Fig. 9A to an appropriate level of depolarization for firing, e.g. 12 mV. This would give an EPSP size of around 125 μV and perhaps a connectivity of 100%. It should be noted that, even if somewhat arbitrary, some such extrapolation would be needed if we assume that of all the excitation is derived from EBSNs. On that assumption, the cross-correlation data are seen to differ from the EPSP data by a factor of about 5.7.
One possible source of error here is the translation of a cross-correlation peak to an EPSP amplitude. As in Kirkwood et al. (1999), we think that an error of a factor of 2 might be possible, but not one of 5.7. Moreover, given other published estimates of this translation (see Kirkwood & Sears, 1991; Kirkwood et al. 1999 for references), the errors would most likely be in the opposite direction. Thus it would seem that firing expiratory neurones most likely do not have all their depolarization derived from the direct EBSN input.
The simplest observation to support this conclusion is that the largest expiratory ramp was only 6 mV in amplitude, yet in these preparations a reasonable number of expiratory motoneurones are known to be firing (Kirkwood, 1995). One might argue that the lowest threshold (smallest) motoneurones were missed, because of a bias towards sampling larger ones. However, this did not seem to be the case for EI or inspiratory IIN motoneurones, where CRDPs up to 15 mV were found. Thus our recordings are likely to have included the smaller, lower threshold motoneurones. Note that we are making no classifications of the intracellularly recorded motoneurones on the basis of whether they were actually firing or not, nor on their absolute membrane potentials, since these are both too readily affected by leak currents related to the penetrations. The lack of large expiratory ramps among the observations here also suggests that the missing input is not likely to be a phasic expiratory one, but more likely therefore to be mainly tonic. Consistent with this, note that the thoracic respiratory interneurones identified by Kirkwood et al. (1993) as excitatory mostly had a pronounced tonic component in their discharges.
Another possible source of depolarization for firing motoneurones could be dendritic amplification via persistent inward currents (Heckman et al. 2005). In the awake animal, no doubt such currents must play a role, but the argument above concerning CRDP amplitudes militates against this possibility in these preparations. Further, although recordings in decerebrate animals have revealed plateau potentials in expiratory thoracic motoneurones, presumably evoked from such currents, they have not yet been found in the anaesthetized animal (Enríquez Denton et al. 2003), a condition where in any case they are expected to be suppressed (Hultborn, 1999). Finally, the unknown modulatory mechanism which we have suggested above to explain the apparent sensitivity to barbiturates, could also play a part.
Whichever way this is argued, the conclusion seems to be that mechanisms in addition to the direct connections from the EBSNs are required to produce expiratory discharges and their spatial patterns. Perhaps this is not surprising. Just as the connections to inspiratory motoneurones here seem inappropriate to respiration, many of the direct connections to EO motoneurones are inappropriate to one of the important non-respiratory roles of these motoneurones, the retching movements of vomiting (Kirkwood & Road, 1995). Thus one must expect other inputs or control mechanisms to be vital in producing the final motor output. Notwithstanding any of the above, however, there could still be some motor acts, such as vocalization, coughing and sneezing, in which the discharges of neurones of nucleus retroambiguus may be more tightly synchronized than in respiration, and where the EBSN discharges, acting only via the connections revealed in the present experiments, might be sufficient on their own to produce the required motoneurone discharge.
Finally, the results here comprise a quantitative set of measurements that can be used as control data for subsequent measurements of possible plasticity following lesions of the spinal cord (Anissimova et al. 2001). However, the suggestions of state dependence also emphasize the need to maintain the experimental conditions as constant as possible.
Acknowledgments
This work was supported by the International Spinal Research Trust and the Jeanne Anderson Fund (Institute of Neurology). S.A.S. and C.F.M. were MRC students. Dr R. Donga is thanked for his assistance in two experiments.
References
- Anissimova NP, Saywell SA, Ford TW, Kirkwood PA. XXXIV Congress of the International Union of Physiological Sciences. 2001. Distributions of EPSPs from individual expiratory bulbospinal neurones in the normal and the chronically lesioned thoracic spinal cord. Abstract 1029. [Google Scholar]
- Appenteng K, O'Donovan MJ, Somjen G, Stephens JA, Taylor A. The projection of jaw elevator muscle spindle afferents to fifth nerve motoneurones in the cat. J Physiol. 1978;279:409–432. doi: 10.1113/jphysiol.1978.sp012353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers J, Kirkwood P, De Weerd H, Holstege G. Ultrastructural evidence for direct excitatory retroambiguus projections to cutaneus trunci and abdominal muscle motoneurons in the cat. Brain Res Bull. 2006;68:249–256. doi: 10.1016/j.brainresbull.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Feldman JL, Sommer D. Caudal medullary expiratory neuron and internal intercostal nerve discharges in the cat: Effects of lung inflation. J Physiol. 1985;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JGMcF, Kirkwood PA, Sears TA. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985a;368:33–62. doi: 10.1113/jphysiol.1985.sp015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JGMcF, Kirkwood PA, Sears TA. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985b;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- Enríquez Denton M, Kirkwood PA, Wienecke J, Hultborn HR. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Do respiratory motoneurons need plateau potentials? Program No. 496.13.2003. [Google Scholar]
- Feldman JL, Sommer D, Cohen MI. Short time scale correlations between discharges of medullary respiratory neurons. J Neurophysiol. 1980;43:1284–1295. doi: 10.1152/jn.1980.43.5.1284. [DOI] [PubMed] [Google Scholar]
- Fleshman JW, Munson JB, Sypert GW. Homonymous projection of individual group Ia-fibers to physiologically characterized medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981;46:1339–1348. doi: 10.1152/jn.1981.46.6.1339. [DOI] [PubMed] [Google Scholar]
- Ford TW, Kirkwood PA. Respiratory drive in hindlimb motoneurones of the anaesthetized female cat. Brain Res Bull. 2006;70:450–456. doi: 10.1016/j.brainresbull.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Ford TW, McWilliam PN, Shepheard SL. A multibarrelled platinum-coated micro-elecrode. J Physiol. 1987;386:9P. [Google Scholar]
- Ford TW, Saywell SA, Kirkwood PA. Relationship between single-fibre EPSPs and respiratory drive potentials in expiratory motoneurones. J Physiol. 1999;518.P:69P. [Google Scholar]
- Ford TW, Vaughan CW, Kirkwood PA. Changes in the distribution of synaptic potentials from bulbospinal neurones following axotomy in cat thoracic spinal cord. J Physiol. 2000;524:163–178. doi: 10.1111/j.1469-7793.2000.t01-1-00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K, Duffin J. Cross correlation of medullary expiratory neurons in the cat. Exp Neurol. 1981;73:451–464. doi: 10.1016/0014-4886(81)90279-x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Taylor A. Individual excitatory post-synaptic potentials due to muscle spindle Ia afferents in cat triceps surae motoneurones. J Physiol. 1981;312:455–470. doi: 10.1113/jphysiol.1981.sp013638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hilaire GG, Nicholls JG, Sears TA. Central and proprioceptive influences on the activity of levator costae motoneurones in the cat. J Physiol. 1983;342:527–548. doi: 10.1113/jphysiol.1983.sp014867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Jack JJB, Miller S, Porter R, Redman SJ. The time course of minimal excitatory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971;215:353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA. Synaptic excitation in the thoracic spinal cord from expiratory bulbospinal neurons in the cat. J Physiol. 1995;484:201–225. doi: 10.1113/jphysiol.1995.sp020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P, Enríquez Denton M, Wienecke J, Nielsen JB, Hultborn H. Physiological roles for persistent inward currents in motoneurones: insights from the central respiratory drive. Biocybernet Biomed Eng. 2005;38:31–38. [Google Scholar]
- Kirkwood PA, Ford TW. Do respiratory neurons control female receptive behavior: a suggested role for a medullary central pattern generator? Prog Brain Res. 2004;143:105–114. doi: 10.1016/S0079-6123(03)43010-0. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Ford TW, Donga R, Saywell SA, Holstege G. Assessing the strengths of motoneuron inputs: Different anatomical and physiological approaches compared. Prog Brain Res. 1999;123:67–82. doi: 10.1016/s0079-6123(08)62845-9. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Lawton M, Ford TW. Plateau potentials in hindlimb motoneurones of female cats under anaesthesia. Exp Brain Res. 2002;146:399–403. doi: 10.1007/s00221-002-1163-0. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol. 1988;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Road JD. On the functional significance of long monosynaptic descending pathways to spinal motoneurones. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor Systems. London: Plenum Press; 1995. pp. 589–592. [Google Scholar]
- Kirkwood PA, Schmid K, Sears TA. Functional identities of thoracic respiratory interneurones in the cat. J Physiol. 1993;461:667–687. doi: 10.1113/jphysiol.1993.sp019535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973;234:87–89P. [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Cross-correlation analyses of motoneurone inputs in a coordinated motor act. In: Kruger J, editor. Neuronal Cooperativity. Berlin: Springer-Verlag; 1991. pp. 225–248. [Google Scholar]
- Kirkwood PA, Sears TA, Tuck DL, Westgaard RH. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Martin-Body RL. Morphological properties of respiratory intercostal motoneurons in cats as revealed by intracellular injection of horseradish peroxidase. J Comp Neurol. 1987;260:423–434. doi: 10.1002/cne.902600308. [DOI] [PubMed] [Google Scholar]
- Meehan CF, Ford TW, Road JD, Donga R, Saywell SA, Anissimova NP, Kirkwood PA. The rostro-caudal distribution of motoneurones and the variation in ventral horn area within a segment of the feline thoracic spinal cord. J Comp Neurol. 2004;472:281–291. doi: 10.1002/cne.20096. [DOI] [PubMed] [Google Scholar]
- Merrill EG. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970;24:11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary respiratory neurons in the cat. J Neurophysiol. 1987;57:1837–1853. doi: 10.1152/jn.1987.57.6.1837. [DOI] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Progr Neurobiol. 1991;37:83–141. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Monteau R, Khatib M, Hilaire G. Central determination of recruitment order: intracellular study of phrenic motoneurons. Neurosci Lett. 1985;56:341–346. doi: 10.1016/0304-3940(85)90266-6. [DOI] [PubMed] [Google Scholar]
- Munson JB, Sypert GW, Zengel JE, Lofton SA, Fleshman JW. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1982;48:1164–1174. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967;30:1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Ford TW, Kirkwood PA. J Physiol. 509.P. 1998. Morphology and projections of thoracic interneurones; p. 170P. [Google Scholar]
- Saywell SA, Ford TW, Kirkwood PA. Reinvestigation of connections from expiratory bulbospinal neurones to thoracic motoneurones. J Physiol. 1999;518.P:68P. doi: 10.1113/jphysiol.2006.122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JG, Mendell LM. Individual EPSPs produced by single triceps surae Ia afferents in homonymous and heteronymous motoneurons. J Neurophysiol. 1976;39:679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Sears TA. The fibre calibre spectra of sensory and motor fibres in the intercostal nerves of the cat. J Physiol. 1964a;172:150–161. doi: 10.1113/jphysiol.1964.sp007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA. The slow potentials of thoracic respiratory motoneurones and their relation to breathing. J Physiol. 1964b;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA. The respiratory motoneuron and apneusis. Fed Proc. 1977;36:2412–2420. [PubMed] [Google Scholar]
- Tani M, Kida MY, Akita K. Relationship between the arrangement of motoneuron pools in the ventral horn and ramification pattern of the spinal nerve innervating trunk muscles in the cat (Felis domestica) Exp Neurol. 1994;128:290–300. doi: 10.1006/exnr.1994.1139. [DOI] [PubMed] [Google Scholar]
- Taylor A. The contribution of the intercostal muscles to the effort of respiration in man. J Physiol. 1960;151:390–402. doi: 10.1113/jphysiol.1960.sp006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst VGJM, de Weerd H, Holstege G. Evidence for monosynaptic projections from the nucleus retroambiguus to hindlimb motoneurons in the cat. Neurosci Lett. 1997;224:33–36. doi: 10.1016/s0304-3940(97)13449-8. [DOI] [PubMed] [Google Scholar]
- VanderHorst VGJM, Terasawa E, Ralston HJ, 3rd, Holstege G. Monosynaptic projections from the nucleus retroambiguus to motoneurons supplying the abdominal wall, axial, hindlimb, and pelvic floor in the female rhesus monkey. J Comp Neurol. 2000;424:233–250. doi: 10.1002/1096-9861(20000821)424:2<233::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Kirkwood PA. Evidence from motoneurone synchronization for disynaptic pathways in the control of inspiratory motoneurones in the cat. J Physiol. 1997;503:673–691. doi: 10.1111/j.1469-7793.1997.673bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]