Abstract
Recent studies have indicated a critical role for STIM (stromal interacting molecule) proteins in the regulation of the store-operated mode of receptor-activated Ca2+ entry. Current models emphasize the role of STIM located in the endoplasmic reticulum membrane, where a Ca2+-binding EF-hand domain within the N-terminal of the protein lies within the lumen and is thought to represent the sensor for the depletion of intracellular Ca2+ stores. Dissociation of Ca2+ from this domain induces the aggregation of STIM to regions of the ER immediately adjacent to the plasma membrane where it acts to regulate the activity of store-operated Ca2+ channels. However, the possible effects of STIM on other modes of receptor-activated Ca2+ entry have not been examined. Here we show that STIM1 also regulates the arachidonic-acid-regulated Ca2+-selective (ARC) channels – receptor-activated Ca2+ entry channels whose activation is entirely independent of store depletion. Regulation of the ARC channels by STIM1 does not involve dissociation of Ca2+ from the EF-hand, or any translocation of STIM1. Instead, a critical role of STIM1 resident in the plasma membrane is indicated. Thus, exposure of intact cells to an antibody targeting the extracellular N-terminal domain of STIM1 inhibits ARC channel activity without significantly affecting the store-operated channels. A similar specific inhibition of the ARC channels is seen in cells expressing a STIM1 construct in which the N-linked glycosylation sites essential for the constitutive cell surface expression of STIM1, were mutated. We conclude that, in contrast to store-operated channels, regulation of ARC channels by STIM1 depends exclusively on the pool of STIM1 constitutively residing in the plasma membrane. These data demonstrate that STIM1 is a more universal regulator of Ca2+ entry pathways than previously thought, and appears to have multiple modes of action.
Despite the demonstrated importance of receptor-activated Ca2+ entry in non-excitable cells (Putney, 1986, 1990; Berridge, 1993; Bootman et al. 2002), much remains to be discovered about its nature and regulation. Such entry can take several different forms, but the most extensively studied is the so-called store-operated entry that is activated following depletion of intracellular Ca2+ stores (Putney, 1986, 1990; Parekh & Putney, 2005). Of the various conductances involved in this mode of entry, the most thoroughly characterized is the Ca2+-release-activated Ca2+ (CRAC) channel (Hoth & Penner, 1992, 1993; Zweifach & Lewis, 1993; Parekh & Putney, 2005). Recent studies, based on screening protocols designed to identify novel proteins involved in this mode of Ca2+ entry, have revealed that a protein named stromal interacting molecule 1 (STIM1) (Parker et al. 1996; Oritani & Kincade, 1996) plays a critical role in the activation of store-operated Ca2+ entry and the activity of the CRAC channels in several different cell types (Roos et al. 2005; Liou et al. 2005).
STIM1 was originally identified as an adhesion molecule in bone marrow stromal cells (Oritani & Kincade, 1996), and as being involved in the suppression of cell growth (Parker et al. 1996; Sabbioni et al. 1997). This protein possesses a single transmembrane spanning region, and is found in both the plasma membrane and the endoplasmic reticulum (ER) (Manji et al. 2000; Williams et al. 2002; Liou et al. 2005). Current models of the role of STIM1 in regulating Ca2+ entry focus on the protein residing in the ER membrane. Studies showed that the depletion of intracellular Ca2+ stores induces a marked change in the distribution of STIM1 in the ER, from a generally diffuse distribution to the formation of discrete clusters of the protein at sites either within (Zhang et al. 2005) or, as now seems more likely, immediately adjacent to the plasma membrane (Liou et al. 2005; Wu et al. 2006). More recently, it has been demonstrated that this translocation process seen on store depletion immediately precedes the activation of the CRAC channels (Wu et al. 2006), and that the clusters of STIM1 are spatially associated with regions of CRAC channel activity (Luik et al. 2006). Examination of the domain structure of STIM1 indicates an N-terminal region containing a putative Ca2+-binding EF-hand which, it is predicted, would lie within the lumen of the ER. Expression of a STIM1 construct in which this EF-hand had been mutated in a way designed to reduce its Ca2+ affinity results in a similar redistribution of the protein to clusters close to the plasma membrane in the absence of store depletion, and to a constitutively active entry of Ca2+ and CRAC channel activity (Liou et al. 2005; Zhang et al. 2005; Spassova et al. 2006). In addition, recent studies have reported that the cytosolic C-terminal of STIM1 alone was able to activate the CRAC channels, and can interact with, and activate, an expressed store-operated TRP channel, TRPC1 (Huang et al. 2006). Moreover, deletions of certain domains within this region blocked both the translocation of STIM1 to sites near the plasma membrane on store depletion (Baba et al. 2006), as well as the constitutive activation of store-operated Ca2+ entry induced by expression of the EF-hand mutant of STIM1 (Huang et al. 2006). Current models therefore propose that the luminal EF-hand of STIM1 in the ER acts as the sensor for depletion of these Ca2+ stores, signalling the translocation of STIM1 to sites close to the plasma membrane, where it acts to regulate the activity of the store-operated Ca2+ entry channels (Putney, 2005; Marchant, 2005; Luik et al. 2006).
Whilst the role of STIM1 in the regulation of store-operated Ca2+ entry has rapidly become well-established, the effects of STIM1 on other modes of receptor-activated Ca2+ entry, specifically those whose activation is independent of any depletion of intracellular Ca2+ stores, have not been examined. We therefore explored whether STIM1 might affect the arachidonic-acid-regulated Ca2+-selective (ARC) channels (Mignen & Shuttleworth, 2000; Mignen et al. 2001; Shuttleworth et al. 2004). These channels represent a well-characterized, and apparently widely expressed, mode of agonist-activated Ca2+ entry that has been shown to play a specific role in the modulation of oscillatory Ca2+ signals in various non-excitable cells (Mignen et al. 2001, 2005; Shuttleworth et al. 2004). Critically, the agonist-induced activation of these Ca2+ entry channels is entirely independent of the depletion of intracellular Ca2+ stores.
Methods
Cell preparation and transfection
HEK 293 cells stably transfected with the human m3 muscarinic receptor (m3-HEK cells) were cultured in Dulbecco's modified Eagle's medium (ATCC) supplemented with 10% newborn calf serum in a 5% CO2 incubator at 37°C. Cells were transfected using a Nucleofector (Amaxa) following the manufacturer's instructions, and were assayed by western blot or used in the various experimental protocols approximately 48 h (range 44–54 h) later.
Western blotting and antibodies
Proteins were resolved on 7% SDS-PAGE gels, transferred onto nitrocellulose, and analysed by standard western blotting techniques using the appropriate primary antibody, and goat antimouse HRP secondary antibodies (1:2000 dilution). Visualization of the labelled bands was by chemiluminescence (Western Lightning; Pierce, Rockford, IL, USA) and exposure to Biomax XAR film. Where appropriate, band densities were quantified from 12-bit images using Scion Image software. GOK/STIM1 mouse primary antibodies targeted to an N-terminal domain (BD Biosciences, Bedford, MA, USA) were used at 1:250 dilution. SERCA2 mouse antibodies (Calbiochem), and calreticulin mouse antibodies (BD Biosciences) were both used at a dilution of 1:2500, and a monoclonal β-actin antibody (Sigma) was used at a 1:5000 dilution.
Expression constructs and siRNA
siRNA duplexes targeting the AGGTGGAGGTGCAATATTA sequence human STIM1 (suggested by Stefan Feske, Harvard Medical School, USA) were obtained from Dharmacon. An AlexaFluor546-labelled siRNA duplex (target sequence –AATTCTCCGAACGTGTCACGT) was used as a control (Qiagen). Human STIM1 in pCMV6-XL5 and the vector backbone were purchased from Origene (Rockville, MD, USA). To generate a siRNA-resistant version of this construct, the siRNA recognition site was silently mutated (two bases changed from G-A and G-C) using QuikChange II (Stratagene). To generate the glycosylation mutant version of STIM1, the two asparagines at positions 131 and 171 in the siRNA-resistant STIM1 construct were mutated to glutamines using the QuikChange Multi kit (Stratagene) as directed by the protocol. Validity of the constructs was confirmed by sequencing. Human STIM1 bearing the D76A/E87A double mutation in the putative EF-hand in the pIRESneo vector was a gift from D. Gill (University of Maryland). For experimental purposes, unless otherwise indicated, all full-length constructs were transfected at a concentration of 0.5 μg (100 μl)−1, and siRNA was transfected at 225 pmol (100 μl)−1.
Whole-cell patch clamp
Patch-clamp recordings of macroscopic whole-cell currents were performed using an Axopatch-200B patch-clamp amplifier (Axon Instruments) essentially as previously described (Mignen & Shuttleworth, 2000; Mignen et al. 2003b). Whole-cell currents were recorded during alternating 250 ms voltage pulses to −80 mV and +60 mV delivered every 2 s from a holding potential of 0 mV. Current–voltage relationships were recorded using 150 ms voltage ramps from −100 to + 60 mV. Data were sampled at 20 kHz during the voltage steps and at 5.5 kHz during the voltage ramps, filtered online at 2 kHz, and digitally filtered offline at 1 kHz. The membrane capacitance of the cells selected for recording was 16.96 ± 0.03 pF (n = 287 cells). Initial current–voltage relationships obtained before activation of the currents were averaged and used for leak subtraction of subsequent current recordings. In the experiments involving cells expressing the EF-hand mutation of STIM1, the presence of a constitutively active CRAC channel current precluded this approach. In this case, the currents obtained after inhibition by La3+ (100 μm) were used for leak subtraction. The extracellular (bath) solution contained (mm) NaCl 140, MgCl2 1.2, CaCl2 10, CsCl 5, d-glucose 30, Hepes 10 (pH 7.4). Unless otherwise indicated, internal (pipette) solutions contained (mm) caesium acetate 140, NaCl 10, MgCl2 3.72, EGTA 10, CaCl2 3.5, Hepes 10 (pH 7.2). Calculated free [Ca2+] and [Mg2+] in this solution were 100 nm, and 3 mm, respectively. This concentration of free Mg2+ was designed to inhibit the activation of MIC/MagNum currents (Kozak et al. 2002; Prakriya & Lewis, 2002; Hermosura et al. 2002), and did not significantly affect the value of either the CRAC currents or the ARC currents. Monitoring at +60 mV provided an additional, highly sensitive, check for activation of these currents, and experiments were terminated on occasions when changes in the currents at this voltage were observed. Where necessary, the external (bath) solution was changed by perfusion of the patch-clamp chamber (approximately 1.5 ml min−1), and all experiments were carried out at room temperature (20–22°C). In experiments examining the effect of the STIM1 N-terminal and IgG2a antibodies, incubations were performed at room temperature for 30–40 min. Because the antibody preparations contained azide, control cells for these experiments were incubated under identical conditions in saline containing the same final concentration of azide (0.002%).
Intracellular Ca2+ measurements
Cells growing on coverslips were loaded with fura-2 by incubation with 2 μm fura-2/AM in Hepes-buffered physiological saline solution containing (mm) glucose 6, NaCl 132.5, MgSO4 1.2, KCl 4.8, KH2PO4 1.2, Hepes 15 (pH 7.4), CaCl2 1.3, for 20 min at 37°C. The loaded cells were then washed with saline and maintained at room temperature for an additional 20 min prior to use. [Ca2+] imaging was performed on an inverted epifluorescence microscope (Nikon 200) equipped with a 40× oil immersion objective lens (NA, 1.3). Alternate excitation of the cells with light at 340 and 380 nm was by a high-speed monochromator (TILL Polychrome IV), and fluorescence images at an emitted wavelength of 500 ± 45 nm were captured and digitized at 12-bit resolution using an interline progressive scan CCD camera (Sensicam QE). Imaging Workbench software, version 5.2 (Indec) controlled both the monochromator and image acquisition by the camera. Images were typically acquired every second with an exposure of 10 ms and stored immediately to hard disk. Background subtraction and calculation of the resulting 340/380 ratio images were performed offline. All experiments were performed at room temperature.
Biotinylation assay
Cell-surface STIM1 was detected using a biotinylation assay in intact cells (Pierce Pinpoint Kit) according to the manufacturer's instructions. In this assay, cell-surface proteins are labelled with a thio-cleavable sulfo-NHS-SS-biotin reagent, followed by lysis and isolation of the labelled proteins on an immobilized NeutrAvid gel column. Retained proteins are released by adding SDS-PAGE sample buffer containing 50 mm DTT and incubating at room temperature for 60 min. Both the flow-through fraction (non-biotinylated) and the subsequently eluted fraction (biotinylated) were analysed by western blotting using the STIM1 antibody. Following stripping, membranes were reprobed with the SERCA2 antibody, and again with the calreticulin antibody.
Statistical analysis
All data are reported as means ±s.e.m. When appropriate, means were compared by Student's t test. P values less than 0.05 were considered significant.
Results
Suppression of STIM1 protein levels reduces currents through both the CRAC channels and ARC channels in HEK 293 cells
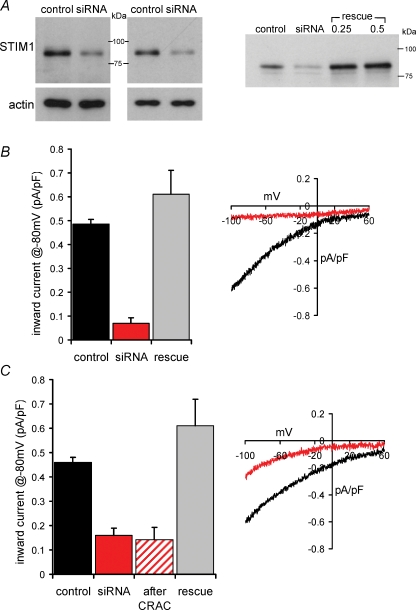
To explore the effects of STIM1 on both store-operated Ca2+ entry and ARC channel-dependent Ca2+ entry, siRNA against STIM1 was transfected into HEK 293 cells stably expressing the human muscarinic m3 receptor (m3-HEK cells). These cells have been extensively used to characterize the ARC channels, and have also been shown to possess a store-operated Ca2+ conductance that is similar in both its biophysical and pharmacological properties to the ‘classic’ CRAC conductance of Jurkat and RBL cells (Mignen & Shuttleworth, 2000; Mignen & Shuttleworth, 2001). Transfection of these cells with siRNA targeted to human STIM1 (225 pmol (100 μl)−1 for 44–50 h) reduced STIM1 protein expression, as confirmed by western blot (Fig. 1A), and indicated a population average reduction in STIM1 of 68 ± 2% (n = 6). To study the store-operated CRAC currents in these cells, they were subjected to whole-cell patch clamp with a Ca2+-free pipette solution containing the potent InsP3 receptor agonist adenophostin A (2 μm), a protocol that maximally activates these currents in these cells (Mignen et al. 2001). Under these conditions, macroscopic inward CRAC currents at −80 mV were reduced from a mean value of 0.49 ± 0.02 pA pF−1 (n = 6) in control-transfected cells, to 0.07 ± 0.02 pA pF−1 (n = 13) in the siRNA-transfected cells (Fig. 1B) – a reduction of more than 85% (P < 3.0 × 10−8). This is consistent with the previous studies using fluorescent measurements of Ca2+ entry in HEK 293 cells (Roos et al. 2005), and with direct measurements of macroscopic store-operated CRAC currents in S2 cells, Jurkat cells and RBL cells (Roos et al. 2005; Spassova et al. 2006), thereby confirming the suitability of the m3-HEK cell system. To examine the specificity of this effect, a STIM1 construct was generated in which the siRNA-binding region had been mutated to render it resistant to siRNA knock-down. The ability of this construct to rescue STIM1 protein levels in the siRNA-treated cells was confirmed by western blot (Fig. 1A). Correspondingly, expression of this construct in siRNA-transfected cells resulted in the complete rescue of CRAC channel currents to 0.61 ± 0.10 pA pF−1 (n = 8) at −80 mV, a value statistically indistinguishable from the controls (P = 0.145) (Fig. 1B).
Figure 1.
siRNA directed against STIM1 inhibits both CRAC channel activity and ARC channel activity in the same cells A, left, representative western blots showing the specific reduction in stromal interacting molecule 1 (STIM1) protein levels in siRNA-expressing cells. Gels were stripped and reprobed with β-actin as a loading control. Right, expression of an siRNA-resistant mutated STIM1 construct (rescue) restores STIM1 protein levels in siRNA-transfected cells. B, the effects of siRNA expression on Ca2+-release-activated Ca2+ (CRAC) channel activity. Mean ±s.e.m. inward current density at −80 mV, and representative current–voltage relationship in cells expressing control siRNA (black, n = 6) are compared with those in cells expressing the siRNA targeted to STIM1 (red, n = 13). Also shown is the effect on mean CRAC currents at −80 mV of expressing an siRNA-resistant construct in the STIM1 siRNA transfected cells (grey, n = 8). CRAC channel currents were activated by use of a Ca2+-free pipette solution containing adenophostin A (2 μm). C, the effects of siRNA expression on arachidonic-acid- regulated Ca2+-selective (ARC) channel activity. Details as in B (control siRNA, n = 6; STIM1 siRNA, n = 11; STIM1 siRNA-resistant, n = 6). Also shown (hatched, n = 6) is the effect on the mean inward ARC channel current density at −80 mV in the same individual siRNA-expressing cells in which reduced CRAC channel currents had been recorded. ARC channel currents were activated by bath addition of arachidonic acid (8 μm).
Macroscopic inward ARC channel currents in these siRNA-transfected cells were examined following activation by exogenous arachidonic acid (8 μm), as previously reported (Mignen & Shuttleworth, 2000). Under these conditions, mean inward ARC channel currents at −80 mV were reduced from a value 0.46 ± 0.02 pA pF−1 (n = 6) in control-transfected cells, to 0.16 ± 0.03 pA pF−1 in the STIM1 siRNA-transfected cells (n = 11) (Fig. 1C) – a 65% inhibition (P < 10−7), which is slightly less than that recorded for the CRAC channel currents. Once again, the specificity of these effects was confirmed by the rescue of ARC channel currents to a value of 0.61 ± 0.11 pA pF−1 (n = 6) at −80 mV, a value statistically indistinguishable from the controls (P = 0.124), by expression of the siRNA-resistant STIM1 construct in the siRNA-transfected cells. Because of the additive nature of the two Ca2+ entry currents in whole-cell patch clamp (Mignen & Shuttleworth, 2000), it was also possible to demonstrate the profound reduction of ARC channel currents (69% inhibition to a value of 0.14 ± 0.05 pA pF−1, n = 6, P < 2 × 10−4) in the same individual cells in which the CRAC currents were inhibited (Fig. 1C). This confirms that siRNA knock-down of STIM1 protein independently reduces both of these coexisting, but entirely distinct, Ca2+ entry pathways within the same cell.
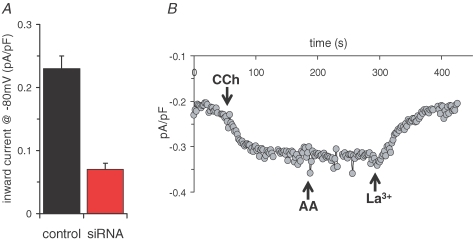
We next examined whether the STIM1 siRNA-induced inhibition of ARC channels was also seen following agonist stimulation. In previous studies, we have shown that activation of the m3-HEK cells with low concentrations (<1 μm) of the agonist carbachol specifically, and uniquely, activates the ARC channels (Mignen et al. 2001). Correspondingly, transfection of siRNA to STIM1 reduced the inward currents activated by 0.5 μm carbachol by approximately 70%, from a value of 0.23 ± 0.05 pA pF−1 (n = 9) at −80 mV, to 0.07 ± 0.01 pA pF−1 (n = 12 (P < 4.0 × 10−8) (Fig. 2A). Importantly, subsequent addition of 8 μm arachidonic acid to siRNA-treated cells after previous addition of carbachol failed to increase the observed current (mean increase in inward current at −80 mV, 0.03 ± 0.03 pA pF−1, n = 3) (Fig. 2B), confirming that this carbachol-activated current specifically reflects the activity of the ARC channels.
Figure 2.
siRNA directed against STIM1 inhibits agonist-activated ARC channel activity A, the effects of siRNA expression on mean inward currents activated by low (0.5 μm) carbachol concentrations. Mean ±s.e.m. carbachol-activated inward current density at −80 mV in cells expressing control siRNA (black, n = 9) are compared with those in cells expressing the siRNA targeted to STIM1 (red, n = 12). B, representative trace showing the activation of inward current at −80 mV following addition of 0.5 μm carbachol (CCh) to a siRNA-expressing cell. Subsequent addition of arachidonic acid (8 μm) failed to further increase the carbachol-activated current, which was fully blocked by La3+ (100 μm).
Overexpression of STIM1 increases currents through both the CRAC channels and ARC channels
In contrast to the effects of reducing STIM1 protein levels by siRNA, previous studies examining overexpression of STIM proteins have produced somewhat contradictory results ranging from little to no effect on store-operated Ca2+ entry in HEK 293 cells, Jurkat cells and S2 cells (Roos et al. 2005; Zhang et al. 2005), to an approximate doubling of the rate of store-operated Mn2+ quench of cytosolic fura-2 (used as a surrogate measurement for Ca2+ entry) in HeLa cells (Liou et al. 2005), and a three- to sixfold increase in CRAC channel currents in RBL cells and Jurkat cells, respectively (Spassova et al. 2006).
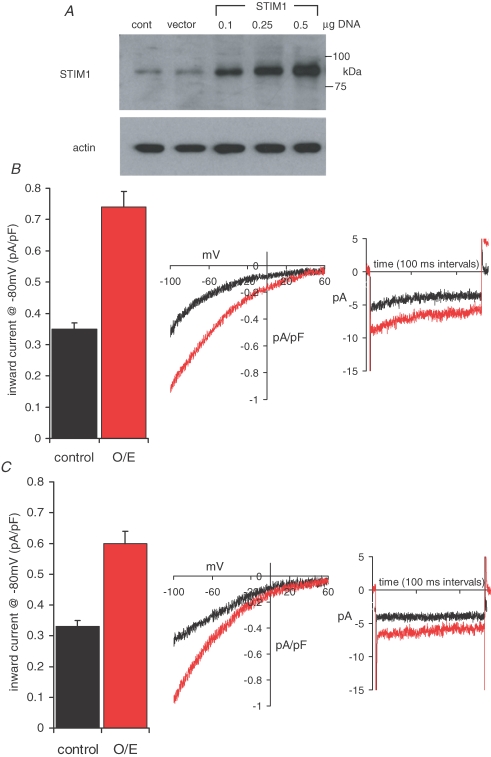
Western blot analysis of m3-HEK cells transfected with a STIM1 construct revealed a robust concentration-dependent increase in STIM1 protein expression (Fig. 3A). For subsequent whole-cell current experiments, cells were transfected with 0.5 μg STIM1 DNA. Parallel western blot analysis indicated that this gave a more than fivefold increase (5.5 ± 0.3, n = 3) in STIM1 protein levels. Measurements of the maximally activated store-operated CRAC currents in these STIM1 overexpressing cells indicated a more than twofold increase (Fig. 3B), from a control value of 0.35 ± 0.02 pA pF−1 (n = 19) at −80 mV, to 0.74 ± 0.05 pA pF−1 (n = 23, P < 2.0 × 10−8). These currents displayed marked inward rectification, reversal potentials of greater than +60 mV (Fig. 3B), and were completely inhibited by La3+ (100 μm). The presence of clear fast-inactivation during brief pulses to −80 mV (Fig. 3B) confirmed that these currents reflected the activity of the CRAC channels, as did their sensitivity to 2-APB (100 μm), which produced a 76 ± 1.8% (n = 9) inhibition of inward currents at −80 mV (data not shown).
Figure 3.
Overexpression of STIM1 increases both CRAC channel activity and ARC channel activity A, representative western blot showing STIM1 protein levels in cells transfected with increasing concentrations of a STIM1 construct. Gels were stripped and reprobed with β-actin as a loading control. B, the effects of STIM1 overexpression (0.5 μg DNA) on CRAC channel activity, shown as the mean ±s.e.m. inward current density at −80 mV, in cells transfected with a control vector (black; n = 19) compared with those in cells transfected with the STIM1 construct (red, n = 23). Also shown are representative traces showing the current–voltage relationship and fast inactivation at −80 mV in the CRAC channel currents in control cells (black) and cells overexpressing STIM1 (red). C, the effects of STIM1 overexpression on ARC channel activity. Details as in B (control vector, n = 13; STIM1 overexpression, n = 32). Note the characteristic absence of fast inactivation in the ARC channel currents.
Examination of the arachidonic-acid-activated macroscopic ARC channel currents showed that overexpression of STIM1 resulted in an approximate 80% increase in this current (Fig. 3C), from a value of 0.33 ± 0.02 pA pF−1 (n = 13) at −80 mV, to 0.60 ± 0.03 pA pF−1 (n = 32, P < 6.0 × 10−9). As previously described for control m3-HEK cells (Shuttleworth & Thompson, 1998), the application of arachidonic acid (8 μm) to cells overexpressing STIM1 failed to induce any detectible release of intracellular Ca2+ (data not shown), indicating that store-operated conductances make no contribution to these increased currents. This, together with their specific activation by arachidonic acid, and the absence of any significant fast-inactivation during brief pulses to −80 mV (Mignen & Shuttleworth, 2000) (Fig. 3C), indicates that the observed currents specifically reflect the activity of the ARC channels. Together these data demonstrate that, much like the store-operated CRAC channels, the activity of the arachidonic-acid-regulated ARC channels is profoundly influenced by the overall level of STIM1 expression in cells.
Expression of an EF-hand mutant STIM1 constitutively activates CRAC channel currents, but not ARC channel currents
As noted, a key feature of current models describing the action of STIM1 on store-operated Ca2+ entry and the activity of the CRAC channels is the translocation of STIM1 to sites close to the plasma membrane. Store depletion induces such a translocation, as does the expression of STIM1 proteins bearing mutations in the N-terminal EF-hand designed to interfere with its proposed ability to bind Ca2+ (Liou et al. 2005; Zhang et al. 2005; Wu et al. 2006). Expression of these mutants has been shown to result in the constitutive activation of Ca2+ entry (Liou et al. 2005; Zhang et al. 2005) and CRAC channel currents (Zhang et al. 2005; Spassova et al. 2006) in the absence of store depletion.
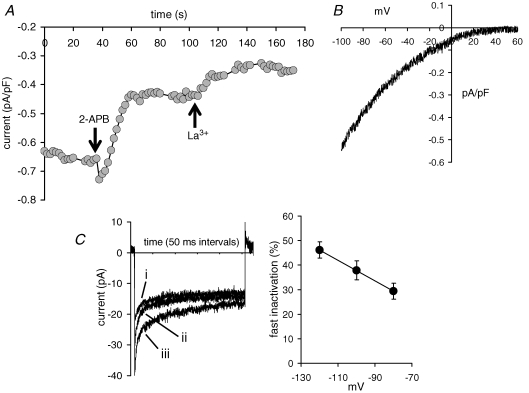
Based on our previous demonstration that activation of ARC channels is entirely independent of depletion of intracellular Ca2+ stores (Shuttleworth & Thompson, 1998; Mignen et al. 2003a, 2005), we predicted that expression of similarly mutated STIM1 would be unlikely to induce any constitutive activity of the ARC channels. To examine this, we used a STIM1 construct in which two sites in the EF-hand domain that are known to be critical for the binding of Ca2+ were mutated (D76A, and E87A) (Liou et al. 2005; Zhang et al. 2005). Expression of this mutant STIM1 in m3-HEK cells resulted in the appearance of a constitutively active, inwardly directed, La3+-sensitive current equal to 0.30 ± 0.05 pA pF−1 (n = 18) at −80 mV (Fig. 4A). This constitutive current displayed marked inward rectification, and reversal potentials greater than +60 mV (Fig. 4B), indicating it represented a specific (likely Ca2+-selective) current and not a non-specific leak. Importantly, these currents also showed clear fast-inactivation during brief pulses to negative potentials, the magnitude of which was dependent on the applied voltage (Fig. 4C) – a key feature of the endogenous store-operated CRAC channels. Moreover, addition of 2-APB (100 μm) resulted in a 76 ± 1.7% (n = 9) inhibition of the constitutive current – a value identical to that seen with the CRAC currents in cells overexpressing the wild-type STIM1 (P = 0.443, see above). Consistent with the published studies described above, these biophysical and pharmacological features confirm that the constitutively active currents observed in cells expressing the EF-hand mutation of STIM1 were exclusively comprised of current through the store-operated CRAC channels. Importantly, as we predicted above, these data demonstrate that there is no corresponding constitutive activation of the ARC channels in the cells expressing the EF-hand mutant of STIM1.
Figure 4.
Expression of an EF-hand mutant STIM1 induces the constitutive activation of a current that exclusively reflects CRAC channel activity A, representative trace from a cell expressing the EF-hand mutant STIM1 showing the constitutive activity of an inward current at −80 mV that is partially inhibited by 2-APB (100 μm) and fully inhibited by La3+ (100 μm). The cell was patched with the normal pipette solution (free Ca2+ concentration, 100 nm; see Methods). B, representative trace of the current–voltage relationship of the constitutively active La3+-sensitive current in a cell expressing the EF-hand mutant STIM1. C, the presence of fast inactivation, and its voltage dependence, in the constitutively active La3+-sensitive current in a cell expressing the EF-hand mutant STIM1. Shown are (left) representative recordings showing fast inactivation in the constitutively active current from an individual cell expressing the EF-hand mutant STIM1 recorded during brief (250 ms) pulses to −80 mV (i), −100 mV (ii), and −120 mV (iii), and (right) a plot of the percentage fast inactivation (mean ±s.e.m.) seen in the constitutively active La3+-sensitive current at different voltages in cells expressing the EF-hand mutant STIM1.
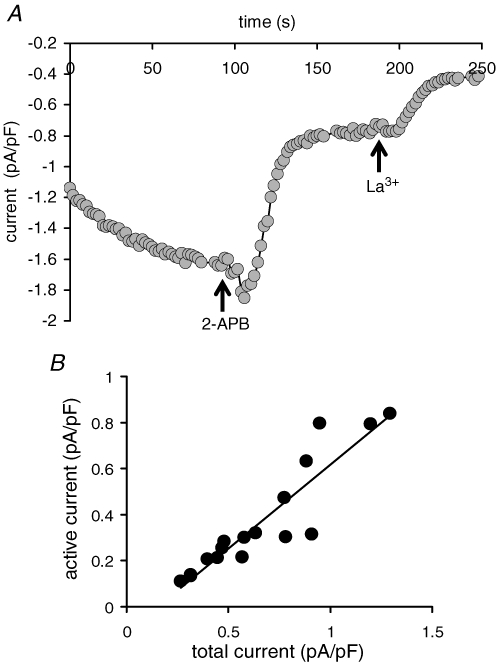
Maximal activation the CRAC channels in the same cells expressing the EF-hand mutant STIM1, using a Ca2+-free pipette solution containing 2 μm adenophostin, resulted in the activation of a total La3+-sensitive current (Fig. 5A) whose magnitude (0.66 ± 0.07 pA pF−1 at −80 mV, n = 17) was similar to that seen following the same maximal activation of CRAC currents in cells overexpressing the wild-type STIM1 (0.74 ± 0.05 pA pF−1, n = 23, see above). Addition of 2-APB (100 μm) resulted in a 75 ± 1.9% (n = 11) inhibition of this maximally activated CRAC current, a value that was statistically indistinguishable from that recorded for the constitutively active current in the same cells (P = 0.428). Further analysis showed that the constitutively active current accounted for 35–84% (mean value 53 ± 3%, n = 17) of the total CRAC current in the same cell, with the magnitude of the constitutively active component being approximately linearly related to the total current (Fig. 5B). We interpret these data as indicating that the total current reflects the combination of a constitutively active CRAC channel component whose magnitude depends on the variable expression of the EF-hand mutant, plus an approximately constant CRAC channel current that is dependent on the endogenous wild-type STIM1 protein and which is only activated upon store depletion.
Figure 5.
Relative contribution of the constitutively active CRAC channel current to total CRAC channel currents in cells expressing the EF-hand mutant STIM1 A, representative trace showing the full activation of CRAC channel currents at −80 mV, and the subsequent effects of 2-APB (100 μm) and La3+ (100 μm) in a cell expressing the EF-hand mutant STIM1. CRAC channels were maximally activated by use of a Ca2+-free pipette solution containing adenophostin (2 μm). B, relationship between the constitutively active current, and the total CRAC channel current measured at −80 mV, for individual cells expressing the EF-hand mutant STIM1.
ARC channel activity is uniquely, and specifically, dependent on STIM1 residing in the plasma membrane
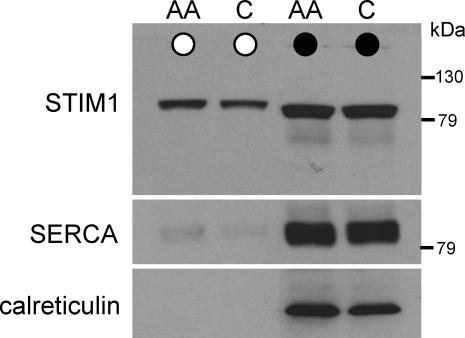
Despite the current emphasis on the importance of STIM1 in the membranes of the ER for the regulation of store-operated Ca2+ entry, STIM1 was originally characterized as a plasma membrane protein (Oritani & Kincade, 1996; Manji et al. 2000; Williams et al. 2002). We therefore sought to examine the presence of STIM1 in the plasma membrane of the m3-HEK cells. To do this, we used a protocol in which surface proteins were labelled by biotinylation of intact cells, followed by probing of the biotinylated fraction in western blots with STIM1-specific antibodies. Similar probing with antibodies directed against the widely expressed ER proteins SERCA2 and calreticulin served as controls. These experiments revealed that a proportion (approximately 10–15%) of the total endogenous cellular STIM1 could be biotinylated in intact m3-HEK cells under control conditions (Fig. 6), a value similar to that previously reported for the K562 myeloid leukaemia cell line (Manji et al. 2000). The validity of this biotinylation protocol was confirmed by the absence of any significant reaction to antibodies against the ubiquitously expressed ER proteins SERCA2, and calreticulin (Fig. 6). Addition of exogenous arachidonic acid (8 μm) to activate the ARC channels in these cells failed to significantly affect the proportion of STIM1 present in the biotinylated fraction, indicating that arachidonic acid does not appear to induce any increase in the incorporation of STIM1 in the plasma membrane (Fig. 6).
Figure 6.
STIM1 is expressed on the cell surface in the m3-HEK cells Representative western blot showing STIM1 in the biotinylated fraction (^), and whole cell lysate (•) in intact control cells (C) and in cells exposed to 8 μm arachidonic acid (AA). Lysate samples were diluted 4.5-fold compared with the biotinylated samples. The gel was stripped and reprobed with antibodies to SERCA2, and again to calreticulin, as controls for contamination with the endoplasmic reticulum.
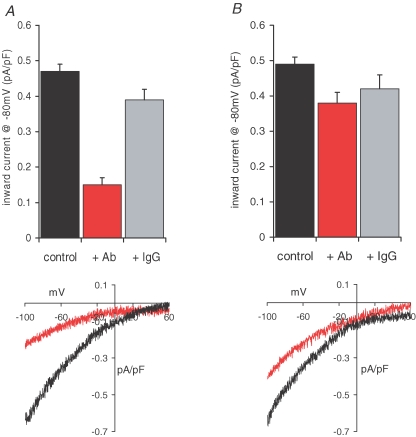
The finding of significant levels of STIM1 in the plasma membrane (or tightly associated with other plasma membrane proteins) even in unstimulated cells, obviously raises the possibility that it is this pool of STIM1 that is critical for the regulation of the activity of the ARC channels. In the plasma membrane, STIM1 will be orientated with the N-terminal domain of the protein exposed to the extracellular medium. Therefore, as an initial approach to explore the possible role of plasma membrane STIM1 in the regulation of Ca2+ entry pathways, we examined whether the binding of an antibody targeted to this extracellular N-terminal might affect the ability of STIM1 to modulate the currents underlying such entry, particularly those reflecting the activity of the ARC channels. Incubation of the cells with such an antibody (5 μg ml−1 for 30–40 min at room temperature) resulted in a 68% inhibition of the subsequently activated inward ARC channel currents (Fig. 7A), from a mean value of 0.47 ± 0.02 pA pF−1 (n = 11) at −80 mV in control cells, to 0.15 ± 0.02 pA pF−1 (n = 10) in the antibody-treated cells (P < 3.0 × 10−11). In contrast, exposure of cells to the same concentration (5 μg ml−1) of IgG2a antibodies resulted in only a 17% inhibition of the inward ARC channel current at −80 mV to a value of 0.39 ± 0.03 pA pF−1 (n = 7, P = 0.023) (Fig. 7A), thereby confirming the specificity of the observed effect. In contrast to the profound effect on ARC channel currents, maximally activated CRAC currents were only modestly affected by the N-terminal antibody (Fig. 7B), reducing inward currents at −80 mV from a mean value of 0.49 ± 0.02 pA pF−1 (n = 11) to 0.38 ± 0.03 pA pF−1 (n = 14) – an inhibition of only 22% (P < 0.001). Moreover, exposure of cells to the same concentration (5 μg ml−1) of control IgG2a antibodies reduced the inward CRAC channel current at −80 mV to a value of 0.42 ± 0.04 pA pF−1 (n = 5), a value that is not statistically different from that recorded with the N-terminal antibody (P = 0.219). Together, these data indicate that the effect of the N-terminal antibody on the CRAC channel currents is minimal, and unlikely to be specific.
Figure 7.
Antibodies targeted to the exposed extracellular N-terminal of plasma membrane STIM1 selectively inhibit ARC channel activity A, mean (±s.e.m.) inward current density at −80 mV, and representative current–voltage relationship in azide-control cells (black, n = 11) are compared with those in cells incubated with the N-terminal STIM1 antibody (5 μg ml−1 for 30–40 min at room temperature, red, n = 10). Also shown is the effect of an identical incubation with control IgG2a antibodies (grey, n = 7). B, the effects of the STIM1 antibody on CRAC channel activity, and a representative current–voltage relationship; details as in A; n = 11, 14 and 5, for azide-control cells, STIM1-antibody-treated cells, and control IgG2a-antibody-treated cells, respectively.
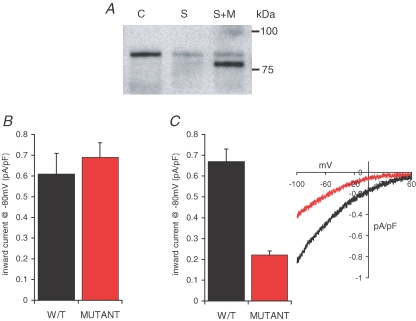
To further examine the possible involvement of STIM1 residing in the plasma membrane in the regulation of currents through the ARC channels, we sought a means to directly impair the expression of STIM1 in the plasma membrane. Previous studies have shown that such expression is critically dependent on a process of N-linked glycosylation at two sites (N131 and N171) localized in the extracellular region of the protein (Williams et al. 2002). We therefore generated a siRNA-resistant STIM1 construct (see above) in which both of these sites had been mutated to glutamines. Successful expression of this mutant construct in siRNA-transfected m3-HEK cells was demonstrated by western blot, where the siRNA-induced depleted level of the endogenous wild-type STIM1 can be seen as a faint band running above the expressed siRNA-resistant glycosylation mutant STIM1 (Fig. 8A). Examination of maximally activated CRAC currents in these cells indicated a mean value of 0.69 ± 0.07 pA pF−1 (n = 7) at −80 mV, a value that was statistically indistinguishable (P = 0.256) from that recorded in similar cells expressing the siRNA-resistant wild-type STIM1 (0.61 ± 0.10 pA pF−1, n = 8) (Fig. 8B). In marked contrast, expression of the siRNA-resistant glycosylation mutant resulted in an almost 70% reduction in measured ARC channel currents, declining from a mean value at −80 mV of 0.67 ± 0.06 (n = 6) in cells expressing the siRNA-resistant wild-type STIM1, to 0.22 ± 0.02 pA pF−1 (n = 11, P < 3.0 × 10−4) in cells expressing the siRNA-resistant glycosylation mutant STIM1 (Fig. 8C). This latter value was very similar to that seen in control siRNA-treated cells (0.16 ± 0.03 pA pF−1, n = 11, P = 0.051) (see Fig. 1C). These data indicate that the expression of STIM1 in the plasma membrane has a critical, and specific, impact on the activity of the ARC channels.
Figure 8.
Preventing constitutive STIM1 expression on the cell surface selectively inhibits ARC channel activity A, representative western blot showing the expression of the N-glycosylation mutant STIM1 protein levels in siRNA-transfected cells. Shown are STIM1 protein in control cells (C), in cells transfected with the STIM1 siRNA (S), and in siRNA-transfected cells expressing the siRNA-resistant N-glycosylation mutant STIM1 (S + M). Note that the glycosylation mutant runs at a lightly lower molecular mass, and that the siRNA-reduced endogenous STIM1 levels can be seen as a faint band in the same lane running at the normal molecular weight. B, the effects of the N-glycosylation mutant STIM1 on CRAC channel activity. Mean ±s.e.m. inward current density at −80 mV in siRNA-transfected cells expressing the siRNA-resistant wild-type STIM1 (black, n = 8) are compared with those in siRNA-transfected cells expressing the siRNA-resistant N-glycosylation mutant STIM1 (red, n = 7). C, the effects of the N-glycosylation mutant STIM1 on ARC channel activity. Mean ±s.e.m. inward current density at −80 mV (n = 6), and representative current–voltage relationship in siRNA-transfected cells expressing the siRNA-resistant wild-type STIM1 (black) are compared with those in siRNA-transfected cells expressing the siRNA-resistant N-glycosylation mutant STIM1 (red, n = 11).
Discussion
In this study, we have demonstrated that the overall levels of cellular STIM1 protein influence the magnitude of both the store-operated CRAC channels and the arachidonic-acid-activated ARC channels in m3-HEK cells in an essentially similar manner. However, these two conductances are pharmacologically and biophysically distinct, and have fundamentally different modes of activation – with the activation of ARC channels being entirely independent of the depletion of intracellular Ca2+ stores (Mignen & Shuttleworth, 2000). In the present context, this difference is clearly reflected in the unique and specific ability of mutations to the EF-hand of STIM1 to induce the constitutive activation of the CRAC channels, whilst no such effect was seen for the ARC channels. Consequently, and consistent with our previous demonstration that activation of ARC channels is entirely independent of depletion of intracellular Ca2+ stores (Shuttleworth & Thompson, 1998; Mignen et al. 2003a, 2005), we conclude that the translocation of STIM1 in the ER to sites close to the plasma membrane, which is clearly critical for the activation of the CRAC channels, appears to play no role in the regulation of ARC channel activity.
How, then, can the shared effect of STIM1 on the store-operated CRAC channels and the store-independent ARC channels be explained? A possible clue may lie in the fact that, although overall changes in STIM1 expression levels (by siRNA or overexpression) influence the magnitude of the currents through both the CRAC channels and the ARC channels, such effects were significantly more apparent with the CRAC channels. In contrast, in experiments designed to examine the specific effect of STIM1 residing in the plasma membrane, effects on the currents through the ARC channels were clear and profound, whilst those on the CRAC channel currents were marginal at best. Moreover, this feature was independently demonstrated using two entirely distinct approaches. The first involved exposure of intact cells to an antibody targeted to an N-terminal epitope that, for STIM1 molecules located in the plasma membrane, would be exposed to the extracellular environment. This resulted in a marked inhibition of ARC channel currents, whilst the same antibody had only a small effect on the CRAC channel currents that was not significantly different from that observed with a control antibody. It should be noted that the same N-terminal antibody was reported to induce a much larger (∼70%) inhibition of CRAC currents in Jurkat cells (Spassova et al. 2006). However, comparisons are difficult as different incubation conditions and higher antibody concentrations were used (incubation at 4°C and antibody concentrations of 20 μg ml−1), and only the monovalent currents observed through the CRAC channels in the absence of external divalent cations were reported.
The second approach involved expression of a mutant STIM1 designed to prevent its constitutive delivery to the plasma membrane. Unfortunately, attempts to directly confirm that the transfected glycosylation mutant fails to be expressed in the plasma membrane using the biotinylation protocol described above proved to be impossible. In these experiments, the presence of a significant reaction to the SERCA2 antibody in the biotinylated fraction of the transfected cells (data not shown) indicated the presence of a small proportion of damaged cells in this population, making any subsequent interpretation of the data obtained unreliable. Nevertheless, expression of this mutant STIM1 resulted in the profound inhibition of currents through the ARC channels, without affecting those through the CRAC channels. Thus, despite inevitable limitations in the protocols used, these two independent approaches produced essentially identical, selective effects on the currents through the ARC channels, whilst having negligible effects on the CRAC conductance.
Together, these findings lead us to two important conclusions. First, the fact that protocols designed to disrupt the action, or eliminate the presence, of STIM1 in the plasma membrane had little or no effect on the activity of the CRAC channels is consistent with those reports demonstrating that the regulation of these, and other, store-operated conductances depend exclusively on STIM1 acting within the ER membrane (Liou et al. 2005; Wu et al. 2006; Luik et al. 2006; Mercer et al. 2006; Baba et al. 2006), and argue against any requirement for STIM1 to become actually inserted into the plasma membrane (Zhang et al. 2005; Spassova et al. 2006). Secondly, and in marked contrast to the STIM1-dependent regulation of the store-operated conductances, the regulation of ARC channel activity appears to exclusively involve the fraction of STIM1 that is constitutively resident in the plasma membrane. Based on this, the simplest models for this action of STIM1 would seem to be either that the plasma membrane STIM1 itself is the target for agonist-generated arachidonic acid, or that STIM1 influences the ability of arachidonic acid to activate the ARC channels. Examination of these two alternatives will obviously be the subject of future studies.
Importantly, these data demonstrate that the effects of STIM1 on Ca2+ entry are not limited to CRAC channels or, indeed, to any Ca2+ entry pathway activated by store-depletion. It could be that ARC and CRAC channels, despite their obvious differences, are ancestrally related proteins that have retained the common feature of regulation by STIM1. However, if so, then such regulation clearly involves entirely distinct mechanisms for the two channel types. In the case of ARC channels, such regulation does not involve the depletion of intracellular Ca2+ stores or any consequence of such depletion, and is independent of any translocation of STIM1 to the sites near the plasma membrane. Instead, the regulation of the ARC channel involves an action of the pool of STIM1 constitutively residing in the plasma membrane. Whilst these findings serve to emphasize the role of STIM1 as a key regulator of Ca2+ entry pathways in non-excitable cells, they demonstrate that such a role involves multiple distinct mechanisms that are capable of acting on diverse Ca2+ entry channels.
Acknowledgments
We thank S. Feske (Harvard Medical School, USA) for generous advice on the siRNA construct, D. Gill (University of Maryland, MD, USA) for the gift of the EF-hand mutant expression plasmid, and Pauline Leakey for excellent technical assistance. This work was supported by a grant from the National Institutes of Health (GM040457) to T.J.S. In addition, O.M. was supported in part by funds from the Alfred and Eleanor Wedd Endowment.
References
- Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kursaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: more messengers, more channels, more complexity. Curr Biol. 2002;12:R563–R565. doi: 10.1016/s0960-9822(02)01055-2. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji SS, Parker NJ, Williams RT, Van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- Marchant JS. Cellular signalling: STIMulating calcium entry. Curr Biol. 2005;15:R493–R495. doi: 10.1016/j.cub.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Shuttleworth TJ. IARC, a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- Mignen O, Shuttleworth TJ. Permeation of monovalent cations through the non-capacitative arachidonate-regulated Ca2+ channels in HEK293 cells. Comparison with endogenous store-operated channels. J Biol Chem. 2001;276:21365–21374. doi: 10.1074/jbc.M102311200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J Biol Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels. J Biol Chem. 2003a;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J Biol Chem. 2003b;278:40088–40096. doi: 10.1074/jbc.M306365200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Yule DI, Shuttleworth TJ. Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells. J Physiol. 2005;564:791–801. doi: 10.1113/jphysiol.2005.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Parker NJ, Begley CG, Smith PJ, Fox RM. Molecular cloning of a novel human gene (D11S4896E) at chromosomal region 11p15.5. Genomics. 1996;37:253–256. doi: 10.1006/geno.1996.0553. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: a gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res. 1997;57:4493–4497. [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Muscarinic receptor activation of arachidonate-mediated Ca2+ entry in HEK293 cells is independent of phospholipase C. J Biol Chem. 1998;273:32636–32643. doi: 10.1074/jbc.273.49.32636. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Senior PV, Van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim Biophys Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SLYuY, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]