Abstract
The homomeric acid-sensing ion channel 1a (ASIC1a) is a H+-activated ion channel with important physiological functions and pathophysiological impact in the central nervous system. Here we show that homomeric ASIC1a is distinguished from other ASICs by a reduced response to successive acid stimulations. Such a reduced response is called tachyphylaxis. We show that tachyphylaxis depends on H+ permeating through ASIC1a, that tachyphylaxis is attenuated by extracellular Ca2+, and that tachyphylaxis is probably linked to Ca2+ permeability of ASIC1a. Moreover, we provide evidence that tachyphylaxis is probably due to a long-lived inactive state of ASIC1a. A deeper understanding of ASIC1a tachyphylaxis may lead to pharmacological control of ASIC1a activity that could be of potential benefit for the treatment of stroke.
Acid-sensing ion channels (ASICs) are Na+ channels gated by extracellular protons. Four genes, asic1–asic4, code for ASIC subunits in mammals (Kellenberger & Schild, 2002). These subunits assemble into homo- and hetero-oligomeric channels in vivo (Baron et al. 2002; Benson et al. 2002; Xie et al. 2002; Askwith et al. 2004; Vukicevic & Kellenberger, 2004; Wu et al. 2004); the number of subunits per channel is probably four (Coscoy et al. 1998; Firsov et al. 1998).
ASIC1a is an ASIC subunit that seems to have important functions for the physiology and pathophysiology of the central nervous system. It contributes to the most abundant ASICs in the brain: homomeric ASIC1a and heteromeric ASIC1a/2a (Baron et al. 2002; Askwith et al. 2004; Vukicevic & Kellenberger, 2004) and knockout of the asic1 gene leads to deficits in spatial memory and learned fear (Wemmie et al. 2002; Wemmie et al. 2003), suggesting a contribution to higher brain functions. Moreover, homomeric ASIC1a seems to be the only ASIC which is permeable for Ca2+ (Waldmann et al. 1997b; Bässler et al. 2001; Yermolaieva et al. 2004). Recently, it has been shown that activation of ASIC1a channels during brain ischaemia leads to Ca2+ influx in neurons and contributes to neuronal death associated with ischaemia (Xiong et al. 2004). This result suggests that the control of ASIC1a activity may be of clinical relevance in the treatment of stroke.
In an early characterization of ASIC currents in mesencephalic neurons and oligodendrocytes, it was noted that the currents showed a run-down phenomenon (Sontheimer et al. 1989). More recently it was noted that ASIC1a channels endogenously expressed in a skeletal muscle cell line (Gitterman et al. 2005) or HEK293 cells (Neaga et al. 2005) or heterologously expressed in Xenopus oocytes (Paukert et al. 2004b) showed tachyphylaxis, a decreased response to successive applications of the ligand. In the present study, we show that tachyphylaxis is specific for homomeric ASIC1a and propose a model in which H+, permeating through the channels, contribute to the induction of a conformational change of the pore that leads to a long-lived inactive state. A detailed understanding of this mechanism could be useful for a pharmacological control of ASIC1a activity.
Methods
Electrophysiology
cDNAs for rat ASIC1a, ASIC1b, and chimeras have been described previously (Bässler et al. 2001; Babini et al. 2002). The cDNAs for rat ASIC2a and ASIC3 were cloned by PCR from rat brain and dorsal root ganglia, respectively, using the Expand High Fidelity PCR system (Roche). Primers had been deduced from the published sequences (Waldmann et al. 1996, 1997a) and were: ASIC2a5′, 5′-CCGCTCGAGCACAGGGTCCCGATGGACC-3′; ASIC2a-3′, 5′-GGGGTACCTCAGCAGGCAATCTCCTCCAG-3′; ASIC3-5′, 5′-CCGCTCGAGTCCCTGGTCCAGCCATGAAAC-3′; and ASIC3-3′, 5′-GGCCTGCAGCTAGAGCCTTGTGACGAGGTAAC-3′. Using terminal restriction sites (ASIC2a, XhoI/KpnI and ASIC3, XhoI/PstI), the PCR products were ligated in the vector pRSSP6009 (Bässler et al. 2001) and entirely sequenced.
Oocytes were surgically removed under anaesthesia from adult Xenopus laevis females. Anaesthetized frogs were killed after the final oocyte collection by decapitation. Animal care and experiments followed approved institutional guidelines at the Universities of Tübingen and Würzburg.
Oocytes were isolated by digestion with collagenase type II (Sigma, 1 mg ml−1 for 60–120 min). Synthesis of cRNA, maintenance of Xenopus laevis oocytes and recordings of whole-cell currents were done as previously described (Chen et al. 2006). For expression of homomeric ASICs, we injected 0.01–0.1 ng of ASIC1a cRNA or 1–10 ng of ASIC1b, ASIC2a or ASIC3 cRNAs. For co-expression of subunits, we injected equal amounts of cRNAs of the two individual subunits, absolute amounts being 0.1 ng (ASIC1a/3) and 0.01 ng (ASIC1a/2a), respectively. Since ASIC1a cRNA leads to much larger current amplitudes than an equal amount of ASIC3 cRNA, we inhibited, in co-expression experiments of ASIC1a and 3, homomeric ASIC1a, which could possibly contaminate heteromeric ASIC1a/3, by incubation of oocytes with 40 nm of the specific ASIC1a-inhibitor psalmotoxin 1 (PcTx1) (Escoubas et al. 2000). Solutions with a low concentration of or without Ca2+ were supplemented with 0.1 mm flufenamic acid or niflumic acid to block the large conductance induced in Xenopus oocytes by low concentrations of divalent cations. Intracellular acidification of oocytes was achieved by replacing 90 mm NaCl with 90 mm NaHCO3; the pH was adjusted to 7.3 by titration with HCl. NaHCO3-containing solutions were freshly prepared prior to each experiment. If not specified differently, the membrane potential was clamped to –70 mV.
Outside-out patch-clamp measurements
Outside-out patches were established as previously described (Paukert et al. 2004a). Following outside-out patch formation, the patch pipette was placed in front of a piezo-driven double-barrelled application pipette enabling fast solution exchange (Bässler et al. 2001). Gravity-driven control and test solution flowing out of the application pipette contained (mm): 140 NaCl, 1.8 CaCl2, 1.0 MgCl2, 10 Hepes, pH 7.4 (control solution) or 140 NaCl, 0.1 CaCl2, 1.0 MgCl2, 10 Mes, pH 5 (test solution). Patches were clamped to –70 mV. Data were acquired using an Axopatch 200B amplifier (Axon Instruments), filtered with the built-in Bessel filter at 2 kHz, digitized at 10 kHz, and stored on hard disk. Data acquisition and control of the application pipette were managed using the open-access software Scanclamp (provided by K. Löffler; http://www.scanclamp.de). All experiments were conducted at room temperature.
Determination of surface expression
We determined surface expression of ASIC1a by the method of Zerangue et al. (1999). The haemagglutinin (HA) epitope (YPYDVPDYA) of influenza virus was inserted in the extracellular loop of ASIC1a between residues F147 and K148.
Oocytes were manually dissected from an isolated portion of an ovary and, on the following day, injected with 1 ng of cRNA. The second day, they were treated with collagenase type II (0.33 mg ml−1 for 60 min) and in the morning of the third day the follicular layer was removed. Oocytes were then stimulated six times for 10 s with either pH 4.0 or, as a control, pH 7.4; the interval between stimulations was 60 s. Oocytes were transferred on ice and kept there for the whole procedure of antibody binding. They were placed for 30 min in ND96 (In mM: 96 NaCl, 2 KCL, 1.8 CaCl2, 2 MgCl2, 5 Hepes; pH 7.4. For pH 4, Hepes was replaced by Mes) with 1% BSA to block unspecific binding, incubated for 60 min with 0.5 μg ml−1 of rat monoclonal anti-HA antibody (3F10, Roche), washed extensively with ND96–1% BSA, and incubated for 90 min with 2 μg ml−1 of horseradish peroxidase-coupled secondary antibody (goat anti-rat Fab fragments, Jackson ImmunoResearch). Oocytes were washed six times with ND96–1% BSA and three times with ND96 without BSA. They were placed individually in wells of a microplate and luminescence was quantified in a Berthold Orion II luminometer (Berthold detection systems; Pforzheim, Germany). The chemiluminescent substrates (50 μl Power Signal Elisa; Pierce) were automatically added and luminescence measured after 2 s for 5 s. Relative light units (RLUs) per second were calculated as a measure of surface expressed channels. RLUs of HA-tagged channels were more than 1000-fold higher than RLUs of untagged channels. Results are from two independent experiments, with oocytes from two different frogs; at least seven oocytes were analysed for each experiment and each condition.
The whole time that elapsed between stimulation of channels and measurement of their surface expression was about 6 h; oocytes were kept on ice for the whole period. In order to exclude that changes in surface expression were reversed during this period, we stimulated oocytes in the same way as for determination of surface expression, kept them on ice for 6 h and measured current amplitudes at pH 5. Results for the current amplitudes are from oocytes of two frogs, at least six oocytes for each experiment and each condition.
Data analysis
Whole-cell data were analysed with the software IgorPro (Wavemetrics, Lake Oswego, OR, USA). The use dependence of ASIC1a tachyphylaxis was analysed with a mono-exponential fit. Before fitting, currents from each measurement were normalized to the current amplitude of the first acid application.
Single-channel recordings were analysed with the software Ana (provided by M. Pusch; http://www.ge.cnr.it/ICB/conti-moran-pusch/programs-pusch/softwaremik.htm). Single channel events, which were visible at the end of the open phase, were analysed with an amplitude histogram. The amplitude distribution was fitted to a sum of Gaussian functions, from which single channel amplitudes were derived. This procedure was done for each trace independently. Values from different traces were then pooled and contributed to the mean single channel amplitude.
The permeability ratio PH/PNa=P′ was calculated from the change in reversal potential when the pH was raised from pH 4 to pH 6, using the following equation derived from the Goldmann-Hodgkin-Katz equations:
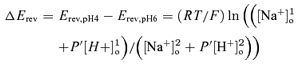 |
where R, T and F have their usual meanings. [Na+]2o=[Na+]1o= 10−3m, [H+]1o= 10−4m, [H+]2o= 10−6m. ΔErev was estimated to be more than 10 mV. We considered the effect of Mg2+ and N-methyl-D-glucamine (NMDG) in the extracellular solution negligible. The intracellular K+ concentration was unknown, but equal under both conditions and therefore did not affect the change in reversal potential ΔErev.
Results are reported as means ±s.e.m. They represent the mean of n individual measurements on different oocytes or different patches. Statistical analysis was done with either Student's t test or one-way analysis of variance (ANOVA).
Results
The currents of homomeric ASIC1a exhibit tachyphylaxis in Xenopus oocytes
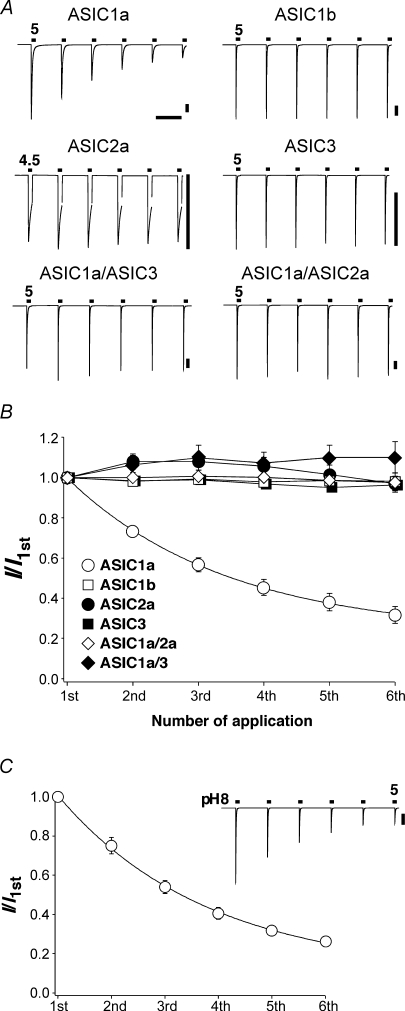
We expressed different ASIC channels in Xenopus oocytes. We repeatedly activated the channels by applying an acidic solution (pH 4.5–5.0) for 10 s every 70 s. Figure 1 shows that all ASIC channels desensitized during the 10 s acidic pulse; ASIC2a desensitized incompletely. We will call this desensitization, which occurs during a single application of the ligand, acute desensitization. After the 60 s interval, acid-induced currents of homomeric ASIC1b, -2a and -3, had fully recovered and were of similar amplitudes as for the preceding stimulus. In contrast, ASIC1a currents did not fully recover and the current amplitude of ASIC1a showed a cumulative reduction with repeated stimulation. Increasing the time interval between successive stimulations did not increase the fraction of current that had recovered and recovery from acute desensitization of ASIC1a is indeed complete in less than 1 min (Babini et al. 2002). Moreover, as shown below, the reduction in current amplitude was not caused by a decreased driving force due to intracellular accumulation of Na+. It did also not correlate with the amplitude of ASIC1a currents: currents with small amplitudes were reduced like currents with large amplitudes. This is also illustrated in Fig. 1A where currents of ASIC1b and heteromeric ASICs were of similar amplitude as ASIC1a currents, yet did not show a decreased response with repeated stimulations. Finally, changing the conditioning pH from pH 7.4–8.0 did not prevent the decrease in the current amplitude (Fig. 1C), demonstrating that this decrease is not due to a shift of the steady-state desensitization curve to higher pH values. Similarly, as shown below, a reduction in the pH sensitivity also cannot account for the reduction in the current amplitude. Hence, ASIC1a responds with a unique decrease to repeated stimulations; such a decreased response is called tachyphylaxis.
Figure 1.
Currents of homomeric ASIC1a exhibit tachyphylaxis A, representative examples of currents from homomeric ASIC1a, -1b, -2a and –3, and from heteromeric –1a/3 and –1a/2a. ASICs were repeatedly activated by application of pH 5 (pH 4.5 for ASIC2a) for 10 s. Channels were allowed to recover in conditioning pH 7.4 for 60 s. The measurements with ASIC1a/ASIC3 were done in the presence of 40 nm PcTx1 (see Methods). Bars correspond to 60 s and 2 μA, respectively. B, current amplitudes were normalized to the first amplitude. The depression of the ASIC1a current amplitude could be well fitted with a single exponential function (continuous line). ASIC1a, n = 10; ASIC1b, n = 6; ASIC2a, n = 7; ASIC3, n = 7; ASIC1a/3, n = 7; ASIC1a/2a, n = 9. C, ASIC1a was repeatedly activated as in A, but the conditioning pH was pH 8.0. n = 11.
We asked whether heteromeric channels containing the ASIC1a subunit also show tachyphylaxis. Co-expressing ASIC1a with either ASIC2a or ASIC3 leads to formation of heteromeric channels in oocytes (Bassilana et al. 1997; Chen et al. 2006). Both ASIC1a-containing heteromeric channels (ASIC1a/3 and ASIC1a/2a) did not display tachyphylaxis (Fig. 1), indicating that it is specific for homomeric ASIC1a.
As can be seen from Fig. 1B, the decreased response to successive stimulations could be fitted with a single exponential function revealing a new steady-state level for the current amplitude. Even by waiting for 10 min between successive stimulations, however, channels did not clearly recover from tachyphylaxis. Thus, it seemed that tachyphylaxis is due to a long-lived inhibition of ASIC1a and that with time this long-lived desensitized state affects all channels. Therefore, a real steady-state level may not be reached (see also below).
Tachyphylaxis of ASIC1a currents depends on the extracellular concentrations of H+
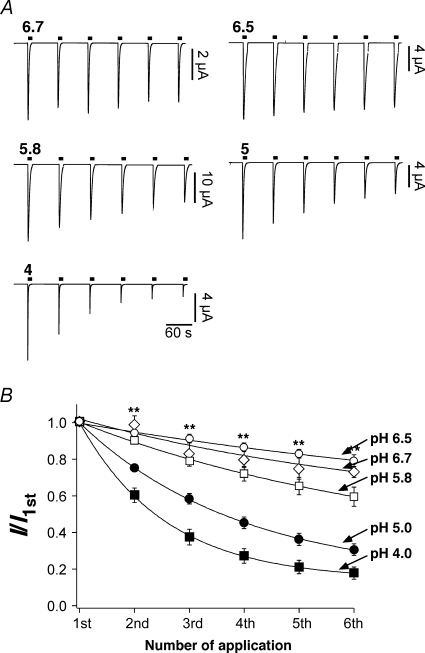
Next we asked whether tachyphylaxis depends on the strength of the activating stimulus. We repeatedly stimulated ASIC1a channels with solutions of varying pH (pH 6.7–4). As shown in Fig. 2, the reduction of ASIC1a current amplitude was indeed a function of pH. The more acidic the stimulation solution, the faster the reduction of the amplitude. Tachyphylaxis was mild with pH 6.7 (Fig. 2A, top left, and 2B), whereas with pH 4 it was pronounced. Moreover, at any pH tachyphylaxis could be fitted with a single exponential function revealing that the current amplitude relaxed to a new quasi steady-state level (Fig. 2B). This steady-state level was a function of pH. It was lowest with pH 4 and highest with pH 6.5. Such behaviour is expected when tachyphylaxis is due to protonation of a titratable site on the channel. Since, as already mentioned before, even at low pH a clear steady-state was not reached during the number of activations we used (usually 6), it was not possible to determine the apparent H+ affinity of the putative binding site; but the effect seemed to be of rather low affinity. It should be stressed, however, that tachyphylaxis was also induced by the physiological pH 6.7 and, if it is irreversible on the time scale of many minutes, tachyphylaxis can therefore have a profound effect of ASIC1a activity even under physiological conditions, provided that channels are activated often enough.
Figure 2.
Tachyphylaxis is pH dependent A, representative traces of ASIC1a currents induced by different extracellular pH values. B, current amplitudes were normalized to the first amplitude. Continuous lines represent fits to a single exponential function. **P < 0.001 by one-way ANOVA test. pH 6.7, n = 7; pH 6.5, n = 9; pH 5.8, n = 9; pH 5, n = 7; pH 4, n = 15.
Tachyphylaxis is due to a reduced number of open channels
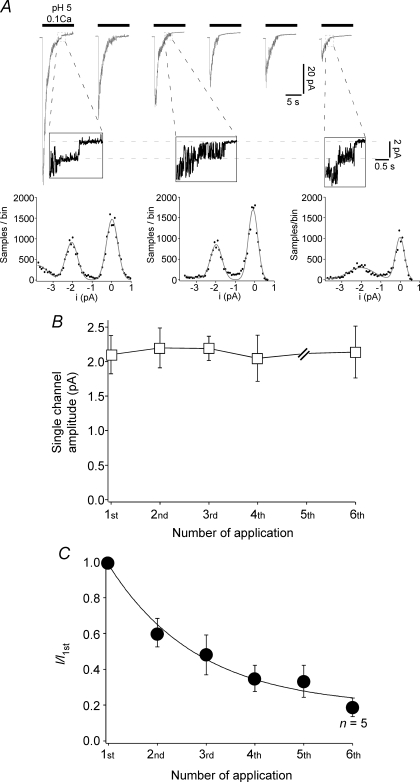
The macroscopic reduction in current amplitude could be due to a reduction of the amplitude of single channels or of the number of open channels. We addressed these possibilities in outside-out patches. Repeated activation of ASIC1a channels in outside-out patches reproduced the reduction in peak current amplitude that we observed in whole cells (Fig. 3C). We analysed the amplitude of the ‘last’ channels that could be seen in such measurements (Fig. 3A). The main amplitude was constantly about 2.1 pA, irrespective of whether it was determined for channels that had been activated for the first or for the sixth time (Fig. 3B). Thus, tachyphylaxis is most probably not due to reduced single channel amplitude and is therefore most likely due to a reduced number of open channels.
Figure 3.
Tachyphylaxis is not due to a reduction of the single channel amplitude A, top, representative current traces recorded from one excised outside-out patch. ASIC1a was repeatedly (six times) activated for 10 s with pH 5. Between each application, channels could fully recover from acute desensitization by application for 60 s of pH 7.4. Activation solution had a reduced Ca2+ concentration (0.1 mm), whereas conditioning solution contained 1.8 mm Ca2+ (in addition, both contained 1 mm Mg2+). Holding potential was –70 mV. At the end of the open phase, single channel events can be observed. For the 1st, 3rd and 6th application, they are shown at higher magnification below the respective trace. Bottom, amplitude histogram of parts of the magnified segments shown above. Amplitude distribution was fitted with a sum of Gaussian functions. B, single channel amplitudes, analysed as shown in A, as a function of the number of acid applications. n = 4–6 individual patches. The fifth application was not analysed due to a low number of discernible single channels. There was no significant change of the single channel amplitude during repetitive low pH stimulation (P = 1 by ANOVA). C, analysis of the peak current amplitude in outside-out patches as a function of the number of acid application. The continuous line is a fit with a mono-exponential function. If not specified differently, n = 7. Recordings with peak current amplitudes ranging from 22 pA to 100 pA (first activation) were analysed. P < 0.001 by ANOVA.
Moreover, the desensitization time constant in these measurements did not significantly change between the first and the sixth activation. It was constantly around 1.3 s (not shown), suggesting that the mean open time of channels did not change with repeated activation.
Tachyphylaxis is not caused by endocytosis
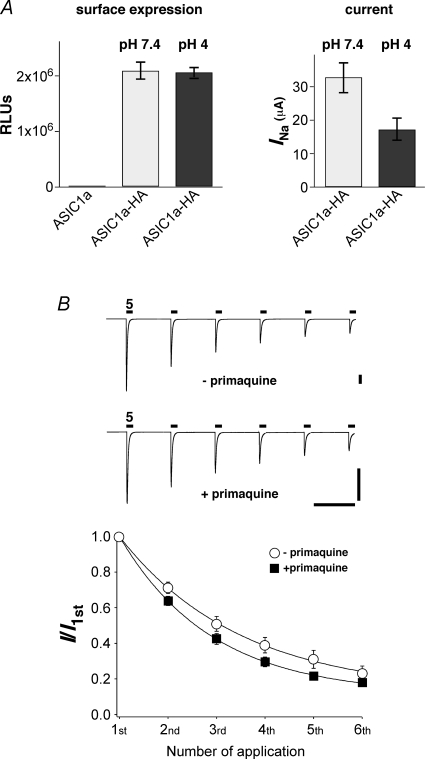
In order to address whether tachyphylaxis is caused by endocytosis of channels, we inserted an HA epitope into the extracellular loop of ASIC1a; HA-tagged channels showed tachyphylaxis like untagged channels (not shown). Using a monoclonal anti-HA antibody and a luminescence assay, we then quantified the surface expression of HA-tagged ASIC1a that had been stimulated six times for 10 s with pH 4 to induce tachyphylaxis. Channels that were exposed to pH 7.4 served as a control. As shown in Fig. 4A, ASIC1a surface expression was not changed by pH 4 stimulation. In contrast, pH 4 stimulation reduced the current amplitude of HA-tagged ASIC1a, which had been treated in a similar way, by about 50% (Fig. 4A). This result shows that endocytosis cannot account for tachyphylaxis.
Figure 4.
Tachyphylaxis is not caused by endocytosis A, left, surface expression of HA-tagged ASIC1a that had been exposed six times to pH 7.4 or pH 4. Untagged ASIC1a served as a control (first column). Results are expressed as relative light units (RLUs) per oocyte. ASIC1a, n = 18; ASIC1a-HA, pH 7.4, n = 17; ASIC1a-HA, pH 4, n = 25. Right, current amplitude of HA-tagged ASIC1a that were treated in a similar way as oocytes for which surface expression was determined. ASIC1a-HA, pH 7.4, n = 13; ASIC1a-HA, pH 4, n = 15. P = 0.01 by t test. B, top, ASIC1a channels were repeatedly activated by pH 5 for 10 s in the absence or presence of 1 mm primaquine. In both conditions, channels were allowed to recover at pH 7.4 for 60 s. Oocytes in the presence of primaquine had been preincubated in primaquine for 4 h. Bars correspond to 60 s and 1 μA, respectively. Bottom, current amplitudes were normalized to the first amplitude. Continuous lines represent fits to a single exponential function. There was no significant difference between the two conditions. –primaquine, n = 6; +primaquine, n = 7.
Confirming this finding, primaquine (1 mm, preincubation for 4 h) did not attenuate tachyphylaxis in whole oocytes (Fig. 4B). Primaquine is a weak base that inhibits vesicle formation (Hiebsch et al. 1991) and rundown of epithelial Na+ channels (ENaCs) in Xenopus oocytes (Volk et al. 2004). Thus, tachyphylaxis of ASIC1a seems to be a fundamentally different process than ENaC rundown. ENaC is a channel that is related to ASICs and ENaC rundown is due to endocytosis of the channels. Moreover, tachyphylaxis in excised membrane patches (Fig. 3) also suggests that endocytosis of channels cannot account for tachyphylaxis.
Channels have to open for tachyphylaxis to ensue
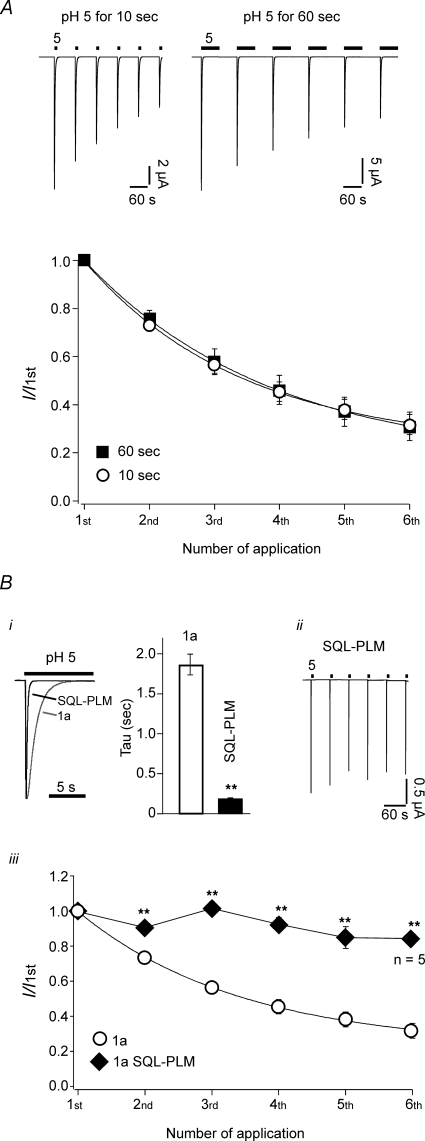
We asked whether protons can access the putative titratable site responsible for tachyphylaxis also when channels are in the desensitized state. At pH 5, ASIC1a desensitizes completely and sustained exposure to pH 5 will keep ASIC1a in the desensitized state. We repeatedly stimulated ASIC1a with pH 5 for either 10 s or 60 s, respectively, and compared tachyphylaxis. As shown in Fig. 5A, tachyphylaxis of ASIC1a was not enhanced when pH 5 was applied for 60 s (P > 0.5 by t test), suggesting that protons cannot induce tachyphylaxis when channels are in the desensitized conformation.
Figure 5.
Tachyphylaxis occurs from the channels open state A, top, ASIC1a channels were repeatedly activated by pH 5 for 10 s (left) or 60 s (right). In both conditions, channels were allowed to recover at pH 7.4 for 60 s. Bottom, current amplitudes were normalized to the first amplitude. Continuous lines represent fits to a single exponential function. 10 s application, n = 10; 60 s application, n = 8. Bi, comparison of desensitization of wt ASIC1a with mutant SQL(83-85)-PLM. The desensitization time constant of ASIC1a wt and SQL-PLM were 1.9 ± 0.1 s (n = 10) and 0.17 ± 0.02 s (n = 10), respectively. ii, representative current traces of repetitive activation with pH 5 of mutant SQL-PLM. iii, current amplitudes were normalized to the first amplitude. For ASIC1a, continuous line represents a fit to a single exponential function. **P < 0.001 by t test. If not specified differently, n = 10 for ASIC1a wt and n = 11 for ASIC1a SQL-PLM.
This result predicts that shortening of the time the channel spends in the open state should reduce tachyphylaxis. For fish ASIC1, it has recently been shown that the speed of desensitization is controlled by three amino acids in the extracellular loop (Coric et al. 2003). We exchanged the corresponding amino acids of rat ASIC1a by those that are found in the faster desensitizing fish channel. The mutant channel ASIC1a SQL(83-85)PLM indeed desensitized more than 10-fold faster (τdesens= 0.17 ± 0.02 s, n = 11) than wild-type ASIC1a (τdesens= 1.9 ± 0.1 s, n = 10; Fig. 5B). As expected, tachyphylaxis was strongly attenuated in mutant SQL-PLM at pH 5 (Fig. 5B). This result is, first, consistent with the interpretation that protons can access the channel only in the open state to induce tachyphylaxis, and second, it is consistent with the interpretation that the rate for modification by H+ is so small that a reduction of the macroscopic open time by a factor of 10 strongly affects tachyphylaxis. Confirming this result, shortening the time of ASIC1a activation from 1 s to 0.3 s in a skeletal muscle cell line reduces tachyphylaxis (Gitterman et al. 2005).
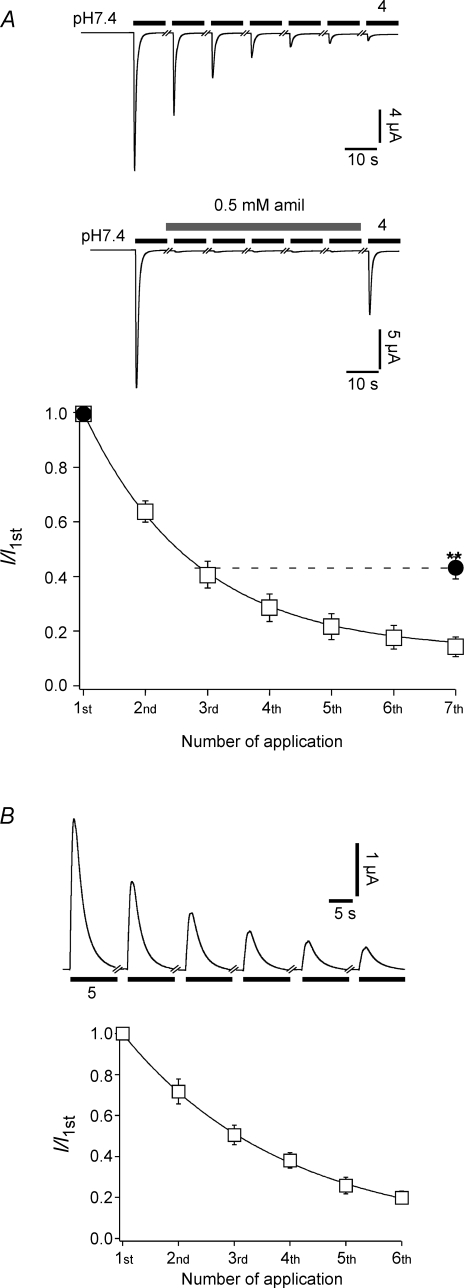
The dependence of tachyphylaxis on the open state prompted the question whether the ion pore needs to be open for tachyphylaxis to ensue. To address this question, we activated ASIC1a repeatedly with pH 4 in the presence of a high concentration (500 μm) of the pore blocker amiloride (Fig. 6A). This concentration of amiloride is about 25-fold higher than IC50 and should keep the ion pore of approximately 95% of the channels blocked. After five stimulations in the presence of amiloride, we activated the channel without amiloride and looked for the decrease of the current amplitude relative to the first test pulse. We found that tachyphylaxis was strongly attenuated but not completely relieved by amiloride (Fig. 6A).
Figure 6.
Blockage of the open pore reduces tachphylaxis A, top, ASIC1a was repeatedly activated with pH 4 either in the absence or in the presence of amiloride, as indicated. Bottom, current amplitudes were normalized to the first amplitude. Continuous line represents a fit to a single exponential function. For the series to assess the effect of amiloride, only the amplitudes of the amiloride-free applications (1st and 7th) are shown. n = 12 without amiloride and n = 9 with amiloride. **P < 0.001 by t test. B, outward currents do show tachyphylaxis. Top, representative traces of ASIC1a outward currents. Extracellular ion concentrations were (mm): 130 NMDGCl, 10 NaCl, 1.8 CaCl2, 1.0 MgCl2, 10 Hepes, pH 7.4. For acidic activation solution (pH 5.0), Hepes was replaced by Mes. Holding potential was +20 mV during the test period. n = 9.
ASIC1 is permeable to H+ and tachyphylaxis is related to H+ permeation
This result suggested that ions flowing through the open pore contribute to tachyphylaxis. We first considered Na+. We substituted most of the Na+ with NMDG and clamped the membrane potential to +20 mV. Under these conditions, acid stimulation of ASIC1a evoked outward currents. If anything, these outward currents displayed enhanced tachyphylaxis (Fig. 6B), ruling out that the influx of Na+ causes tachyphylaxis.
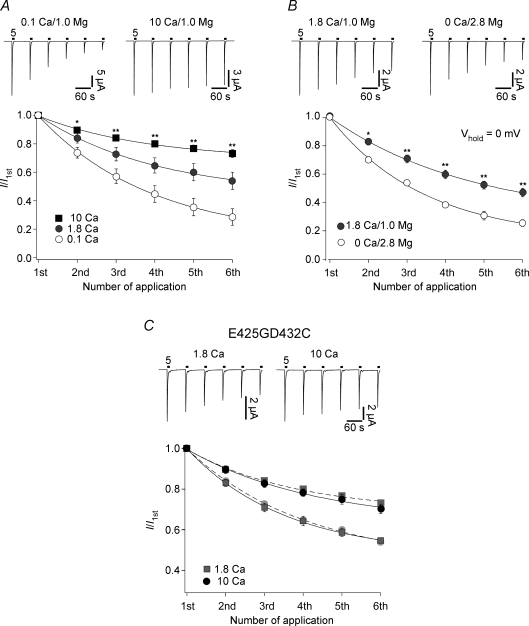
We then considered Ca2+. Homomeric ASIC1a is the only ASIC subtype to be slightly permeable to Ca2+ (Bässler et al. 2001) and for the vanilloid-receptor TRPV1, tachyphylaxis depends on the influx of Ca2+ (Koplas et al. 1997). However, as shown in Fig. 7A, tachyphylaxis of ASIC1a currents was attenuated (P << 0.01) at a higher concentration of extracellular Ca2+. We further investigated a possible role of Ca2+ by changing the experimental protocol to avoid Ca2+ influx. We replaced the extracellular Ca2+ with the same concentration of Mg2+. This should reduce the concentration of free Ca2+ in the extracellular solution to ∼10 nm. Oocytes have an intracellular Ca2+ concentration of 10–100 nm (Brooker, 1990) so that the reversal potential of calcium should be 0 mV or lower. We clamped the oocyte membrane to 0 mV, preventing any strong influx of Ca2+, and assessed for tachyphylaxis. As shown in Fig. 7B, tachyphylaxis was enhanced under these conditions. Extracellular Ca2+ has many effects on ASIC1a. It competes with H+, shifting the H+ dependence of activation and steady-state desensitization (Babini et al. 2002), and it blocks the open pore (Paukert et al. 2004a). In our experiments we changed the Ca2+ concentration only during channel activation, ruling out any influence on steady-state desensitization. Moreover, at the acidic pH we used for channel activation (pH 5.0) Ca2+ has no influence on H+ activation (Babini et al. 2002). Finally, in the experiment shown in Fig. 7B the total concentration of extracellular divalent cations was constant. Whereas Mg2+ can largely substitute Ca2+ for its effect on steady-state desensitization, H+ activation and channel block (Babini et al. 2002; Paukert et al. 2004a), it very likely cannot permeate ASIC1a. In order to further rule out any indirect effect of Ca2+ on tachyphylaxis through a block of the open pore, we measured tachyphylaxis with an ASIC1a mutant (ASIC1aE425GD432C) that is no longer blocked by Ca2+ (Paukert et al. 2004b). The ASIC1aE425GD432C mutant showed tachyphylaxis that was indistinguishable from ASIC1a wild-type (Fig. 7C). Thus, extracellular Ca2+ does not attenuate tachyphylaxis through a block of the open pore. Together, these data show that the influx of Ca2+ cannot explain tachyphylaxis and that Ca2+ rather attenuates tachyphylaxis.
Figure 7.
Calcium attenuates tachyphylaxis A, top, ASIC1a was repeatedly activated by pH 5 either with 0.1 mm extracellular Ca2+ (left) or with 10 mm extracellular Ca2+ (right). Bottom, current amplitudes were normalized to the first amplitude. Continuous lines represent fits to a single exponential function. Calcium attenuated tachyphylaxis: 0.1 Ca2+, n = 10; 1.8 Ca2+, n = 10; 10 Ca2+, n = 11. *P < 0.01, **P < 0.001 by ANOVA. B, top, ASIC1a was repeatedly activated by pH 5 either with 1.8 Ca2+–1.0 Mg2+ (left) or with 0 Ca2+–2.8 Mg2+ (right). Holding potential was 0 mV. Tachyphylaxis was enhanced when extracellular Ca2+ was replaced by Mg2+. n = 7 for 1.8 Ca2+–1.0 Mg2+ and n = 9 for 0 Ca2+–2.8 Mg2+. *P < 0.01, **P < 0.001 by t test. C, block of the ion pore by Ca2+ is not involved in tachyphylaxis. Top, ASIC1a mutant E425GD432C was repeatedly activated by pH 5 either with 1.8 mm (left) or with 10 mm extracellular Ca2+ (right). Extracellular concentration of Mg2+ was always 1 mm. Calcium attenuated tachyphylaxis as for ASIC1a wt: 1.8 Ca2+, n = 6; 10 Ca2+, n = 8. For direct comparison, the results for ASIC1a wt from Fig. 7A are shown as light symbols and the respective fits as dashed lines.
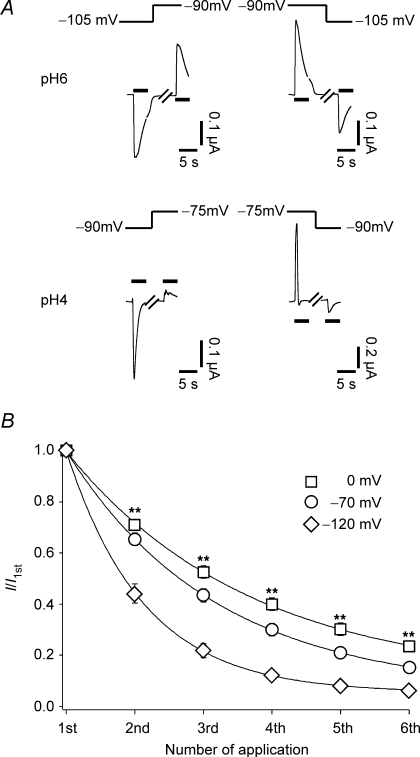
Finally we considered H+. We assessed H+ permeability of ASIC1a by strongly reducing the extracellular Na+ (1 mm) and Ca2+ (0 mm) concentration and looking for a dependence of the reversal potential on the H+ concentration. Under these conditions, tachyphylaxis was very pronounced, allowing only a few successive measurements. Reversal potentials could therefore not be determined with precision. However, it was evident that the reversal potentials shifted by at least 10 mV when the H+ concentration was raised from 1 μm to 100 μm (pH 6 to pH 4, Fig. 8A). This corresponds to a relative permeability PH/PNa of at least 5 (see Methods). We observed a similar 10 mV shift for ASIC1b (not shown). Hence, ASIC1 is not only gated by H+, it is also highly permeable to H+.
Figure 8.
ASIC1a is H+ permeable A, ASIC1a was repeatedly activated by either 1 μm H+ (pH 6, top) or 100 μm H+ (pH 4, bottom). The concentrations of the other ions were fixed at (mm): 1 NaCl, 90 NMDGCl, 0 CaCl2, 3 MgCl2, 10 Mes, 10 Hepes. The holding potential was changed as indicated. Black bars indicate application of acid. As can be seen, the potential at which the current reversed its sign shifted by at least 10 mV when the H+ concentration was raised from 1 μm to 100 μm. Note that desensitization was faster at pH 4. B, ASIC1a was repeatedly activated by pH 5. The holding potential was 0 mV (n = 19), –70 mV (n = 17) or –120 mV (n = 13). Relative current amplitudes normalized to the first amplitude are shown. Continuous lines represent fits to a single exponential function. The solution contained (mm): 140 NaCl, 0 CaCl2, 3 MgCl2, 10 Hepes. For acidic activation solution, Hepes was replaced by Mes. **P < 0.001 by ANOVA.
In the experiments just described the contribution of H+ to the total inward current was relatively large, due to the low Na+ concentration and the absence of Ca2+. The pronounced tachyphylaxis in these experiments therefore suggests that permeating H+ may contribute to tachyphylaxis. This would predict that tachyphylaxis should be reduced by reducing the driving force for H+. Indeed, depolarizing the cell to 0 mV significantly reduced tachyphylaxis and hyperpolarizing the cell to –120 mV enhanced tachyphylaxis (Fig. 8B).
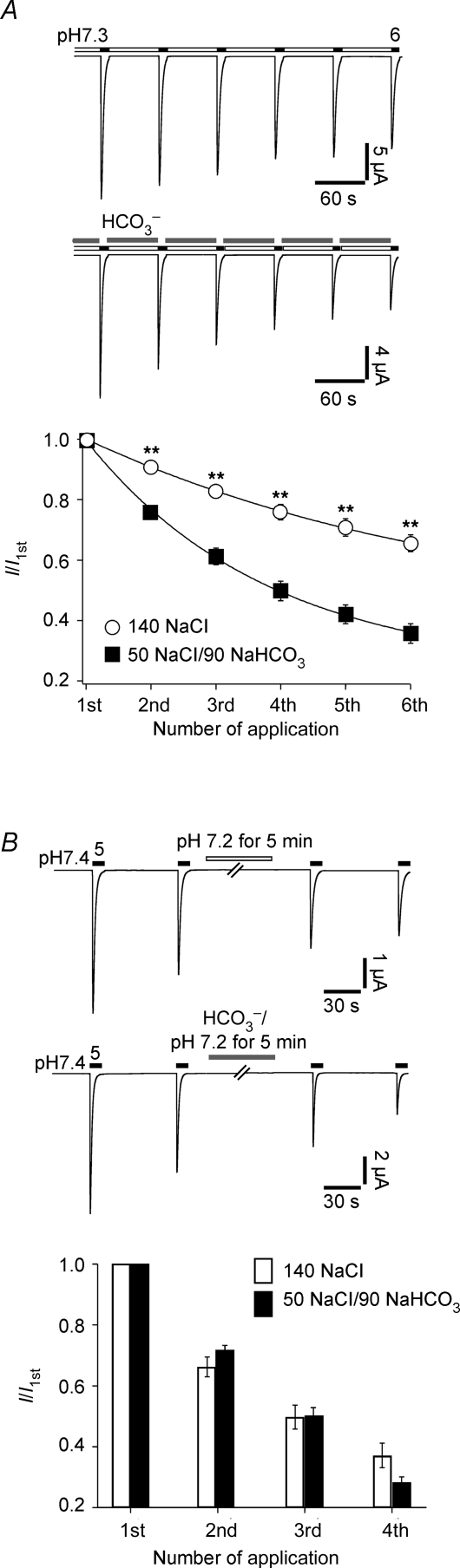
Since tachyphylaxis was related to H+ permeation, we next asked whether tachyphylaxis also depends on the intracellular concentration of H+. We addressed this question by exposing the oocytes to a CO2– buffer, which will reduce the intracellular pH of oocytes by approximately 1.0 pH unit in approximately 2 min (Fakler et al. 1996). Application of the solution during the interval between acid stimulations (pH 6.0) significantly (P < 0.001) enhanced tachyphylaxis (Fig. 9A), showing that also a decrease of the intracellular pH enhances tachyphylaxis. In order to address whether intracellular access to the H+ binding site depends on the conformation (closed, open, desensitized) of the channel, we applied the solution continuously for 5 min with no channel opening (Fig. 9B). The first current amplitude after this long application was not significantly smaller (Fig. 9B, third column; P = 0.9) than in a control cell that was not exposed to . These results are in agreement with the idea that the putative H+ binding site is accessible also from the inner side of the channel, but only when channels are in the open conformation.
Figure 9.
Intracellular acidification enhances tachyphylaxis A, top, ASIC1a was repeatedly activated by pH 6; conditioning pH was pH 7.3. In the experimental group, during the 60 s intervals, 90 mm was applied to acidify the intracellular compartment. Bottom, current amplitudes were normalized to the first amplitude. Continuous lines represent fits to a single exponential function. n = 13 for the control group, n = 11 for the experimental group. **P < 0.001 by t test. B, top, ASIC1a was repeatedly activated by pH 5. In the experimental group, 90 mm was applied for 5 min. Bottom, relative current amplitudes, normalized to the first amplitude, are shown as bars. The first amplitude after incubation (‘3rd application’) was not smaller than in the control group. n = 11 for the control group, n = 10 for the experimental group.
Tachyphylaxis is also related to Ca2+ permeability of ASIC1
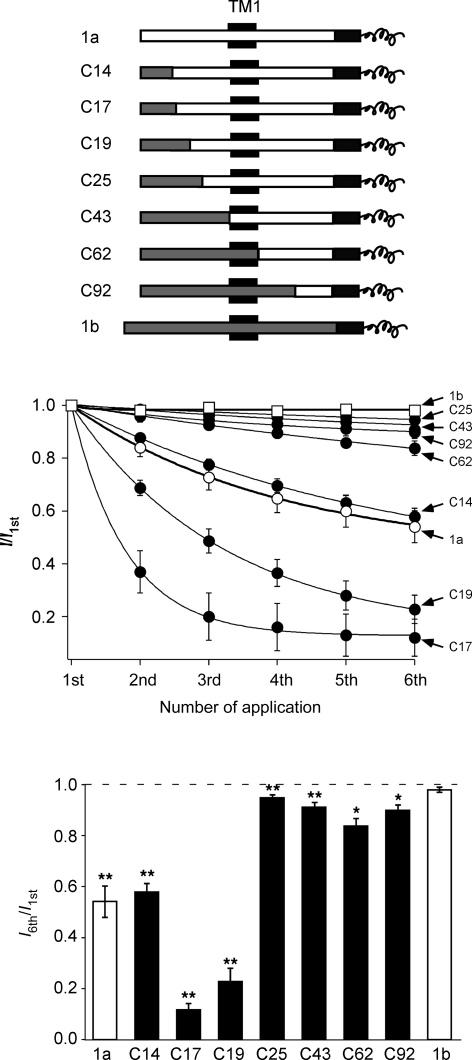
Finally, we addressed the question why only ASIC1a but not other ASICs display tachyphylaxis. We used a series of chimeric channels that exchange increasing parts of the ASIC1a N-terminus with the ASIC1b N-terminus. The chimeras could be clearly divided into two groups: those chimeras that had not more than the first 19 amino acids from ASIC1b showed robust tachyphylaxis (chimera C19) and those that had at least 25 amino acids from ASIC1b at their N-terminus (C25) showed much attenuated tachyphylaxis (Fig. 10). Only two amino acids are different between chimeras C19 and C25. One of these two amino acids is a serine in ASIC1a (S23) and an asparagine in ASIC1b, the other is a serine in 1a (S25) and a cysteine in 1b. Importantly, all these chimeric channels, except C17, desensitized slowly like ASIC1a, ruling out differences in the open time as the main reason for differences in tachyphylaxis. Previously, we had shown that the same chimeras that now showed reduced tachyphylaxis have a reduced permeability for divalent cations whereas those that now showed robust tachyphylaxis are permeable for divalent cations like ASIC1a wild-type (Bässler et al. 2001). Thus, tachyphylaxis correlated with divalent cation permeability of the chimeras. Moreover, the results obtained with the chimeras suggest an intracellular mechanism for tachyphylaxis.
Figure 10.
Tachyphylaxis is related to permeability for divalent cations Top, chimeric channels are schematically drawn. NH2 terminal sequences from ASIC1a are shown as open bars, those from ASIC1b as grey bars. The first transmembrane domain is indicated as a black box and the common C-terminus is shown as a black bar; only its first part is shown. The number denotes the number of exchanged amino acids; for example, in chimera C22 the NH2 terminal 22 amino acids of ASIC1a had been exchanged by the corresponding amino acids of ASIC1b. The chimeras did have a shorter NH2 terminus than ASIC1b; this shorter NH2 terminus corresponds to M3 in Bässler et al. (2001). Chimeras have already been reported in Bässler et al. (2001) and Babini et al. (2002). Middle, current amplitudes were normalized to the first amplitude. Continuous lines represent fits to a single exponential function. Activation was with pH 5. n = 6–14. Bottom, ratio of the sixth current amplitude to the first amplitude. The sixth amplitude was always significantly smaller than the first, except for ASIC1b. *P < 0.01, **P < 0.001 by paired t test.
Discussion
Homomeric ASIC1a is the only ASIC that shows tachyphylaxis (Fig. 1). Tachyphylaxis of the ASIC1a current had already previously been noted (Paukert et al. 2004a; Gitterman et al. 2005; Neaga et al. 2005). However, our study for the first time investigates this phenomenon systematically. Beyond a detailed description, our results also provide some clues to the mechanism of tachyphylaxis. First, they show that tachyphylaxis depends on the concentration of extracellular H+. Second, the amiloride and voltage dependence of tachyphylaxis suggest that H+, at least partially, exerts its effect by permeating through the channel. Probably permeating H+ locally decreases the cytoplasmic pH, which somehow induces tachyphylaxis. In agreement with this model, intracellular acidification by a CO2– buffer facilitated tachyphylaxis despite the fact that it reduced the driving force for H+ permeation through ASIC1a.
But why should permeating H+ induce tachyphylaxis when also the related ASIC1b is permeable to H+, yet does not show tachyphylaxis? One clue to this apparent paradox comes from the chimeras, which identify an intracellular region that is probably close to the inner ion permeation pathway (Coscoy et al. 1999; Bässler et al. 2001) and somehow controls tachyphylaxis in ASIC1 (Fig. 10). This region could structure the inner pore in a way that is unique to ASIC1a. Since the same intracellular region is responsible for the Ca2+ permeability of ASIC1a (Bässler et al. 2001), tachyphylaxis and Ca2+ permeability, two features that distinguish homomeric ASIC1a from other ASICs, would be linked by a unique pore structure of ASIC1a. Such a relation between tachyphylaxis and Ca2+ permeability is supported by a recent study reporting that treatment of ASIC1a with trypsin reduces tachyphylaxis and Ca2+ permeability (Neaga et al. 2005). Exactly how this unique structure favours tachyphylaxis is presently unknown. We speculate that H+ may interact with the inner pore of ASIC1a to induce tachyphylaxis. It is even possible that Ca2+ exerts its negative effect on tachyphylaxis (Fig. 7) by interacting with this same site, competing with the interaction of H+. At present we have, however, no direct evidence for an intracellular or ion pore effect of Ca2+ on tachyphylaxis. Therefore, although our results suggest that Ca2+ did not affect tachyphylaxis by changing steady-state desensitization, H+ activation or blocking the pore, we cannot rule out that Ca2+ affects tachyphylaxis via an extracellular mechanism.
We propose that binding of H+ induces with a low efficacy a conformational change, leading to a long-lived desensitized state. We think this state is long-lived because we did not obtain consistent evidence for recovery from tachyphylaxis (not shown). The hypothesis of a long-lived blocked state of the channel is consistent with the unchanged single channel amplitude during repetitive stimulation in excised patches (Fig. 3). Interestingly, tachyphylaxis could be induced by either extra- or intracellular acidification only when the pore was open. This suggests that the putative site where binding of H+ induces tachyphylaxis is accessible only in the open conformation, but not in the closed and desensitized conformations of the channel.
The following simple scheme illustrates the relation of the long-lived desensitized state D2 to the other basic states of the channel: 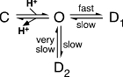
According to this scheme, upon binding of H+, channels would open. From the open state, O, the majority of the channels would undergo acute desensitization entering state D1. A smaller fraction, however, would enter the long-lived desensitized state D2. Since the fraction of channels that enters D2 is pH dependent, channels would bind additional H+ during the transition from O to D2 (not shown in the scheme). This explains why high H+ concentrations would increase the fraction of channels entering the long-lived state D2 whereas an increased rate for acute desensitization (transition from O to D1), as observed in ASIC1a mutant SQL(83-85)PLM (Fig. 5B), would increase the fraction of channels entering state D1, attenuating tachyphylaxis. Although this scheme does not take into account the existence of multiple closed, open and desensitized states, it illustrates the basic features of the long-lived state D2.
We confirmed an earlier finding (Waldmann et al. 1997b) that ASIC1a is permeable for H+. Moreover, ASIC1b is also H+ permeable (not shown). Since the related ENaC is also H+ permeable (Gilbertson et al. 1993), H+ permeability may be a general feature of ASICs. Usually, the H+ concentration is small compared with the Na+ concentration, and therefore the contribution of the H+ current to the total ASIC current will be small. However, under some circumstances, H+ could substantially contribute to the depolarizing current passing through ASICs. For example, ASIC2a/2b heteromers are expressed in rat taste cells (Ugawa et al. 2003) and H+ permeating through this channel could lead to excitation of the taste cell even when there is only a small Na+ concentration on the tongue.
Another finding of our study is the dependence of ASIC1a tachyphylaxis on the intracellular pH (pHi). Several studies reported an intracellular acidification of mammalian neurons associated with Ca2+ permeation through NMDA receptors (Irwin et al. 1994; Wang et al. 1994; Canzoniero et al. 1996) or through TRPV1 (Hellwig et al. 2004). Our study suggests that a reduced pHi in neuronal cells would negatively feed back on ASIC1a activity, contributing to the control of ASIC1a activity. Very recently, inhibition by acidic pHi has indeed been shown for ASICs from mouse cortical neurons (Wang et al. 2006). Such an inhibition could be especially relevant in nociceptors where ASIC1a is co-expressed with TRPV1 (Olson et al. 1998; Alvarez De La Rosa et al. 2002; Ugawa et al. 2005; Poirot et al. 2006). TRPV1 shares the property of H+ permeability with ASIC1a, and TRPV1 activation leads to a robust decrease of pHi (Hellwig et al. 2004). Thus, TRPV1 activity could control ASIC1a activity in nociceptors.
Our study shows that the impact of tachyphylaxis on ASIC1a activity depends on many parameters (pHo, pHi, [Ca2+]o, duration of the open state, number of stimulations). Therefore, the impact of tachyphylaxis under in vivo conditions is hard to predict. At normal body temperature, where acute desensitization is faster than at the temperature at which our experiments have been performed (approximately 22°C) (Askwith et al. 2001), tachyphylaxis is probably attenuated. However, understanding tachyphylaxis of ASIC1a may be a way to control ASIC1a activity, which is of potential therapeutic value for the treatment of stroke (Xiong et al. 2004).
Acknowledgments
We thank P. Seeberger and M. Siba for expert technical assistance, M. Paukert for advice on outside-out patches, K. Löffler and M. Langer for providing and supporting Scanclamp software, and M. Pusch for providing Ana software, many helpful discussions and comments on the manuscript. This work was supported by grants GR1771/3-3 and /3-4 of the Deutsche Forschungsgemeinschaft to S.G.
References
- Alvarez De La Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci U S A. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci U S A. 2001;98:6459–6463. doi: 10.1073/pnas.111155398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Babini E, Paukert M, Geisler HS, Gründer S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J Biol Chem. 1997;272:28819–28822. doi: 10.1074/jbc.272.46.28819. [DOI] [PubMed] [Google Scholar]
- Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- Brooker G, Seki T, Croll D, Wahlestedt C. Calcium wave evoked by activation of endogenous or exogenously expressed receptors in Xenopus oocytes. Proc Natl Acad Sci USA. 1990;87:2813–2817. doi: 10.1073/pnas.87.7.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzoniero LM, Sensi SL, Choi DW. Recovery from NMDA-induced intracellular acidification is delayed and dependent on extracellular bicarbonate. Am J Physiol. 1996;270:C593–C599. doi: 10.1152/ajpcell.1996.270.2.C593. [DOI] [PubMed] [Google Scholar]
- Chen X, Paukert M, Kadurin I, Pusch M, Gründer S. Strong modulation by RFamide neuropeptides of the ASIC1b/3 heteromer in competition with extracellular calcium. Neuropharmacology. 2006;50:964–974. doi: 10.1016/j.neuropharm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Coric T, Zhang P, Todorovic N, Canessa CM. The extracellular domain determines the kinetics of desensitization in acid-sensitive ion channel 1. J Biol Chem. 2003;278:45240–45247. doi: 10.1074/jbc.M304441200. [DOI] [PubMed] [Google Scholar]
- Coscoy S, de Weille JR, Lingueglia E, Lazdunski M. The pre-transmembrane 1 domain of acid-sensing ion channels participates in the ion pore. J Biol Chem. 1999;274:10129–10132. doi: 10.1074/jbc.274.15.10129. [DOI] [PubMed] [Google Scholar]
- Coscoy S, Lingueglia E, Lazdunski M, Barbry P. The Phe-Met-Arg-Phe-amide-activated sodium channel is a tetramer. J Biol Chem. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson TA, Roper SD, Kinnamon SC. Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: enhancement by vasopressin and cAMP. Neuron. 1993;10:931–942. doi: 10.1016/0896-6273(93)90208-9. [DOI] [PubMed] [Google Scholar]
- Gitterman DP, Wilson J, Randall AD. Functional properties and pharmacological inhibition of ASIC channels in the human SJ-RH30 skeletal muscle cell line. J Physiol. 2005;562:759–769. doi: 10.1113/jphysiol.2004.075069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schäfer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- Hiebsch RR, Raub TJ, Wattenberg BW. Primaquine blocks transport by inhibiting the formation of functional transport vesicles. Studies in a cell-free assay of protein transport through the Golgi apparatus. J Biol Chem. 1991;266:20323–20328. [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Long RT, Paul SM. N-methyl-D-aspartate induces a rapid, reversible, and calcium-dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J Neurosci. 1994;14:1352–1357. doi: 10.1523/JNEUROSCI.14-03-01352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaga E, Amuzescu B, Dinu C, Macri B, Pena F, Flonta ML. Extracellular trypsin increases ASIC1a selectivity for monovalent versus divalent cations. J Neurosci Meth. 2005;144:241–248. doi: 10.1016/j.jneumeth.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport. 1998;9:1109–1113. doi: 10.1097/00001756-199804200-00028. [DOI] [PubMed] [Google Scholar]
- Paukert M, Babini E, Pusch M, Gründer S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol. 2004a;124:383–394. doi: 10.1085/jgp.200308973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukert M, Sidi S, Russell C, Siba M, Wilson SW, Nicolson T, Gründer S. A family of acid-sensing ion channels from the zebrafish: widespread expression in the central nervous system suggests a conserved role in neuronal communication. J Biol Chem. 2004b;279:18783–18791. doi: 10.1074/jbc.M401477200. [DOI] [PubMed] [Google Scholar]
- Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Perouansky M, Hoppe D, Lux HD, Grantyn R, Kettenmann H. Glial cells of the oligodendrocyte lineage express proton-activated Na+ channels. J Neurosci Res. 1989;24:496–500. doi: 10.1002/jnr.490240406. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Yamamura H, Shimada S. In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Brain Res Mol Brain Res. 2005;136:125–133. doi: 10.1016/j.molbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S, Minami Y, Guo W, Saishin Y, Takatsuji K, Tohyama M. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J Neurosci. 2003;23:3616–3622. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, Konstas AA, Bassalay P, Ehmke H, Korbmacher C. Extracellular Na+ removal attenuates rundown of the epithelial Na+-channel (ENaC) by reducing the rate of channel retrieval. Pflugers Arch. 2004;447:884–894. doi: 10.1007/s00424-003-1193-x. [DOI] [PubMed] [Google Scholar]
- Vukicevic M, Kellenberger S. Modulatory effects of acid-sensing ion channels on action potential generation in hippocampal neurons. Am J Physiol Cell Physiol. 2004;287:C682–C690. doi: 10.1152/ajpcell.00127.2004. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Randall RD, Thayer SA. Glutamate-induced intracellular acidification of cultured hippocampal neurons demonstrates altered energy metabolism resulting from Ca2+ loads. J Neurophysiol. 1994;72:2563–2569. doi: 10.1152/jn.1994.72.6.2563. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Chu XP, Li MH, Seeds J, Simon RP, Xiong ZG. Modulation of asic currents, acid-induced increase of intracellular Ca2+ and acidosis mediated neuronal injury by intracellular pH. J Biol Chem. 2006;281:29369–29378. doi: 10.1074/jbc.M605122200. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ, Leonard AS, Yermolaieva O, Hruska-Hageman A, Price MP, Liu L, Johnson WA. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–43724. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J Neurophysiol. 2002;87:2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]