Abstract
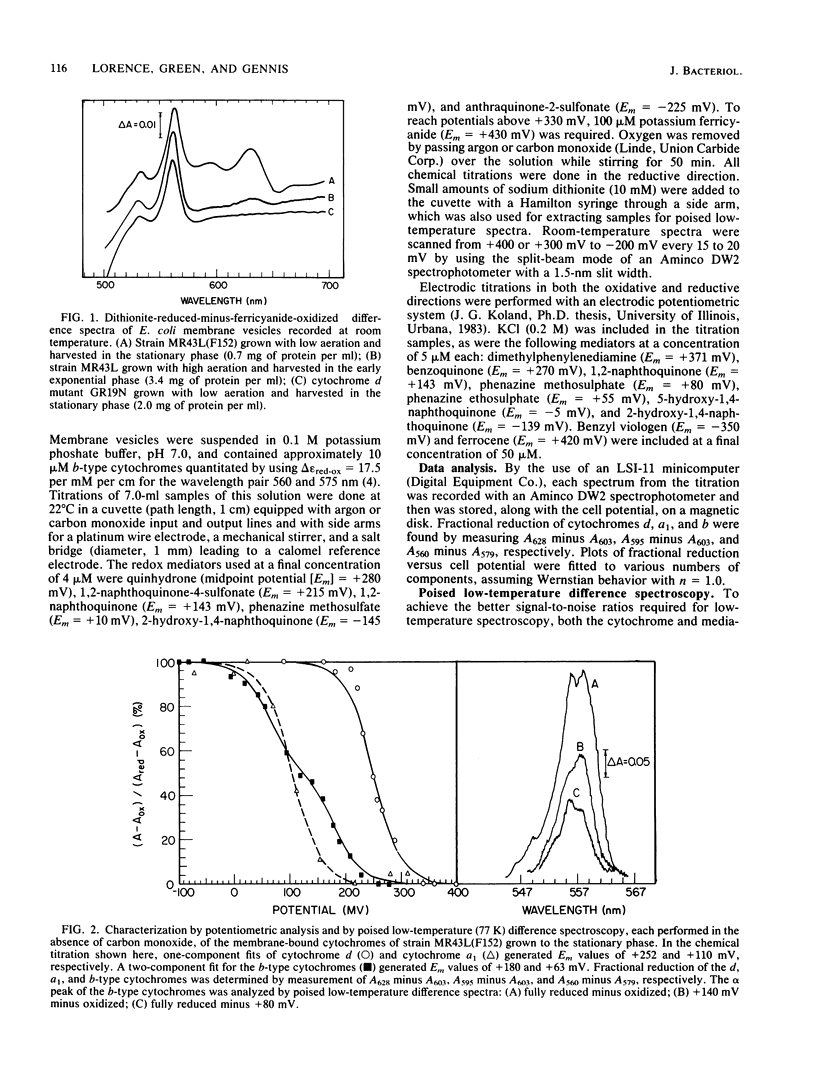
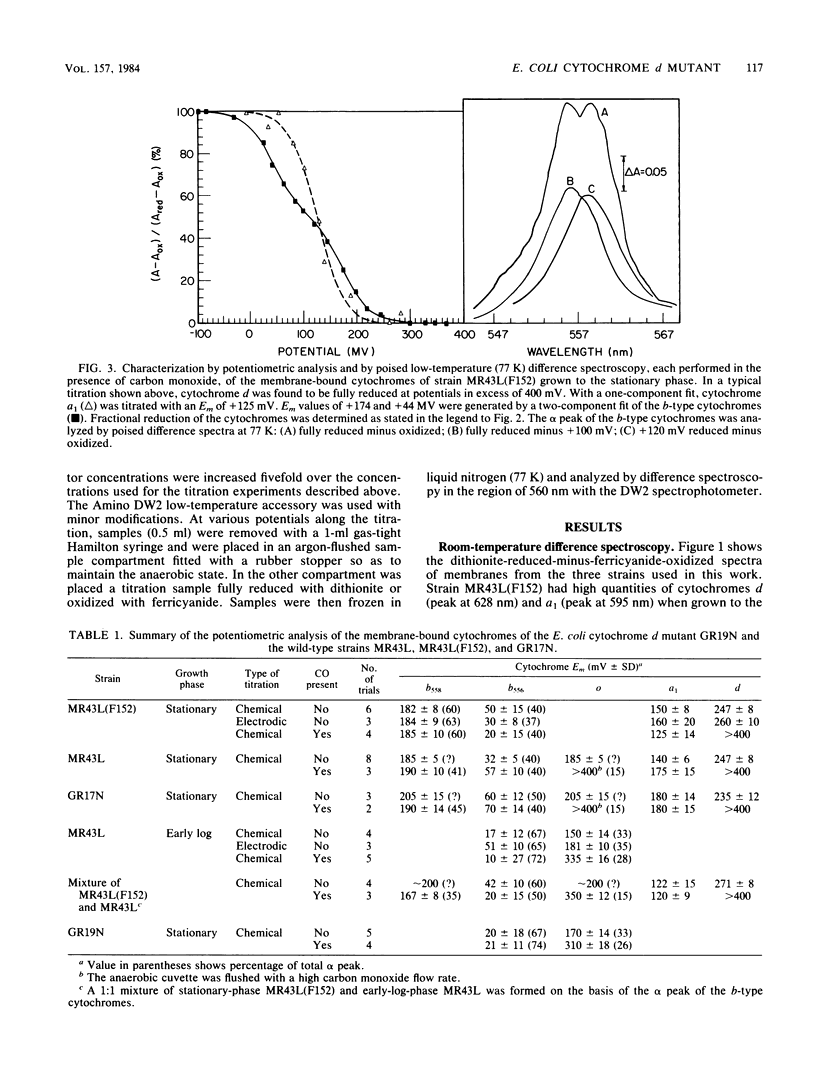
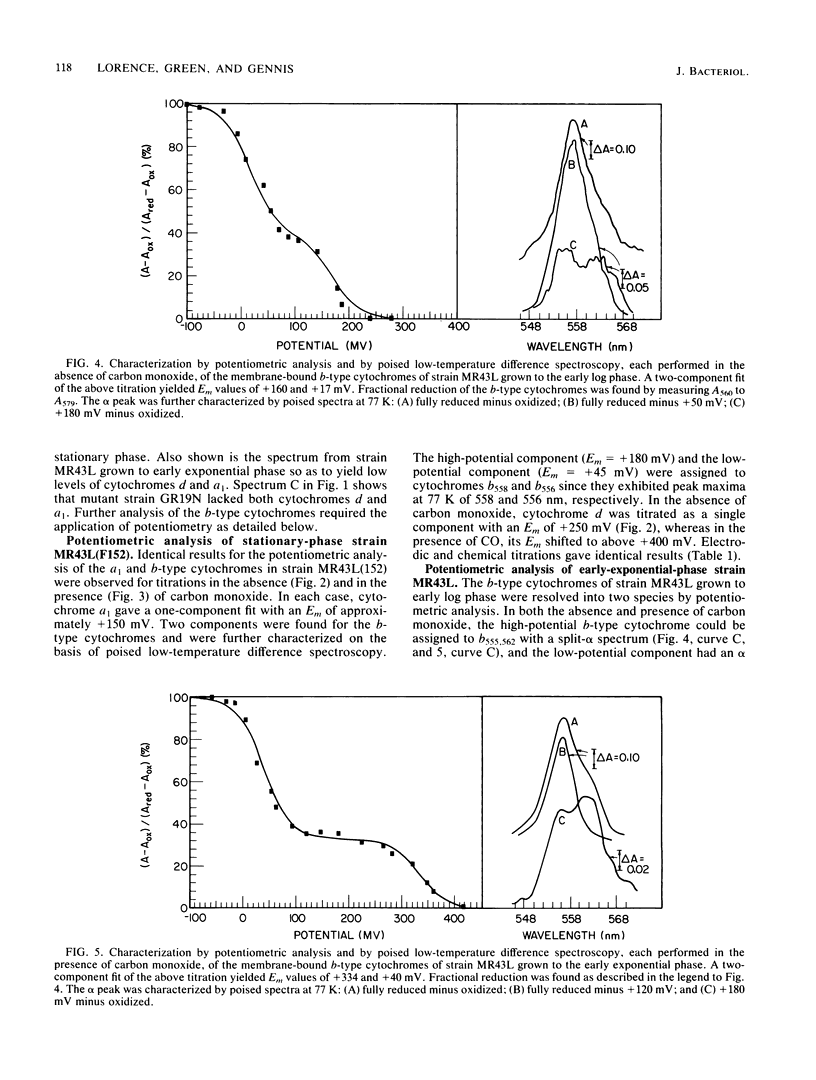
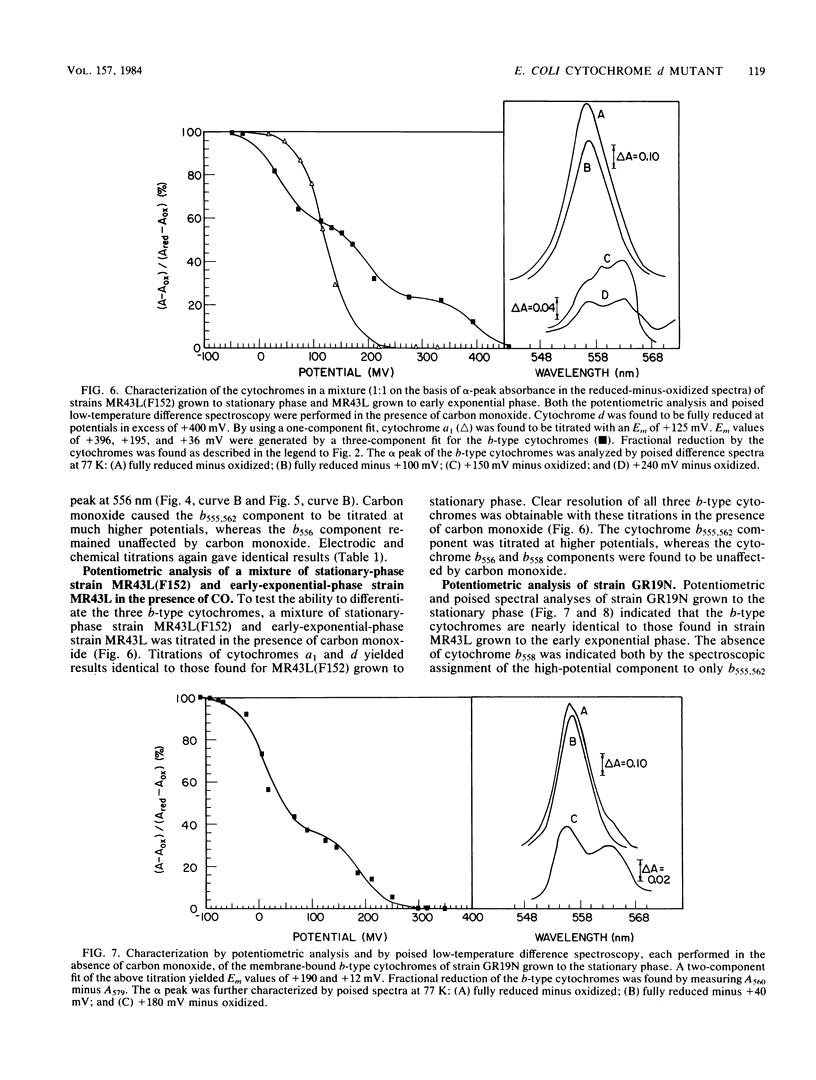
A combination of potentiometric analysis and electrochemically poised low-temperature difference spectroscopy was used to examine a mutant strain of Escherichia coli that was previously shown by immunological criteria to be lacking the cytochrome d terminal oxidase. It was shown that this strain is missing cytochromes d, a1, and b558 and that the cytochrome composition of the mutant is similar to that of the wild-type strain grown under conditions of high aeration. The data indicate that the high-aeration branch of the respiratory chain contains two cytochrome components, b556 (midpoint potential [Em] = +35 mV) and cytochrome o (Em = +165 mV). The latter component binds to CO and apparently has a reduced-minus-oxidized split-alpha band with peaks at 555 and 562 nm. When the wild-type strain was grown under conditions of low aeration, the components of the cytochrome d terminal oxidase complex were observed: cytochrome d (Em = +260 mV), cytochrome a1 (Em = +150 mV) and cytochrome b558 (Em = +180 mV). All cytochromes appeared to undergo simple one-electron oxidation-reduction reactions. In the absence of CO, cytochromes b558 and o have nearly the same Em values. In the presence of CO, the Em of cytochrome o is raised, thus allowing cytochromes b558 and o to be individually quantitated by potentiometric analysis when they are both present.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Green G. N., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983 Jun;154(3):1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett N. R., Bragg P. D. Membrane cytochromes of Escherichia coli grown aerobically and anaerobically with nitrate. J Bacteriol. 1983 May;154(2):708–718. doi: 10.1128/jb.154.2.708-718.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Shrager R. I. Potentiometric analysis of Escherichia coli cytochromes in the optical absorbance range of 500 nm to 700 nm. J Biol Chem. 1979 Nov 25;254(22):11288–11299. [PubMed] [Google Scholar]
- Hendler R. W., Towne D. W., Shrager R. I. Redox properties of beta-type cytochromes in Escherichia coli and rat liver mitochondria and techniques for their analysis. Biochim Biophys Acta. 1975 Jan 31;376(1):42–62. doi: 10.1016/0005-2728(75)90203-0. [DOI] [PubMed] [Google Scholar]
- Kita K., Kasahara M., Anraku Y. Formation of a membrane potential by reconstructed liposomes made with cytochrome b562-o complex, a terminal oxidase of Escherichia coli K12. J Biol Chem. 1982 Jul 25;257(14):7933–7935. [PubMed] [Google Scholar]
- Kita K., Yamato I., Anraku Y. Purification and properties of cytochrome b556 in the respiratory chain of aerobically grown Escherichia coli K12. J Biol Chem. 1978 Dec 25;253(24):8910–8915. [PubMed] [Google Scholar]
- Kranz R. G., Barassi C. A., Miller M. J., Green G. N., Gennis R. B. Immunological characterization of an Escherichia coli strain which is lacking cytochrome d. J Bacteriol. 1983 Oct;156(1):115–121. doi: 10.1128/jb.156.1.115-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. Immunological characterization of the cytochrome o terminal oxidase from Escherichia coli. J Biol Chem. 1983 Sep 10;258(17):10614–10621. [PubMed] [Google Scholar]
- Matsushita K., Patel L., Gennis R. B., Kaback H. R. Reconstitution of active transport in proteoliposomes containing cytochrome o oxidase and lac carrier protein purified from Escherichia coli. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4889–4893. doi: 10.1073/pnas.80.16.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Effects of sulphate-limited growth in continuous culture on the electron-transport chain and energy conservation in Escherichia coli K12. Biochem J. 1975 Dec;152(3):537–546. doi: 10.1042/bj1520537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Redox potentials of the cytochromes in the respiratory chain of aerobically grown Escherichia coli. Arch Biochem Biophys. 1976 Jun;174(2):546–552. doi: 10.1016/0003-9861(76)90382-9. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Haddock B. A., Ingledew W. J. Assembly of functional b-type cytochromes in membranes from a 5-aminolaevulinic acid-requiring mutant of Escherichia coli. FEBS Lett. 1981 Aug 31;131(2):346–350. doi: 10.1016/0014-5793(81)80400-0. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Ingledew W. J. Characterization and phenotypic control of the cytochrome content of Escherichia coli. Biochem J. 1979 Aug 15;182(2):465–472. doi: 10.1042/bj1820465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. I., Poole R. K. A re-examination of the cytochromes of Escherichia coli using fourth-order finite difference analysis: their characterization under different growth conditions and accumulation during the cell cycle. J Gen Microbiol. 1982 Aug;128(8):1685–1696. doi: 10.1099/00221287-128-8-1685. [DOI] [PubMed] [Google Scholar]
- Shipp W. S. Cytochromes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):459–472. doi: 10.1016/0003-9861(72)90063-x. [DOI] [PubMed] [Google Scholar]
- Shipp W. S., Piotrowski M., Friedman A. E. Apparent cytochrome gene dose effects in F-lac and F-gal heterogenotes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):473–481. doi: 10.1016/0003-9861(72)90064-1. [DOI] [PubMed] [Google Scholar]
- Van Wielink J. E., Oltmann L. F., Leeuwerik F. J., De Hollander J. A., Stouthamer A. H. A method for in situ characterization of b- and c-type cytochromes in Escherichia coli and in complex III from beef heart mitochondria by combined spectrum deconvolution and potentiometric analysis. Biochim Biophys Acta. 1982 Aug 20;681(2):177–190. doi: 10.1016/0005-2728(82)90021-4. [DOI] [PubMed] [Google Scholar]



