Abstract
This review describes the rationale, early stage development, and initial human application of neural interface systems (NISs) for humans with paralysis. NISs are emerging medical devices designed to allow persons with paralysis to operate assistive technologies or to reanimate muscles based upon a command signal that is obtained directly from the brain. Such systems require the development of sensors to detect brain signals, decoders to transform neural activity signals into a useful command, and an interface for the user. We review initial pilot trial results of an NIS that is based on an intracortical microelectrode sensor that derives control signals from the motor cortex. We review recent findings showing, first, that neurons engaged by movement intentions persist in motor cortex years after injury or disease to the motor system, and second, that signals derived from motor cortex can be used by persons with paralysis to operate a range of devices. We suggest that, with further development, this form of NIS holds promise as a useful new neurotechnology for those with limited motor function or communication. We also discuss the additional potential for neural sensors to be used in the diagnosis and management of various neurological conditions and as a new way to learn about human brain function.
Neural interface (NI) systems offer a novel approach to restore lost function and to diagnose or manage nervous system disorders. A NI system (NIS) couples the nervous system to a device that may either stimulate tissue or record neural activity, or perform both in a closed loop system. The NI sensor or stimulator can either be directly in contact with the neural tissue or remotely located. An NIS also typically includes a processor for signal conditioning or stimulus delivery and may include a user interface. Stimulation has the potential to provide missing sensory signals, or could be used to modulate neural function, while recordings can be used to assess the brain's state or intentions, or to provide commands that can be used as a control signal to restore or replace lost motor functions. Advances in neuroscience, engineering, computer science and mathematics have accelerated the development of a range of NISs that could access a variety of biological elements or physical devices. NI systems that stimulate electrically are already in wide clinical use. More than 50 000 people have received cochlear implants to restore hearing and more than 30 000 have received deep brain stimulators to treat the symptoms of movement disorders such as Parkinson's disease and dystonia. The NI portion of these systems consists of millimetre-scale surfaces in contact with the tissue that is being stimulated. On the other hand, NISs to read out neural activity are at a much earlier stage of development. NIs with sensing capabilities have the potential to be used as the input side of a system that could serve as a diagnostic aid in neurological and psychiatric disorders if they could detect or predict abnormal brain function. There is also considerable interest in using a sensory NI to provide a command signal source for a system that could restore communication or control abilities following paralysing injuries or limb loss.
Devices that transform a neurally based motor intention into a command signal that can operate physical systems have been called brain–computer interfaces (BCIs), brain machine interfaces (BMIs), and neuromotor prostheses (NMPs), among other names. No single term has yet been established in this emerging field. We use the term neural interface system here because all of these systems rely on successful sensing of neural activity to provide a command signal to control computers, machines, or any of a range of prosthetic devices that span from physical to biological elements. Thus, a sensing NIS is agnostic to whether the detected signal is used to control a wheelchair, a prosthetic limb, a computer, or biological elements including voluntary muscles or viscera such as bowel and bladder. Such devices would be useful to a large number of people with physical disability that interferes with mobility, communication or independence. Target populations include those with spinal cord injury, muscular dystrophy, stroke, cerebral palsy, amyotrophic lateral sclerosis (ALS) and other motor neuron diseases, limb loss, or any other condition where the limbs are largely unable to perform useful body actions or speech, but there remains cerebral function.
Types of NI sensors
Sensors that provide the source signal to an NIS vary in design and have been classified in a number of different ways. In one schema, NIs for sensing can be distinguished as being intracortical or extracortical. While NISs have been developed using either interface, differences between them may affect the nature and form of the NIS and the type of external device control that is attained. Intracortical and extracortical NIs are mainly distinguished by the nature of their contact with neural tissue, affording certain differences in the signals they can detect. We define an intracortical NI (iNI) as one that is in direct contact with cortical parenchyma, in very close proximity to neurons. By using different bandpass filtering such NIs are capable of recording multiple forms of electrical potentials, including single or multineuron spiking, as well as lower frequency electrical components called local field potentials (LFPs). A sensor able to record all of these features currently requires the use of microelectrodes which have micrometre scale recording surfaces. One particular advantage of an iNI is that it has the potential to record at higher spatial resolution and to obtain a greater variety of signals compared to an extracortical NI (eNI).
Action potentials are widely held to be the major form of information coding in the nervous system (Stevens & Zador, 1995). At least by current methodologies, recording the spiking (action potential) activity of individual neurons in vivo requires a fine-tipped microelectrode that is placed in close proximity to a neuron, and hence the requirement for an iNI. Most neurons generate an ∼1 ms long spike at rates in the range of < 1 Hz up to 100–300 Hz. Information related to spiking appears mainly to be carried in the spike rate, typically measured as the number of spikes within a defined interval (e.g. count in a 50 ms bin), although some other aspects of spiking such as relative timing (synchronization or coherence between neuron pairs) may also carry significant information. Spike rate in motor cortical areas modulates in conjunction with various aspects of movement, such as hand position, speed, direction, or force, and thus each of these parameters might be potentially extracted as control signals. The fact that spiking correlates with specific motor plans and intentions indicates that it should provide a rich source of movement information. If sufficient samples are acquired across multiple cells and/or multiple areas, these signals should be able, for example, to reveal many details of the bilateral hand actions used to control a computer mouse and keyboard.
Spikes alone do not provide all information about ongoing neural processing (consider early ‘spikeless’ processing in the retina, for example.) One form of slower intracerebral electrical signals are typically labelled local field potentials. LFPs arise from transmembrane currents driven by combinations of spiking, subthreshold synaptic currents, electrical interactions, and the biophysical properties of cell membranes; aspects of these signals can be recorded, in a filtered version, extracortically as well (using eNIs). The relative contribution of biological elements to the field recording is complex and dependent on the physical properties of recording devices and filtering. For example, the contribution of spikes to the LFP would increase when neurons fire highly time-locked spikes or when frequency components closer to 1 kHz are included in the measured signal. LFPs can spread various distances across tissue in ways that are related to their frequency, amplitude, synchrony or other features. LFPs appear to carry different types of information in different frequencies in the cortex (e.g. Donoghue et al. 1998; Andersen et al. 2004). These complex features have made it difficult to understand the full significance of LFPs and their relationship to spiking (see Bullock, 1997). However, both LFPs and spiking are potentially rich sources of control signals for a motor prosthesis. Although it is widely held that spiking patterns hold the greatest amount of information related to neural coding and computation (Stevens, 1995), there are now many renewed attempts to determine how much of the same or new information can be obtained from the LFP, because these signals are thought to be easier to record than spiking (Andersen et al. 2004). Hence they may possibly be a more pragmatic source of commands for neural prosthetic applications, whether or not they contain all of the information contained in population spiking. It is most likely that the full bandwidth of signals available in the cortex will ultimately provide valuable information sources for NISs.
Signals recorded by extracortical sensors
An eNI is a sensor placed outside the parenchyma that detects spatially and temporally summed electrical or field potentials generated by the same mechanisms that produce the intracortically recorded LFP. Examples of eNI signals available for a NIS are the volitionally regulated EEG, event-locked or evoked potentials (EPs), and the electrocorticogram (ECoG). Brain-derived electrical potentials from the scalp are typically called the EEG, while the signals recorded from the cortical surface are called the electrocortigram (ECoG). These signals are also related to the LFP, but are filtered and volume averaged to varying degrees compared to what can be recorded by an iNI. Differences relate to the type of electrode used, the local filtering of tissues and cerebrospinal fluid, neuronal orientation relative to the cortical surface, and other factors. For example, ECoGs contain higher frequency potentials and information content because they avoid the substantial lowpass filtering of extracortical tissues (including skull and skin). Thus, signals recorded using iNIs that include ‘LFPs’ in frequency bands up to those generated during spiking appear to provide even more information than eNIs. In one case, intracortically recorded potentials have been shown to carry about twice as much information about arm movement direction as those recorded from the cortical surface (Mehring et al. 2004).
In the case of EEG, a command signal is derived from a relation between a learned brain state and the modulation of frequency bands in the EEG. For example, there is a beta frequency suppression related to the onset of movement (Neuper & Pfurtscheller, 2001), as noted for the LFP. Different EEG frequency bands appear to carry different information and some can be controlled independently to provide a multidimensional control signal, as discussed by Wolpaw (2004, 2007). The ability to learn imagery which sufficiently modulates these EEG signals to promote brief epochs of cursor control is discussed elsewhere in this volume. EPs provide another way to obtain information from eNIs. The P300 wave is a scalp-measured response evoked to an ‘oddball’ or cognitively valent stimulus; it does not depend on learning a new association between the stimulus and EEG signal. These signals have been used effectively to identify screen location or letters of interest in eNISs (Krusienski et al. 2006; Sellers, 2006).
There are a variety of other differences in sensors that may affect their use in prosthesis systems. Sensors can vary in their ‘invasiveness’. An iNI is invasive by definition. This is necessary, at least at present, to detect spiking, which requires close proximity of a microelectrode to a neuron; eNis may be either non-invasive, by recording signals from the scalp, or invasive if they are placed at various levels below the skin, skull or meninges. The non-invasiveness of some eNIs make it comparatively easy for many healthy control subjects and patients to try NI systems or to adopt them without a surgical procedure, a clear benefit of this approach. However, scalp-based NISs must be applied to the scalp daily (by a caregiver), may cause discomfort, can be affected by head movement, and are conspicuous, while iNi and subcutaneous eNi systems can be made fully implantable and thus less susceptible to cosmetic or movement-related limitations. External eNI sensors need to be donned recurrently, while fully implanted systems could be always ‘on’, a critical advantage for users who desire immediate and 24 h access to a communication or mobility system. A potential further advantage of an iNI for prosthesis applications is that they provide signals that contain movement information ordinarily generated when movements are performed, without requiring the user to learn arbitrary associations or concentrate on stimuli. Further, iNIs are directly scalable, and thus potentially able to derive signals related to multiple arm and hand, or leg and foot actions, if sensors are placed in each of these brain representations, including those on each cerebral hemisphere. The mutual independence of volitionally controlled intracortical signals is well established; achieving this degree of independence for volitionally modulated EEG waves is a more challenging endeavor.
Development of an intracortical neural interface system for humans with paralysis
The BrainGate neural interface system, which is being developed by Cyberkinetics Neurotechnology Systems, Inc. (CKI), is a NIS based upon an intracortical neural interface. Below, we describe the concept of a NIS that employs and intracortical sensor, review progress that has been recently published related to pilot clinical trials, and discuss how neural interfaces may develop into useful devices to restore function, and diagnose and manage neurological disorders.
One can think of a motor NIS (or motor neuroprosthesis; Leuthardt, 2006) as a series of interfaces: the neural interface (for signal detection), a decoder interface between the brain and the device to be controlled (for signal interpretation) and a user interface (to make practical use of the control signal to perform actions). Fundamentally, the largest challenges for an iNIS at present are the neural interface and decoders, which must together be stable and reliable for any user interface to work. An intracortical sensor-based NIS requires a placement near a source of neural movement command signals and the creation of a long-lasting sensor to detect these signals. Once signals are obtained, specific methods are required to decode, interpret, or translate the recorded pattern of neural activity into a useful command signal. Utility of this command signal can be judged in terms of both its signal richness and its reliability. Decoding processes must be optimized to operate within the same time frame as actual neural processing to allow real time control. The command signal must also be able to operate useful devices, such as a prosthetic limb or a computer, each of which present distinct challenges. In addition, the signal could be used to command muscles, by activating a functional electrical stimulation (FES) system (Peckham & Knutson, 2005), to restore direct brain-controlled limb movement. Although not reviewed here, sensory feedback in a closed loop system is highly desirable and might be achieved by either intra- or extracortical interfaces.
Sources of neural movement command signals
Neural prosthesis work has heavily focused on attempting to restore upper limb function. More than a dozen distinct areas related to control of limb movement appear to be present in the primate brain (Kalaska, 1992; Burnod et al. 1999). Cortical regions spanning the frontal and parietal lobes are engaged by various types of movement preparation and action. Among these areas, the primary motor cortex (MI), located in the posterior part of the precentral gyrus, is generally believed to be the most closely coupled to the production of movement. This conclusion is supported by the confluence of electrical stimulation, lesion, recording, connectional, and architectonic studies. Separate MI regions control the leg, which is located most medially in MI, the arm, and the face, which is most laterally placed in MI. Thus, each may provide separate command signal sources for axial or limb musculature on each side of the body. The vast majority of neuroscientific data about motor cortex has been obtained from experimental investigation of arm regions. This bias is possibly based upon the fundamental significance of reach, grasp and manipulation actions to humans and, with the development of prosthesis systems, the clear and powerful utility of restoring arm and hard control.
Signal source and sensor
The goal for an iNI to record spikes places specific design constraints on the sensor. The only readily available method to detect spikes is a microelectrode; small recording surfaces placed near neurons can detect spiking extracellularly if it is less than a few hundred micrometres from the soma of the cell emitting the spike. Waveform shapes distinguish separate neurons that may be differentialted on a single electrode. Most neurophysiological studies of primate cortex employ single microelectrodes and therefore provide restricted amounts of information. A reliable estimate of movement features in real time requires an array of many microelectrodes to obtain simultaneously the individual spiking patterns of a population of neurons.
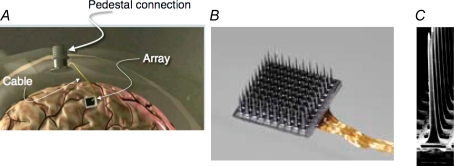
It has been challenging to develop reliable microelectrode arrays. The task is made especially difficult for brain interface applications where the goal is not only to record many cells, but to maintain recording, ideally, for decades. There are various forms of multielectrode arrays under development (Donoghue, 2002). A multielectrode array created by Richard Normann and colleagues (Rousche & Normann, 1998) has been developed further and is now being evaluated in a pilot clinical trial (Hochberg et al. 2006). The array consists of 100 tapered 1 or 1.5 mm long microelectrodes in a 10 × 10 grid, with electrodes spaced by 400 μm; the assembly forms a 4 × 4 mm base (Fig. 1). The entire array is carved from a block of boron-doped silicon, with each electrode isolated by a glass layer. The electrodes have a single recording site at their tip. The arrays have been evaluated with different forms of connectors and surgical methods in a longitudinal series of preclinical studies leading up to the pilot human trial (Maynard et al. 1999; Serruya et al. 2002, 2003; Suner et al. 2005). In its current design the implant consists of the array, internal microcabling, and a Ti pedestal. The pedestal is mounted on the skull and passes through the skin for connection to external electronics (Suner, 2005). The array is implanted by first making a small craniotomy and reflecting the dura. It is then tapped into place via a pneumatic inserter, so that the electrode tips lie within the pyramidal cell layers of the cortex and the common base rests on the cortical surface. Once inserted, the bone flap is replaced. The cabling from the array passes extracranially through the skull to the pedestal. The skin is then closed around the pedestal base, leaving only the top of the pedestal exposed. Although human data are currently being collected, a prospective study in three of the monkeys examined up to 513 days showed that multiple neurons could be recorded throughout the test period with a mean of about 60 neurons being detected at any time (Suner, 2005). Importantly, there was no indication that the recordings in this preclinical study declined as a result of tissue responses. Significant impedance increases, which would be expected with tissue reaction (Williams et al. 1999), did not occur, suggesting that these arrays have features suitable for human evaluation. Recent comparisons between single moveable microelectrodes and the array demonstrated substantial similarities in recording characteristics (Kelly et al. 2007). Data from the first participant using this same sensor have shown that many months of recording are feasible in humans as well (Hochberg et al. 2006).
Figure 1.
NIS implant and sensor A, parts of the implant include the array, skull mounted percutaneous pedestal, and a 96 wire cable that connects them. B, 10 × 10 array of electrodes, each separated by 400 μm. C, scanning electron micrograph one electrode showing its shape and pointed, platinum (Pt)-coated tip.
The multielectrode array used in the BrainGate NIS differs from others being developed in that the array is designed to float with the cortical surface. By contrast, assemblies of microwires, another form of iNI, are typically affixed to the skull's surface. This arrangement would be expected to produce damage as a result of relative motion between the skull and the brain in humans. Flexible microwire or thin film silicon electrodes, a new form of iNIs, can be difficult to insert and to stabilize because they have no support substrate at the cortical surface (Lee et al. 2005; Johnson et al. 2005). Multisite silicon thin film electrodes, which provide many recording sites per probe are being developed, and may become suitable for human clinical use (Vetter et al. 2004). Design flexibility in thin film electrodes not only could provide more sample sites per electrode, but the ability to incorporate electronics or other features directly on the electrode.
Decoding
Creation of a control signal from the spiking pattern of a population of neurons requires the ability to decode or translate that pattern. Such a decoding method must exploit fundamental properties of the neural code. We know from studies of MI neurons recorded one at a time in the pioneering work of Evarts, Humphrey, Fetz, Schmidt, Georgopoulos and their colleagues that neurons in the MI arm area carry information about hand kinematics and forces (Hatsopoulos, 2005). In addition, hand motion can be reasonably well reconstructed from the weighted average of neural firing, even when only a small sample of the neurons engaged in action is available (McIlwain, 2001). Surprisingly, as few as six MI neurons enable some prediction of the motion of the hand through space during reaching; this improves substantially as the number approaches around 100 (Serruya et al. 2003; Wessberg & Nicolelis, 2004), even though millions of neurons are engaged during such actions. Presumably, sampling more neurons provides information about a wider range of actions, but defining the full range of information that can be extracted from any one sample of neurons is an area of ongoing inquiry. The decoder effectively serves as a replacement for the missing parts of the nervous system. The sample of neurons obtained is a small subset of all the neurons engaged in even the simplest voluntary actions and this sparse sampling can result in a noisy decoded output signal. A decoder must exploit prior information, for example that the decoded hand movement should be smooth, to estimate reliable control parameters. In most cases researchers have attempted to replicate the action of the hand (its position or motion in space) from the recorded activity of a neural population.
Various methods have been used to decode spiking patterns into behavioural or motor correlates (Serruya, 2003). Algorithms are being evaluated that extract hand position, direction and speed or grasping actions using linear and non-linear classes of filters; state classifiers are also being used to decode neural activity (Maynard, 1999; Santhanam et al. 2006). These decoding algorithms must analyse large amounts of neural data and still be efficient enough to work in real time for prosthetics applications. Work in able-bodied monkeys showed that decoding can be implemented with sufficient speed and accuracy for a hand controlled mouse-driven cursor to be replaced with one that was driven by the decoded firing patterns from the MI arm area (see e.g. Serruya et al. 2002; Taylor et al. 2002). Extending these results to obtain higher dimensional control of devices such as robot arms and hands remains an open challenge. Decoding of LFPs also provides hand motion information that can augment, or potentially substitute for, aspects of spiking information (Scherberger, 2005); how spikes and LFPs compare as useful signals in neuroprosthetic applications for persons with motor disabilities is an active area of inquiry.
BrainGate pilot trial: translation to humans
An iNIS that allows neuronal ensemble activity to serve as a control signal has substantial potential benefit for those with limited movement abilities. An iNIS could connect one or multiple areas of cortex to external devices to restore communication, mobility, or other forms of functional independence. Preclinical data in able-bodied monkeys demonstrated the efficacy of the core elements of an NIS: the efficacy of the interface, the ability to decode the population information, decoding and use of this information (Maynard et al. 1999; Serruya et al. 2002; Suner et al. 2005; Paninski et al. 2004; Wu et al. 2006). The human version of the iNI based prosthesis system was granted an investigational device exemption by the US Food and Drug Administration (FDA) for two pilot clinical trials. The first permits up to five persons with spinal cord injury, brainstem stroke or muscular dystrophy to be enrolled; the second is a similar trial for up to five persons with motor neuron diseases, including ALS. Four participants with tetraplegia have been enrolled – two with high cervical spinal cord injury, one with a brainstem stroke and one with ALS. All were implanted with the BrainGate sensor in the MI arm area as located by anatomical criteria (Yousry et al. 1997). Initial data from two of the participants revealed several important findings essential for the development of a successful iNIS (Hochberg, 2006b). First, action potentials were readily recorded in MI years after spinal cord injury. This demonstrates that injury does not silence motor cortex spiking, despite inability to move the limbs. Second, immediate modulation of MI neurons was possible merely by attempting or imagining action. Thus, neuronal spiking in MI can still be activated by movement intentions years after injury. Further, limb movement is not required for this neural modulation to occur. In addition, LFPs were also simultaneously recorded and these signals appeared to contain movement intention related information (Hochberg et al. 2006b). These findings, which are essential to the development of iNISs, had not been previously demonstrated for persons with spinal cord injury.
It was also found that MI neurons were engaged by a diverse set of intended actions that include the hand and arm. Different neurons had distinctive properties so that some correlated with imagined or intended opening or closing of the hand, while others nearby became active with intended reaching movements of the arm. Examples of single neurons that were active both with actual shoulder movement and with imagined arm actions were also identified. It was possible to create decoders that translated intended actions into a command signal sufficiently quickly to be used in real time. This signal was used to demonstrate the ability to operate computer software, assistive technologies, and robotic devices (see Hochberg, 2006b for video demonstrations). No time was required for participants to gain neural control of these devices, except for that necessary to create the decoding filter that related MI neural activity patterns to the desired action (approximately 20 min). That is, motor learning is not required to gain initial control, presumably because control signals were driven by the brain's natural neuronal signals for arm control. Whether learning by the participant can play a role in further changes in performance has not yet been explored.
These preliminary results provide initial proof of concept that a neuronally based control system is feasible: signals can be detected, decoded and used for real time operation of computer software, assistive technologies, and other devices. The presence of LFP signals not unlike those seen in able-bodied monkeys suggests that iNIs will provide a rich signal source that ranges from LFPs to spikes. However, additional evaluation is necessary to show that reliable performance of the iNIS can be obtained in multiple participants. Our more recent preliminary observations in one participant with brainstem stroke and one participant with ALS suggest that the findings in spinal cord injury may generalize across a broad population of persons with tetraplegia arising from various causes (Donoghue et al. 2006; Hochberg et al. 2006a). Day-to-day differences in the number of neurons (and presumably the composition of neurons) will present additional challenges to providing a consistently reliable command signal for an iNIS which will be based on a small sample of the neurons that ordinarily generate movement. Such challenges are also substantial for eNI based systems: there are considerable instabilities related to electrode placement and attachment, individual variability, artifacts, and brain state changes that will need to be overcome (Krause et al. 2001; McFarland et al. 2005).
Next-step developments for an NIS
A practical iNIS to provide assistive actions would require advances beyond the current BrainGate pilot system. First, a fully implantable NIS would eliminate tethering of the patient that results from the physical connection of the percutaneous pedestal to the signal processor cart. A fully implantable system also reduces the concern of infection at the pedestal site. Second, automated set up and operation is needed to eliminate the need for a skilled technician. Third, miniaturization is necessary to allow greater mobility. These latter two advances are necessary for eNIS as well. Additional improvements in the neural interface itself may also improve the reliability, stability and richness of neural signals available for control. Steps are already being taken towards many of these goals. A number of groups are working on fully implantable, active sensors, which require integrated electronics, self-powering and high bandwidth signal transmission. Nurmikko and colleagues are developing iNIs with signal amplification mounted on the array itself and signal processing contained in a subcutaneous microscale platform that can transmit wirelessly across the skin (Song et al. 2005). Another novel advance of this iNI is its implantable fibre optic powering and signal transmission. Light delivered by the optical fibre can be converted to power and the same fibre can convey all of the high bandwidth signal generated by a 100+ electrode array.
The ongoing human trial has also provided a rare opportunity to observe human neural function at a new level and to create much more powerful decoders. The stability and smoothness of the control signal has been improved using Kalman filtering approaches (Wu et al. 2006) and the ability to stop the cursor and click at desired locations has been added, although the long-term reliability of this control remains to be validated (Kim, 2006). This advance would approximate the functions of a mouse input device. Developments in modern electronics make it possible to reduce the current bulky signal processing hardware to very compact and portable components. These initial successes for the iNIS, and the potential promise for those who have limited ability to move, seem to further pursuing these engineering improvements.
Beyond connecting to a wide variety of physical devices ranging from computers to robots, this same neural interface system could also be used to reanimate paralysed limbs. Functional electrical stimulation (FES) systems can activate paralysed but otherwise normal muscles (for example, in people with cervical spinal cord injury), through implanted wires that deliver electrical currents (Peckham & Knutson, 2005). Current FES systems typically use external switches or sensors on muscles that remain under voluntary control. Connecting a brain interface system to an FES system could create a physical bridge from the brain's motor areas to the muscles, replacing an absent biological path. Research to develop a brain to muscle connection is already underway in collaboration with researchers at the Cleveland FES centre through a contract with the National Center for Medical Rehabilitation Research.
Future application of neural interfaces
The rapid expansion of efforts in the area of intracortical neural interfaces and the proof of concept demonstration of their feasibility indicates that this technology is likely to expand and develop into an integral part of the management of neurological disorders, just as cardiac pacemakers grew from a bold new concept that began with a cart of external stimulators and oscilloscopes into a small implantable disk with on-board electronics that is now readily accepted as a safe way to address cardiac arrhythmias (Kirk, 2001). This is not to say that there will not be setbacks in NIS development. This is very complex technology that requires a stable interface between man-made components and a dynamic biological system that responds over time in ways that we do not fully understand. Physical devices break and materials can degrade, despite efforts to prevent failure. However, there is a growing body of knowledge about the biocompatibility and biostability of various materials in the body, from artificial joints, cardiac and neural pacemakers (i.e. deep brain stimulators), and cochlear implants, that is likely to lead to continuous improvement of the biological–device interface.
NI systems based on intracranial and extracranial sensors share many goals, challenges and benefits. Both could provide utility to those with limited motor function. Advances in signal processing and human user interfaces will aid in the development of both forms of NIS. In addition, the iNI can potentially also provide a new form of high resolution sensor to report abnormal spiking or LFP patterns in diseased or damaged brains, with many potential clinical applications. Abnormal neuronal ensemble activity is at the base of many neurological and psychiatric disorders, but there is actually very little neuroscientific evidence that describes the nature of that abnormal activity. Epilepsy presents a potential use for NISs, where we know that a transition from usual brain electrical patterns to pathologically synchronous discharges leads to seizures. We could envision a neural sensor near an epileptic focus that would be a sensitive measure of the transition from the ‘normal’ to the abnormal state. Such a signal, if identified early enough could provide a valuable warning of an impending seizure. Of course, we do not know how to detect the signatures of these abnormal electrical events at present. However, if such events could be reliably detected it might further be possible to create a device that interferes with the transition to abnormal activity patterns through either electrical or pharmacological interventions. An initial effort at such a closed loop system using an eNIS is now in clinical trials (Morrell, 2006). These ideas require considerable additional evaluation, but the exquisite sensitivity of a multielectrode iNI is a promising tool to measure neural events at very high resolution that extends beyond anything now available.
Acknowledgments
The authors would like to thank the following for ongoing and past research support: the US Department of Veterans Affairs, NINDS Javits Award, Office of Naval Research, DARPA, National Institute of Child Health and Development, The Keck Foundation and Cyberkinetics Neurotechnology Systems, Inc. The authors would also like to thank Beth Travers for her help in preparing this manuscript.
Conflict of Interest
J.D. is also a director, paid consultant and shareholder of Cyberkinetics Neurotechnology Systems, Inc. Products in clinical trials that are in commercial development by Cyberkinetics are discussed in this review. L.R.H. is a principal investigator in the BrainGate IDE clinical trials described in this article. He receives clinical trial support from Cyberkinetics Neurotechnology Systems, Inc.
References
- Andersen RA, Musallam S, Pesaran B. Selecting the signals for a brain-machine interface. Curr Opin Neurobiol. 2004;14:720–726. doi: 10.1016/j.conb.2004.10.005. Review. [DOI] [PubMed] [Google Scholar]
- Bullock TH. Signals and signs in the nervous system: the dynamic anatomy of electrical activity is probably information-rich. Proc Natl Acad Sci U S A. 1997;94:1–6. doi: 10.1073/pnas.94.1.1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnod Y, Baraduc P, Battaglia-Mayer A, Guigon E, Koechlin E, Ferraina S, Lacquaniti F, Caminiti R. Parieto frontal coding of reaching: an integrated framework. Exp Brain Res. 1999;129:325–346. doi: 10.1007/s002210050902. Review. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Dougherty DD, Rauch SL. Functional imaging of mood and anxiety disorders. J Neuroimaging. 2006;16:1–10. doi: 10.1177/1051228405001474. Review. [DOI] [PubMed] [Google Scholar]
- Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nat Neurosci. 2002;5(Suppl.):1085–1088. doi: 10.1038/nn947. Review. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Friehs GM, Caplan AH, Stein J, Mukand JA, Chen D, Penn RD, Hochberg LR. BrainGate neuromotor prosthesis: first experience by a person with brainstem stroke. Soc Neurosci Abs. 2006:256.10. [Google Scholar]
- Donoghue JP, Sanes JN, Hatsopoulos NG, Gaal G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J Neurophysiol. 1998;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos NG. Encoding in the motor cortex. Was Evarts right after all? Focus on ‘motorcortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks’. J Neurophysiol. 2005;94:2261–2262. doi: 10.1152/jn.00533.2005. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Friehs GM, Brown RH, Cudkowicz ME, Krivickas LS, Donoghue JP. Voluntary modulation of motor cortical activity by a person with amyotrophic lateral sclerosis: initial braingate experience. Soc Neurosci Abs. 2006a:13.1. [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006b;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Otto KJ, Kipke DR. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. IEEE Trans Neural Syst Rehabil Eng. 2005;13:160–165. doi: 10.1109/TNSRE.2005.847373. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Cerebral cortical mechanisms of reaching movements. Science. 2002;255:1517–1523. doi: 10.1126/science.1549781. Review. [DOI] [PubMed] [Google Scholar]
- Kelly RC, Smith MA, Samonds JM, Kohn A, Bonds AB, Movshon JA, Lee TS. Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. J Neurosci. 2007;27:261–264. doi: 10.1523/JNEUROSCI.4906-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Simeral JD, et al. A comparison of decoding models for imagined motion from human motor cortex. Soc Neurosci Abs. 2006:256.11. [Google Scholar]
- Kirk J. Machines in Our Hearts: The Cardiac Pacemaker, the Implantable Defibrillator, and American Health Care. Baltimore, London: Johns Hopkins University Press; 2001. p. 387. [Google Scholar]
- Krause CM, Sillanmaki L, Haggqvist A, Heino R. Test-retest consistency of the event-related desynchronization/event-related synchronization of the 4–6, 6–8, 8–10 and 10–12 Hz frequency bands during a memory task. Clin Neurophysiol. 2001;112:750–757. doi: 10.1016/s1388-2457(01)00501-6. [DOI] [PubMed] [Google Scholar]
- Krusienski DJ, Sellers EW, Cabestaing F, Bayoudh S, McFarland DJ, Vaughan TM, Wolpaw JR. A comparison of classification techniques for the P300 speller. J Neural Eng. 2006;3:299–305. doi: 10.1088/1741-2560/3/4/007. [DOI] [PubMed] [Google Scholar]
- Lee H, Bellamkonda RV, Sun W, Levenston ME. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J Neural Eng. 2005;2:81–89. doi: 10.1088/1741-2560/2/4/003. [DOI] [PubMed] [Google Scholar]
- Leuthardt EC, Schalk G, Moran D, Ojemann JG. The emerging world of motor neuroprosthetics: a neurosurgical perspective. Neurosurgery. 2006;59:1–14. doi: 10.1227/01.NEU.0000221506.06947.AC. discussion 1–14. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Sarnacki WA, Vaughan TM, Wolpaw JR. Brain-computer interface (BCI) operation: signal and noise during early training sessions. Clin Neurophysiol. 2005;116:56–62. doi: 10.1016/j.clinph.2004.07.004. [DOI] [PubMed] [Google Scholar]
- McIlwain JT. Population coding: a historical sketch. Prog Brain Res. 2001;130:3–7. doi: 10.1016/s0079-6123(01)30002-x. [DOI] [PubMed] [Google Scholar]
- Maynard EM, Hatsopoulos NG, Ojakangas CL, Acuna BD, Sanes JN, Normann RA, Donoghue JP. Neuronal interactions improve cortical population coding of movement direction. J Neurosci. 1999;19:8083–8093. doi: 10.1523/JNEUROSCI.19-18-08083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehring C, Nawrot MP, de Oliveira SC, Vaadia E, Schulze-Bonhage A, Aertsen A, Ball T. Comparing information about arm movement direction in single channels of local and epicortical field potentials from monkey and human motor cortex. J Physiol (Paris) 2004;98:498–506. doi: 10.1016/j.jphysparis.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. Review. [DOI] [PubMed] [Google Scholar]
- Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–360. doi: 10.1146/annurev.bioeng.6.040803.140103. Review. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Meth. 1998;82:1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain–computer interface. Nature. 2006;442:195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- Sellers EW, Krusienski DJ, McFarland DJ, Vaughan TM, Wolpaw JR. A P300 event-related potential brain–computer interface (BCI): The effects of matrix size and inter stimulus interval on performance. Biol Psychol. 2006;73:242–252. doi: 10.1016/j.biopsycho.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Serruya MD, Donoghue JP. Design principles for intracortical neuromotor prosthetics. In: Horch KW, Dhillon GS, editors. Neuroprosthetics: Theory and Practice. Imperial College Press; 2004. [Google Scholar]
- Serruya M, Hatsopoulos N, Fellows M, Paninski L, Donoghue J. Robustness of neuroprosthetic decoding algorithms. Biol Cybern. 2003;88:219–228. doi: 10.1007/s00422-002-0374-6. [DOI] [PubMed] [Google Scholar]
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- Song YK, Patterson WR, Bull CW, Beals J, Hwang N, Deangelis AP, Lay C, McKay JL, Nurmikko AV, Fellows MR, Simeral JD, Donoghue JP, Connors BW. Development of a chipscale integrated microelectrode/microelectronic device for brain implantable neuroengineering applications. IEEE Trans Neural Syst Rehabil Eng. 2005;13:220–226. doi: 10.1109/TNSRE.2005.848337. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Zador A. Neural coding: The enigma of the brain. Curr Biol. 1995;5:1370–1371. doi: 10.1016/s0960-9822(95)00273-9. Review. [DOI] [PubMed] [Google Scholar]
- Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Neural Syst Rehabil Eng. 2005;13:524–541. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- Vetter RJ, Williams JC, Hetke JF, Nunamaker EA, Kipke DR. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans Biomed Eng. 2004;51:896–904. doi: 10.1109/TBME.2004.826680. [DOI] [PubMed] [Google Scholar]
- Wessberg J, Nicolelis MA. Optimizing a linear algorithm for real-time robotic control using chronic cortical ensemble recordings in monkeys. J Cogn Neurosci. 2004;16:1022–1035. doi: 10.1162/0898929041502652. [DOI] [PubMed] [Google Scholar]
- Williams JC, Rennaker RL, Kipke DR. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res Brain Res Protoc. 1999;4:303–313. doi: 10.1016/s1385-299x(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Brain–computer interfaces as new brain output pathways. J Physiol. 2007;579:613–619. doi: 10.1113/jphysiol.2006.125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Gao Y, Bienenstock E, Donoghue JP, Black MJ. Bayesian population decoding of motor cortical activity using a Kalman filter. Neural Comput. 2006;18:80–118. doi: 10.1162/089976606774841585. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]