Abstract
Objective
To estimate the incremental cost-effectiveness of improving diabetes care with the Health Disparities Collaborative (HDC), a national collaborative quality improvement (QI) program conducted in community health centers (HCs).
Data Sources/Study Setting
Data regarding the impact of the Diabetes HDC program came from a serial cross-sectional follow-up study (1998, 2000, 2002) of the program in 17 Midwestern HCs. Data inputs for the simulation model of diabetes came from the latest clinical trials and epidemiological studies.
Study Design
We conducted a societal cost-effectiveness analysis, incorporating data from QI program evaluation into a Monte Carlo simulation model of diabetes.
Data Collection/Extraction Methods
Data on diabetes care processes and risk factor levels were extracted from medical charts of randomly selected patients.
Principal Findings
From 1998 to 2002, multiple processes of care (e.g., glycosylated hemoglobin testing [HbA1C] [71→92 percent] and ACE inhibitor prescribing [33→55 percent]) and risk factor levels (e.g., 1998 mean HbA1C 8.53 percent, mean difference 0.45 percent [95 percent confidence intervals −0.72, −0.17]) improved significantly. With these improvements, the HDC was estimated to reduce the lifetime incidence of blindness (17→15 percent), end-stage renal disease (18→15 percent), and coronary artery disease (28→24 percent). The average improvement in quality-adjusted life year (QALY) was 0.35 and the incremental cost-effectiveness ratio was $33,386/QALY.
Conclusions
During the first 4 years of the HDC, multiple improvements in diabetes care were observed. If these improvements are maintained or enhanced over the lifetime of patients, the HDC program will be cost-effective for society based on traditionally accepted thresholds.
Keywords: Quality improvement, cost-effectiveness analysis, safety net providers
Deficiencies in the quality of care of chronic conditions such as diabetes are well recognized as a major public health problem (Committee on Quality of Health Care in America 2001; McGlynn et al. 2003). Concerns regarding these deficiencies have led to significant public investment in quality improvement (QI) programs and related disease management programs (Fleming et al. 2001; Casalino 2005). One of the longest-running national QI programs is the Health Disparities Collaboratives (HDC) initiated in 1998 by the Health Resources and Services Administration's Bureau of Primary Health Care (HRSA's BPHC) in federally qualified community health centers (HCs). This program began with a focus on improving the quality of diabetes care. Short-term studies of the Diabetes HDC have shown that the program improved processes of diabetes care after 1 year of implementation (Chin et al. 2004). Long-term follow-up of the Diabetes HDC over 4 years has more recently demonstrated improvements in processes of care such as use of angiotensin-converting enzyme inhibitors and aspirin as well as intermediary outcomes including glucose and lipid control (Chin et al. forthcoming).
Although many QI programs and disease management programs have been found to improve chronic care (Chodosh et al. 2005), the value of such programs has been rarely assessed with cost-effectiveness analyses (CEA). CEA is used to illustrate the economic value of new treatments, tests, and programs and these results provide policy makers with a common metric for comparing diverse technologies and programs. CEA of QI programs would allow comparison of QI programs with other health care technologies and inform resource allocation decisions. In addition, CEA results can help identify the components of chronic care that are likely to produce the largest health benefits for patients. These analyses can help those implementing QI programs decide where to target future improvements.
We set out to evaluate the cost-effectiveness of the Diabetes HDC program combining data from a study of the program's effectiveness with a comprehensive simulation model of diabetes complications.
METHODS
The Diabetes HDC
The HDC trains HC staff to utilize the tools of rapid QI (Wagner et al. 2001) and chronic disease management (Von Korff et al. 1997; Bodenheimer, Wagner, and Grumbach 2002a, Bodenheimer, Wagner, and Grumbach 2002b). HC staff members acquire skills and share best practices at learning sessions and develop programs tailored to their centers. The typical Diabetes HDC program has included use of diabetes flow sheets in medical charts to remind physicians of processes of care to be completed and patient management software provided by HRSA's BPHC to track patients over time. Beyond these features, the actual type and number of QI or disease management methods that centers have employed has varied across centers, as encouraged by rapid QI. QI program components have included community-health center collaboration, patient-self-management support, and delivery system redesign (e.g., group visits). More detail about the HDC intervention is described in a prior publication (Chin et al. 2004). We evaluated the impact of the Diabetes HDC for 17 Midwestern HCs enrolled in the standard intensity arm of a randomized controlled trial comparing a standard and high-intensity Diabetes HDC program. We utilized the findings for the standard program because it was the program that was deployed nationally.
Data Sources and HDC Effect
Data on the quality of diabetes care were collected in serial cross sections in 1998, 2000, and 2002. For each year, centers were asked to identify nonpregnant adult patients (18–75 years of age) with diabetes and perform chart review on 80 randomly selected patients. HC staff abstracted information on demographics, process of care, and laboratory values from medical charts.
Both crude and adjusted analyses showed that diabetes care improved across each time interval (Chin et al. forthcoming). For this analysis, we used processes of care and clinical outcomes from 1998 to 2002, the longest follow-up period of any evaluation of the HDC. Baseline (1998) rates of processes of care were estimated using mixed logistic regression (Zeger, Liang, and Albert 2004) of outcome on intercept, with HC as a random effect. Changes in rates of performance from 1998 to 2002 were estimated using three-level hierarchical logistic regression (Raudenbush et al. 2001; Raudenbush and Bryk 2002) of outcome on year, with HC and year within HC as random effects, controlling for strata (e.g., region) and patient and HC characteristics. Candidate covariates were urban versus rural location, age, sex, race, insulin treatment, comorbidities, and complications of diabetes. The structure of the models reflects the cross-sectional sampling of patients nested within HCs over time. Baseline rates and changes in values of risk factor levels were obtained using analogous mixed and hierarchical linear regression models.
Cost-Effectiveness Analysis
We adopted the societal perspective for this analysis. We assumed that the population of interest was HC patients with demographic and clinical characteristics of those patients seen in 1998. For this population, we compared the projected complications, quality of life, and costs of care in two hypothetical scenarios for the base case. The first scenario was life with the risk factor levels and processes of care as they were in 1998 at the start of Diabetes HDC. The second scenario was life with the risk factors levels and processes of care as they were in 2002 with the Diabetes HDC in place. These risk factor levels and processes of care reflect the adjusted improvements in care from 1998 to 2002. We will refer to these scenarios as the pre-HDC and HDC scenarios.
Diabetes Complication Simulation Model
We incorporated data from the program evaluation into a previously validated model of the cost-effectiveness of intensive glucose control for type 2 diabetes (courtesy R. Eastman) (Eastman, Javitt, Herman, Dasbach, Copley-Merriman et al. 1997; Eastman, Javitt, Herman, Dasbach, Zbrozek et al. 1997). This Markov Monte Carlo simulation model is framed by simultaneous progression of disease through microvascular complications, cardiovascular complications, and mortality. Within a 1-year cycle length, patients move from one disease state to another or stay in the current disease state until death or age 95. For each distinct model setting, the model is run 10,000 iterations. We used Microsoft Excel 2000 (Microsoft, Seattle, WA) and @Risk 4.5.4 for Windows (Palisades Inc., Newfield, NY) to conduct the simulations.
We revised the original model for the purposes of this analysis. Please see the Technical Appendix for detailed description of model inputs. We updated the transition probabilities for microvascular complications with data from the U.K. Prospective Diabetes Study (UKPDS), conducted in type 2 diabetes patients (DCCT Research Group 1993, DCCT Research Group, 1995; UKPDS Group 1998). For the nephropathy module, we used observations from the control arm of UKPDS for the probability of developing microalbuminuria and proteinuria for the pre-HDC scenario. For these complications we fitted data from UKPDS to the survival function form used in the original NIH model, based on Diabetes Control and Complication Trial data (DCCT Research Group 1996). This functional form allowed us to relate the probability of a complication to both duration of diabetes as well as glycosylated hemoglobin levels (HbA1C). To determine the transition probabilities for the HDC scenario and lower sugar levels, we used UKPDS again, using the form 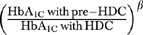 with the β coefficient derived from the comparison of the overall results of UKPDS for individual complications. This is the method utilized in the CDC Diabetes Cost-Effectiveness Model (CDC Group 2002). This approach was also applied to the neuropathy and retinopathy modules. For the neuropathy module, we added a new health state of foot ulcer (Young et al. 1994; Gregg et al. 2004) and incorporated an international scoring system for diabetic foot disease (Peters and Lavery 2001). For the retinopathy module, progression beyond background retinopathy was updated with data from the Liverpool Diabetic Eye study (Younis et al. 2003).
with the β coefficient derived from the comparison of the overall results of UKPDS for individual complications. This is the method utilized in the CDC Diabetes Cost-Effectiveness Model (CDC Group 2002). This approach was also applied to the neuropathy and retinopathy modules. For the neuropathy module, we added a new health state of foot ulcer (Young et al. 1994; Gregg et al. 2004) and incorporated an international scoring system for diabetic foot disease (Peters and Lavery 2001). For the retinopathy module, progression beyond background retinopathy was updated with data from the Liverpool Diabetic Eye study (Younis et al. 2003).
A new stroke module was added to the model with the probability of stroke based on a Framingham prediction model (Wolf et al. 1991). The module for coronary heart disease was revised with the inclusion of coronary heart disease Framingham prediction model (Wilson et al. 1998). We also adopted changes made by Eastman and colleagues after the original publication that included subsequent cardiovascular event probabilities and model structure based on the Harvard Coronary Heart Disease Policy Model (Hunink et al. 1997). The race- and gender-specific background mortality rates were updated with U.S. life table statistics from 1999 (Andersen and DeTurk 2002).
Intervention Effects
For the base case, we conservatively assumed that intensive glucose control had a beneficial effect on the probability of microvascular events but no effect on cardiovascular events in patients with type 2 diabetes because clinical trials of glucose control have yet to show a definitive cardiovascular benefit (UKPDS Group 1998; Huang, Meigs, and Singer 2001; DCCT Research Group 2005). However, observational studies demonstrate an association between glucose levels and cardiovascular events (Stevens et al. 2001). We utilized the Framingham prediction models of coronary heart disease events for the base case and evaluated the impact of this assumption by using the UKPDS risk prediction model for coronary heart disease in sensitivity analyses (Stevens et al. 2001). The benefits of ACE inhibitors were based on the findings from the Heart Outcomes Prevention Evaluation (HOPE) Study (HOPE Study Investigators 1999; Rosen et al. 2005). Aspirin was assumed to reduce the probability of coronary heart disease (Hansson et al. 1998) but increase the probability of gastrointestinal bleed (American Diabetes Association 2004). We assumed that the joint effect of aspirin and an ACE inhibitor on cardiovascular effects was multiplicative. We did not assume any clinical benefit of processes of care that have not been found to independently improve outcomes (Singh, Armstrong, and Lipsky 2005).
Outcome Values
For the base case, we used utilities for complications found in prior CEA (Technical Appendix) (Dasbach, Fryback, and Thornbury 1992; DCCT Research Group 1996; Tennval and Apelqvist 2001; CDC Group 2002; Redekop et al. 2004; Rosen et al. 2005). We assumed no disutility of life with intensified treatments in the base case but evaluated this in sensitivity analyses (Huang et al. 2006). When multiple health states occurred, we used the minimum health state method (Naglie et al. 1997).
Costs
We estimated the program costs of the HDC (Year 1 $712/patient, Year 2 $600/patient, Year 3 $472/patient, and Year 4 $378/patient) based on case studies of selected HCs in conjunction with data obtained from HRSA's BPHC (Huang et al. in press) (Technical Appendix). We assumed that the costs in Year 4 were required for the remainder of the patient's life to sustain HDC benefits.
The costs of diabetes care were based on observed utilization of services with a few exceptions. The frequency of physician office visits was not collected, so we assumed that the average number of office visits was 3 under pre-HDC care and 4 under HDC care based on the protocol of a trial of comprehensive diabetes care (Gaede et al. 2003). The general categories of glucose-lowering therapies (insulin, insulin and oral medications, oral medications, diet) were documented, but without more details regarding the use of specific agents. We assumed that the distribution of use of different oral agents was the same as that observed in national studies of diabetes care (Cohen et al. 2003). The dosing of oral medications was assumed to be doubled in the HDC scenario. The dosing of insulin was assumed to be 65 units/day under both scenarios. The actual utilization of statins was not collected, so we assumed that the observed utilization of statins in the nation in 1998 (Grant et al. 2004) doubled as a result of the HDC. We estimated the costs of drugs based on the average type and frequency of drug prescriptions, dosage of medications, and wholesale drug prices (Red Book 2004). The costs of diabetes complications were common to both scenarios. We updated the yearly costs of all microvascular (Boulware et al. 2003; Gordois et al. 2003) and cardiovascular complications (Cooper et al. 1999; Cundiff 2002; Brandle et al. 2003; Nichol et al. 2003; Hlatky et al. 2004; Van Alem et al. 2004; Rosen et al. 2005) in the original model.
Effectiveness, Costs, and Cost-Effectiveness
We determined the expected lifetime probability of each of the major complications along with differences in costs, life years, and quality-adjusted life years (QALYs). The main outcome of interest is the incremental cost-effectiveness ratio (ICER) comparing the pre-HDC and HDC scenarios. All costs are expressed in 2004 dollars and we used a discount rate of 3 percent for both costs and outcomes. A discount rate of 5 percent was evaluated in sensitivity analysis.
Sensitivity Analyses
We separately assessed the impact and cost-effectiveness of improvements in individual components of care. For these analyses, we assumed that the entire program cost was devoted to improving each individual component of care. In one-way sensitivity analyses, we evaluated a range of values for probabilities, utilization rates, or costs that had significant uncertainty. The impact of different program costs assumptions was of particular interest. We also evaluated the impact of possible secular trends by systematically altering the proportion of change in each component of care attributable to baseline secular trends. We separately evaluated the effect of future costs, including medical costs for unrelated illnesses, nonmedical costs, and future earnings, on the overall cost-effectiveness results (Meltzer et al. 2000). For the future cost analysis we used the national averages for consumption and earnings for given age groups. Because average consumption and earnings for patients in the HC population might differ from those in the population as a whole, we also performed sensitivity analysis around these estimates.
RESULTS
Baseline Patient Characteristics
In 1998, the mean age of patients was approximately 55 years (Table 1). The majority of individuals were nonwhite and about two-thirds were female.
Table 1.
Health Center and Baseline (1998) Patient Characteristics
| Age (mean, standard deviation) | 54.5 (13.6) |
| Female (%) | 67 |
| Race/ethnicity (%) | |
| American Indian/Alaska native | 1 |
| Asian/Pacific Islander | 1 |
| Black (non-Hispanic) | 29 |
| Hispanic/Latino (all races) | 32 |
| White (non-Hispanic) | 37 |
| Other | 1.5 |
| Comorbidities/complications (%) | |
| Hypertension | 59 |
| Myocardial infarction | 4 |
| Retinopathy | 7 |
| Neuropathy | 11 |
| Peripheral vascular disease | 5 |
| Renal failure | 2 |
| Proteinuria | 7 |
Processes of Care and Outcomes
Multiple components of care improved from 1998 to 2002 during the implementation of the diabetes HDC (Table 2). Among screening tests, annual glycosylated hemoglobin testing (HbA1C) (71→92 percent), lipid testing (52→70 percent), microalbumin assessment (15→44 percent), eye exams (25→44 percent) all improved significantly. Similarly, ACE inhibitor (33→55 percent), and aspirin prescribing (22→45 percent), also increased significantly. Mean HbA1C (8.53 percent in 1998, mean difference −0.45 percent [95 percent confidence intervals (CI) −0.72, −0.17]) and cholesterol levels (total cholesterol 212 mg/dL in 1998, mean difference −13.5 [95 percent CI −20.4, −6.7]) decreased significantly, but blood pressure levels, which were relatively low at baseline (133/79 mmHg in 1998), did not.
Table 2.
Processes of Care and Outcome Measures by Year
| Process of Care | Percentage in 1998* | Percentage in 2002† |
|---|---|---|
| At least one HbA1c | 71 | 92 |
| Lipid assessment | 52 | 70 |
| Microalbumin assessment | 15 | 44 |
| Eye exam or referral | 25 | 44 |
| Foot exam or referral | 31 | 63 |
| ACE inhibitor | 33 | 55 |
| Aspirin | 22 | 45 |
| Insulin alone (observed) | 17 | 12 |
| Insulin and oral therapy (observed) | 12 | 16 |
| Oral glucose-lowering therapy alone (observed) | 61 | 64 |
| Outcome Measure | Mean (Standard Deviation), 1998 | Mean Difference between 1998 and 2002 (95% Confidence Intervals)‡ |
| HbA1c (%) | 8.53 (2.14) | −0.45 (−0.72, −0.17) |
| Total cholesterol (mg/dl) | 212 (55) | −13.5 (−20.4, −6.7) |
| LDL cholesterol (mg/dl) | 127 (51) | −19.7 (25.8, −13.6) |
| HDL cholesterol (mg/dl) | 47 (36) | −6.4 (−16.9, 4.0) |
| Systolic blood pressure (mmHg) | 133 (19) | −1.81 (−4.03, 0.40) |
| Diastolic blood pressure (mmHg) | 79 (11) | −0.79 (−1.98, 0.40) |
Estimated rate from univariate mixed logistic regression that incorporates correlation due to clustering of patients within HCs.
Estimated from multivariate mixed logistic regression that adjusts for covariates and incorporates correlation due to clustering of patients within HCs.
Three-level hierarchical regression of patient-level outcomes on year, with health center and year within health center as random effects, controlling for region (Midwest, West Central) and Diabetes Collaborative (I, II). Candidate covariates: urban versus rural location, age, sex, race, insulin treatment, comorbidities, and complications of diabetes (hypertension, myocardial infarction, peripheral vascular disease, retinopathy, neuropathy, and renal failure).
HCs, health centers
Base Case Cost-Effectiveness Results
In the base case, the HDC was found to reduce the expected lifetime incidence of intermediate and end-stage complications (Table 3). For example, for diabetic retinopathy, background retinopathy (56→54 percent), proliferative diabetic retinopathy (32→31 percent), and blindness (17→15 percent) were all reduced. Similar benefits were observed for the lifetime incidence of end-stage renal disease (18→15 percent) and coronary artery disease (28→24 percent). The probability of amputation and stroke did not change. The average improvement in QALYs was 0.35. The ICER of the base case was $33,386/QALY.
Table 3.
Base Case Results
| Lifetime Probability of | Pre-Health Disparities Collaborative Scenario (1998) | Health Disparities Collaborative Scenario (2002) |
|---|---|---|
| Background diabetic retinopathy (%) | 56 | 54 |
| Macular edema (%) | 30 | 30 |
| Proliferative diabetic retinopathy (%) | 32 | 31 |
| Blindness (%) | 17 | 15 |
| Microalbuminuria (%) | 63 | 56 |
| Proteinuria (%) | 36 | 30 |
| End-stage renal disease (%) | 18 | 15 |
| Peripheral neuropathy (%) | 61 | 58 |
| Foot ulcers (%) | 30 | 29 |
| Amputation (%) | 20 | 20 |
| Coronary heart disease (%) | 28 | 24 |
| Stroke (%) | 20 | 20 |
| Life years (mean) | 11.68 | 11.98 |
| Quality-adjusted life years (mean) | 10.58 | 10.93 |
| Life-time costs (mean $) | 90,085 | 101,770 |
Sensitivity Analysis
The individual improvements in care produced variable effects on measures of morbidity and mortality (Table 4). Lowering glucose control levels and increasing rates of associated testing led to a lower lifetime incidence of blindness, end-stage renal disease, and amputations. The improvements in cholesterol control led to a lower lifetime incidences of cardiovascular events. Similarly, enhanced aspirin prescribing led to a slight decline in coronary heart disease events. Only ACE inhibitor utilization led to benefits in both microvascular and cardiovascular complications. The improvements in glucose control and ACE inhibitor utilization produced the largest individual increases in QALYs (0.19). The cost-effectiveness of individual improvements of diabetes care varied (Table 4). The increase in utilization of ACE inhibitors was clearly cost effective (ICER $23,653/QALY), while each of the other improvements were individually not cost-effective based on a $100,000/QALY threshold. The ICERs for improvements in individual therapies were higher than ICERs found from CEA of individual therapies based on original clinical trial results (e.g., intensive glucose-control ICER $41,384/QALY and intensive cholesterol-control ICER $51,889/QALY) (CDC Group 2002).
Table 4.
Impact of Individual Improvements in Diabetes Care
| Glucose Control and Associated Testing | Cholesterol Control | ACE Inhibitor | Aspirin | |
|---|---|---|---|---|
| Absolute risk reduction (%) | ||||
| Blindness | 2 | 0 | 0 | 0 |
| ESRD | 1 | 0 | 3 | 0 |
| Amputation | 1 | 0 | 0 | 0 |
| Coronary heart disease | 0 | 1 | 0 | 2 |
| Stroke | 0 | 0 | 1 | 0 |
| Cost-effectiveness results | ||||
| Quality-adjusted life years (QALYs) gained (mean) | 0.09 | 0.02 | 0.19 | 0.03 |
| Cost difference (mean $)* | 9,433 | 8,337 | 4,494 | 4,553 |
| Incremental cost-effectiveness ratio ($/QALY)* | 104,811 | 416,850 | 23,653 | 151,767 |
These individual treatment analyses assume that the entire program cost was devoted to improving an individual component of diabetes care.
Other sensitivity analyses support the overall conclusion that the HDC program is cost-effective. We considered the effects of potential secular trend by systematically improving processes of care in the pre-HDC scenario, thereby decreasing the difference between the pre-HDC and HDC scenarios. If 50 percent of the observed improvement in every component of care was a result of secular trends, the impact of the HDC would still be cost-effective ($54,839/QALY). Even if secular trends accounted for over 75 percent of observed improvements, the ICER would still remain below the $100,000/QALY threshold ($93,563/QALY). An alternative approach to viewing this sensitivity analysis is to begin with secular improvements and ask how much more improvements would have to be in order for the program to be cost-effective. If 50 percent of observed improvements were already present due to secular trends, improvements with the HDC would have to be greater than 70 percent of observed improvements to generate an ICER below $100,000/QALY and would have to be approximately 110 percent of observed improvements in order to achieve an ICER below $50,000/QALY. We also tested a wide range of assumptions regarding program costs and found that the ICER remained at $54,060/QALY even when assuming high and constant program costs ($1,000/patient/year). The increased costs of the HDC scenario compared with the pre-HDC scenario are largely related to the costs of routine diabetes care. When using the UKPDS risk engine in place of the Framingham risk equation for coronary heart disease, the difference in lifetime probability of coronary heart disease increased (34 versus 29 percent) and the ICER decreased to $26,276/QALY. When accounting for future costs with base case assumptions, the overall ICER decreased to $29,437/QALY. These results did not change significantly with additional sensitivity analyses regarding assumptions such as earnings or consumptions.
DISCUSSION
During the first 4 years of the Diabetes HDC program, we observed improvements in multiple dimensions of diabetes care in community healthy center patients; this study joins a growing body of literature illustrating the potential benefits of QI programs. Although multiple QI programs have been found to produce improvements of similar or greater magnitude (Chodosh et al. 2005), the economic value of these programs is generally unknown because CEA of QI programs, designed to improve the delivery of care, have been rarely conducted (Mason et al. 2001). To our knowledge, this is one of the first studies to examine the cost-effectiveness of QI of diabetes care. It is also one of the first studies to examine the clinical and economic impact of comprehensive diabetes care (CDC Group 2002). Given the ubiquitous nature of disease management programs and QI programs, the lessons learned from this evaluation of the HDC program can provide important insight for policy makers allocating health care dollars and for those who implement QI programs.
Based on improvements in diabetes care observed during the first 4 years of the HDC, we find that the Diabetes HDC program is cost-effective at a level comparable with that of other health care technologies (Coffield et al. 2001). This basic knowledge is valuable for policy makers who are deciding whether to continue or expand QI programs. Our results provide additional lessons for ongoing QI programs regarding how to maximize their effectiveness and cost-effectiveness (Casalino 2005). First, our results illustrate how the effectiveness of improving individual elements of diabetes care can vary widely. The individual improvements that produced the largest health benefits were the lowering of glucose levels and the increase in ACE inhibitor utilization. ACE inhibitor utilization was clearly the most cost-effective area of improvement in care. Our results illustrate that improving diabetes care comprehensively remains the ideal, and that it is crucial not to neglect components of care such as ACE inhibitor prescribing or blood pressure control that have wide ranging health effects and are relatively cost-effective (CDC Group 2002; Vijan and Hayward 2003; Rosen et al. 2005). Second, our sensitivity analysis on program costs show that while the QI program costs certainly influenced results, they were not the major determinants of the overall cost-effectiveness of the program. Our case studies actually indicate that QI program costs are lower than the $1,000/patient/year we used in sensitivity analyses and that program costs typically decline over time as centers gain experience in implementing QI initiatives. Our program cost assumptions were also conservative in that we assumed that secular improvements in diabetes care did not require any additional resource use.
Establishing the societal cost-effectiveness of the Diabetes HDC program is valuable; however, this knowledge does not ensure that the benefits or cost-effectiveness of this program will be realized over the long term. Our study assumed a traditional societal perspective but no one entity represents society in our fractured health care system. Individual organizations make the decisions to support QI programs or sustain their effects. The costs of the HDC program itself are borne by HCs and HRSA's BPHC. We have found that HCs routinely experience financial losses while operating the HDC program, raising concerns about its sustainability (Huang et al. in press). More importantly, the costs of routine diabetes care of HC patients are borne by HRSA's BPHC and Medicaid. Without such programs, HC patients may not be able to receive regular health care, obtain medications regularly, and maintain optimal control of risk factors. Therefore, the basic provision of chronic care for vulnerable patients is a crucial ingredient for sustaining the health care benefits of the HDC program and, in turn, will ultimately determine the cost-effectiveness of this QI program.
The chief limitation of our study is that we based the effect of the HDC program on changes in care over time, which raises the question, to what extent are secular trends driving improvement. Assessing changes over time is a very common approach to outcome assessment for most QI programs. The national HDC program was intended to be delivered to as many vulnerable patients as possible and a randomized controlled trial of the basic program was not considered. To address the possible effect of secular trends, we conducted sensitivity analyses and found that secular trends in diabetes care would have to account for the majority of improvements in order to make the HDC cost-ineffective. In addition, comparisons with studies of national trends lead us to believe that a majority of the changes observed in these HCs are distinct from general secular trends. For example, a contemporaneous study of diabetes care using NHANES data found improvements in cholesterol levels and systolic blood pressure but no improvements in mean glucose levels (Saydah, Fradkin, and Cowie 2004). In our study, improvements occurred in HbA1c and cholesterol levels but not for blood pressure. In addition, the increases in ACE inhibitor and aspirin utilization observed in our study are larger than those observed in national studies (e.g., aspirin 40 percent national increase versus 105 percent, ACE inhibitor 40 versus 66 percent) (Nau, Garber, and Herman 2004; Stafford, Monti, and Ma 2005). When considering the possible effects of secular trends, it is also important to note that HCs are systematically different from other care settings by virtue of the large proportion of uninsured and Medicaid patients that they serve and that these patients may actually present greater challenges to the routine delivery of chronic care compared with other health care systems (Kerr et al. 2004; Pham et al. 2005). We also know from prior study of diabetes patients in HCs that the population is relatively stable with 72 percent of patients remaining from 1 year to the next (Chin et al. 2001), a fact that helps to lessen concerns regarding the effects of secular trends.
These results should be considered in light of this study's other limitations. The main results of our study come from 17 Midwestern HCs and their experiences may not be representative of centers across the nation. In addition, the cost-effectiveness of improving diabetes care in HCs cannot be assumed to be the same for other clinical settings and patient populations. HC patients are younger and have greater minority representation than the general population. Given both of these characteristics, these patients are more likely to benefit from improvements in diabetes care during the course of their lifetimes. At the same time, this population presents unique challenges to care management. To what extent these factors shift the balance of the cost-effectiveness of QI is unclear and deserves further study. Apart from the unique features of HC patients, our results are also based on a simulation model of diabetes-related complications that is based upon multiple assumptions regarding the nature of diabetes. However, simulation models are the only feasible way of estimating the long-term impact of diabetes-related treatments. We also had some incomplete information regarding patients and their care; for example, we did not know the exact utilization of statins over time. Where data were missing, we made conservative assumptions. In addition, our analysis underestimates the overall health impact and social value of the HDC program. We focus on improvements in diabetes care when in fact the HDC is intended to improve care across multiple conditions.
Despite these limitations, this analysis provides valuable lessons regarding the value of improving chronic disease management. The initial impact of the HDC on diabetes care has led to improvements across multiple components of diabetes care that are, in aggregate, substantial. If these benefits are sustained or enhanced over the lifetime of patients, the HDC program will be cost-effective for society. What resources will be required to accomplish this in HCs is an important area of policy analysis and investigation. Our results also indicate that future QI efforts may be particularly valuable if they lead to improved delivery of treatments that produce benefits in multiple areas of health.
Acknowledgments
This study was supported by an AHRQ R01 (Dr. Chin, R01-HS010479), an AHRQ U01 with the participation of the Health Resources and Services Administration (Ms. Brown and Drs. Huang, Drum, Meltzer, and Chin, U01-HS013635), a NIA Career Development Award (Dr. Huang, K23-AG021963), a NIDDK Midcareer Investigator Award in Patient-Oriented Research (Dr. Chin, K24-DK071933), a NIDDK Diabetes Research and Training Center (Ms. Brown and Drs. Huang, Drum, Meltzer, and Chin, P60 DK20595), CDC-PEP (Ms. Brown and Drs. Zhang, Huang, and Meltzer, U36-CCU319276), the Chicago Center of Excellence in Health Promotion Economics (Drs. Huang, Chin, and Meltzer, P30-CD000147), and two Robert Wood Johnson Generalist Physician Faculty Scholar Awards (Drs. Chin and Meltzer).
Disclosures: None.
Disclaimers: None.
Supplementary material
The following supplementary material for this article is available:
Diabetes Complication Simulation Model.
This material is available as part of the online article from: http://www.blackwellsynergy.com/doi/abs/10.1111/j.1475-6773.2007.00734.x (this link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- American Diabetes Association. “Aspirin Therapy in Diabetes.”. Diabetes Care. 2004;27(suppl 1):S72–3. doi: 10.2337/diacare.27.2007.s72. [DOI] [PubMed] [Google Scholar]
- Andersen R, DeTurk P. National Vital Statistics Report. Vol. 50, No. 6. Hyattesville, MD: National Center for Health Statistics; 2002. [Google Scholar]
- Bodenheimer T, Wagner EH, Grumbach K. “Improving Primary Care for Patients with Chronic Illness.”. Journal of the American Medical Association. 2002a;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Wagner EH, Grumbach K. “Improving Primary Care for Patients with Chronic Illness: The Chronic Care Model, Part 2.”. Journal of the American Medical Association. 2002b;288(15):1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. “Screening for Proteinuria in US Adults: A Cost-Effectiveness Analysis.”. Journal of the American Medical Association. 2003;290(23):3101–14. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- Brandle M, Zhou H, Smith BRK, Marriott D, Burke R, Tabaei BP, Brown MB, Herman WH. “The Direct Medical Cost of Type 2 Diabetes.”. Diabetes Care. 2003;26(8):2300–4. doi: 10.2337/diacare.26.8.2300. [DOI] [PubMed] [Google Scholar]
- Casalino LP. “Disease Management and the Organization of Physician Practice.”. Journal of the American Medical Association. 2005;293(4):485–8. doi: 10.1001/jama.293.4.485. [DOI] [PubMed] [Google Scholar]
- CDC Diabetes Cost-Effectiveness Group (CDC Group) “Cost-Effectiveness of Intensive Glycemic Control, Intensified Hypertension Control, and Serum Cholesterol Level Reduction, for Type 2 Diabetes.”. Journal of the American Medical Association. 2002;287(19):2542–51. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- Chin MH, Cook S, Drum ML, Jin L, Guillen M, Humikowski CA, Koppert J, Harrison JF, Lippold S, Schaefer CT. “Improving Diabetes Care in Midwest Community Health Centers with the Health Disparities Collaborative.”. Diabetes Care. 2004;27(1):2–8. doi: 10.2337/diacare.27.1.2. [DOI] [PubMed] [Google Scholar]
- Chin MH, Cook S, Jin L, Drum ML, Harrison JF, Koppert J, Thiel F, Harrand AG, Schaefer CT, Takashima HT, Chiu S. “Barriers to Providing Diabetes Care in Community Health Centers.”. Diabetes Care. 2001;24(2):268–74. doi: 10.2337/diacare.24.2.268. [DOI] [PubMed] [Google Scholar]
- Chin MH, Drum ML, Guillen M, Rimington A, Levie JR, Kirchhoff AC, Quinn MT, Schaefer CT. Forthcoming. “Improving and Sustaining Diabetes Care in Community Health Centers with the Health Disparities Collaborative.” Submitted manuscript. [DOI] [PubMed]
- Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, Rhodes S, Shekelle P. “Meta-Analysis: Chronic Disease Self-Management Programs for Older Adults.”. Annals of Internal Medicine. 2005;143:427–38. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- Coffield AB, Maciosek MV, McGinnis JM, Harris JR, Caldwell MB, Teutsch SM, Atkins D, Richland JH, Haddix A. “Priorities among Recommended Clinical Preventive Services.”. American Journal of Preventive Medicine. 2001;21(1):1–9. doi: 10.1016/s0749-3797(01)00308-7. [DOI] [PubMed] [Google Scholar]
- Cohen FJ, Neslusan CA, Conklin JE, Song X. “Recent Antihyperglycemic Prescribing Trends for U.S. Privately Insured Patients with Type 2 Diabetes.”. Diabetes Care. 2003;26(6):1847–51. doi: 10.2337/diacare.26.6.1847. [DOI] [PubMed] [Google Scholar]
- Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington, DC: National Academy Press; 2001. [Google Scholar]
- Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, Moore JA. “Effect of Transradial Access on Quality of Life and Cost of Cardiac Catheterization: A Randomized Comparison.”. American Heart Journal. 1999;138(3, part 1):430–6. doi: 10.1016/s0002-8703(99)70143-2. [DOI] [PubMed] [Google Scholar]
- Cundiff DK. “Coronary Artery Bypass Grafting: Reassessing Efficacy, Safety, and Cost.”. Medscape General Medicine. 2002;4(2):7. [PubMed] [Google Scholar]
- Dasbach EJ, Fryback DG, Thornbury JR. “Health Utility Preference Differences.”. Medical Decision Making. 1992;12(4):351. [Google Scholar]
- Diabetes Control and Complications Trial Research Group (DCCT Research Group) “The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus.”. New England Journal of Medicine. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group (DCCT Research Group) “The Relationship of Glycemic Exposure (HbA1c) to the Risk of Development and Progression of Retinopathy in the Diabetes Control and Complications Trial.”. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group (DCCT Research Group) “Lifetime Benefits and Costs of Intensive Therapy as Practiced in the Diabetes Control and Complications Trial.”. Journal of the American Medical Association. 1996;276:1409–15. [PubMed] [Google Scholar]
- Diabetes Control and Complication Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. “Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes.”. New England Journal of Medicine. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Copley-Merriman C, Maier W, Dong F, Manninen D, Zbrozek AS, Kotsanos J, Garfield SA, Harris MI. “Model of Complications of NIDDM: II. Analysis of the Health Benefits and Cost-Effectiveness of Treating NIDDM with the Goal of Normoglycemia.”. Diabetes Care. 1997;20(5):735–44. doi: 10.2337/diacare.20.5.735. [DOI] [PubMed] [Google Scholar]
- Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Zbrozek AS, Dong F, Manninen D, Garfield SA, Copley-Merriman C, Maier W, Eastman JF, Kotsanos J, Cowie CC, Harris MI. “Model of Complications of NIDDM: I. Model Construction and Assumptions.”. Diabetes Care. 1997;20(5):725–34. doi: 10.2337/diacare.20.5.725. [DOI] [PubMed] [Google Scholar]
- Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA for the DQIP Group. “The Diabetes Quality Improvement Project.”. Diabetes Care. 2001;24(10):1815–20. doi: 10.2337/diacare.24.10.1815. [DOI] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. “Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes.”. New England Journal of Medicine. 2003;348(5):383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- Gordois A, Suffman P, Shearer A, Oglesby A, Tobian JA. “The Health Care Costs of Diabetic Peripheral Neuropathy in the U.S.”. Diabetes Care. 2003;26(6):1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- Grant RW, Pirraglia PA, Meigs JB, Singer DE. “Trends in Complexity of Diabetes Care in the United States from 1991 to 2000.”. Archives of Internal Medicine. 2004;163:1134–9. doi: 10.1001/archinte.164.10.1134. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L. “Prevalence of Lower-Extremity Disease in the U.S. Adult Population >=40 Years of Age with and without Diabetes.”. Diabetes Care. 2004;27(7):1591–7. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S for the HOT Study Group. “Effects of Intensive Blood-Pressure Lowering and Low-Dose Aspirin in Patients with Hypertension: Principal Results of the Hypertension Optimal Treatment (HOT) Randomised Trial. HOT Study Group.”. Lancet. 1998;351(9118):1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study Investigators (HOPE Study Investigators) “Effects of Ramipril on Cardiovascular and Microvascular Outcomes in People with Diabetes Mellitus: Results of the HOPE Study and MICRO-HOPE Substudy.”. Lancet. 1999;355:253–9. [PubMed] [Google Scholar]
- Hlatky MA, Boothroyd DB, Melsop KA, Brooks MM, Mark DB, Pitt B, Reeder GS, Rogers WJ, Ryan TJ, Whitlow PL, Wiens RD. “Medical Costs and Quality of Life 10 to 12 Years after Randomization to Angioplasty or Bypasss Surgery for Multivessel Coronary Artery Disease.”. Circulation. 2004;110:1960–6. doi: 10.1161/01.CIR.0000143379.26342.5C. [DOI] [PubMed] [Google Scholar]
- Huang ES, Brown SES, Zhang JX, Kirchhoff A, Schaeffer C, Casalino LP, Chin MH. “The Cost Consequences of Improving Diabetes Care: The Community Health Center Experience.”. Joint Commission Journal on Quality and Patient Safety. doi: 10.1016/s1553-7250(08)34016-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ES, Jin L, Shook M, Chin MH, Meltzer DO. “The Impact of Patient Preferences on the Cost-Effectiveness of Intensive Glucose Control in Older Patients with New Onset Diabetes.”. Diabetes Care. 2006;29(2):259–64. doi: 10.2337/diacare.29.02.06.dc05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ES, Meigs JB, Singer DE. “The Effect of Interventions to Prevent Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus.”. American Journal of Medicine. 2001;111:633–42. doi: 10.1016/s0002-9343(01)00978-0. [DOI] [PubMed] [Google Scholar]
- Hunink MGM, Goldman LL, Tosteson ANA, Mittleman MA, Goldman PA, Williams LW, Tsevat J, Weinstein MC. “The Recent Decline in Mortality from Coronary Heart Disease, 1980–1990.”. Journal of the American Medical Association. 1997;277(7):535–42. [PubMed] [Google Scholar]
- Kerr EA, Gerzoff RB, Krein SL, Selby JV, Piette JD, Curb JD, Herman WH, Marrero DG, Narayan KMV, Safford MM, Thompson T, Mangione CM. “Diabetes Care Quality in the Veterans Affairs Health Care System and Commercial Managed Care: The TRIAD Study.”. Annals of Internal Medicine. 2004;141(4):272–81. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- Mason J, Freemantle N, Nazareth I, Eccles M, Haines A, Drummond M. “When Is It Cost-Effective to Change the Behavior of Health Professionals?.”. Journal of the American Medical Association. 2001;286(23):2988–92. doi: 10.1001/jama.286.23.2988. [DOI] [PubMed] [Google Scholar]
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. “The Quality of Health Care Delivered to Adults in the United States.”. New England Journal of Medicine. 2003;348(26):2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- Meltzer DO, Egleston B, Stoffel D, Dasbach EJ. “Effect of Future Costs on Cost-Effectiveness of Medical Interventions among Young Adults: The Example of Intensive Therapy for Type 1 Diabetes Mellitus.”. Medical Care. 2000;38(6):679–85. doi: 10.1097/00005650-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Naglie G, Krahn MD, Naimark D, Redelmeier DA, Detsky AS. “Primer on Medical Decision Analysis: Part 3—Estimating Probabilities and Utilities.”. Medical Decision Making. 1997;17:136–41. doi: 10.1177/0272989X9701700203. [DOI] [PubMed] [Google Scholar]
- Nau DP, Garber MC, Herman WH. “The Intensification of Drug Therapy for Diabetes and Its Complications: Evidence from 2 HMOs.”. American Journal of Managed Care. 2004;10(part 2):118–23. [PubMed] [Google Scholar]
- Nichol G, Valenzuela T, Roe D, Clark L, Huszti E, Wells GA. “Cost Effectiveness of Defibrillation by Targeted Responders in Public Settings.”. Circulation. 2003;108:697–703. doi: 10.1161/01.CIR.0000084545.65645.28. [DOI] [PubMed] [Google Scholar]
- Peters EJG, Lavery LA. “Effectiveness of the Diabetic Foot Risk Classification System of the International Working Group on the Diabetic Foot.”. Diabetes Care. 2001;24(8):1442–7. doi: 10.2337/diacare.24.8.1442. [DOI] [PubMed] [Google Scholar]
- Pham HH, Schrag D, Hargraves JL, Bach PB. “Delivery of Preventive Services to Older Adults by Pimary Care Physicians.”. Journal of the American Medical Association. 2005;294(4):473–81. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A. Hierarchical Linear Models, Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Raudenbush S, Bryk A, Cheong Y, Congdon R., Jr . HLM 5. Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International; 2001. [Google Scholar]
- Red Book. 2004 Drug Topics Red Book. Montvale, NJ: Medical Economics Company, Inc; 2004. [Google Scholar]
- Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. “Diabetic Foot Ulcers and Amputations: Estimates of Health Utility for Use in Cost-Effectiveness Analyses of New Treatments.”. Diabetes Medicine. 2004;30(6):549–56. doi: 10.1016/s1262-3636(07)70154-4. [DOI] [PubMed] [Google Scholar]
- Rosen A, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. “Cost-Effectiveness of Full Medicare Coverage of Angiotensin-Converting Enzyme Inhibitors for Beneficiaries with Diabetes.”. Annals of Internal Medicine. 2005;143(2):89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- Saydah SH, Fradkin JE, Cowie CC. “Poor Control of Risk Factors for Vascular Disease among Adults with Previously Diagnosed Diabetes.”. Journal of the American Medical Association. 2004;291(3):335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- Singh N, Armstrong DG, Lipsky BA. “Preventing Foot Ulcers in Patients with Diabetes.”. Journal of the American Medical Association. 2005;293(2):217–28. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- Stafford RS, Monti V, Ma J. “Underutilization of Aspirin Persists in US Ambulatory Care for the Secondary and Primary Prevention of Cardiovascular Disease.”. PLoS Medicine. 2005;2(12):e353. doi: 10.1371/journal.pmed.0020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Kothari V, Adler AI, Stratton IM. “The UKPDS Risk Engine: A Model for the Risk of Coronary Heart Disease in Type II Diabetes (UKPDS 56).”. Clinical Science (London) 2001;101(6):671–9. [PubMed] [Google Scholar]
- Tennvall GR, Apelquist J. “Prevention of Diabetes-Related Foot Ulcers and Amputations: A Cost-Utility Analysis Based on Markov Model Simulations.”. Diabetologia. 2001;44:2077–87. doi: 10.1007/s001250100013. [DOI] [PubMed] [Google Scholar]
- U.K. Prospective Diabetes Study Group (UKPDS Group) “Intensive Blood–Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33).”. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- Van Alem AP, Dijkgraaf MGW, Tijssen JGP, Koster RW. “Health System Costs of Out-of-Hospital Cardiac Arrest in Relation to Time to Shock.”. Circulation. 2004;110:1967–73. doi: 10.1161/01.CIR.0000143150.13727.19. [DOI] [PubMed] [Google Scholar]
- Vijan S, Hayward RA. “Treatment of Hypertension in Type 2 Diabetes Mellitus: Blood Pressure Goals, Choice of Agents, and Setting Priorities in Diabetes Care.”. Annals of Internal Medicine. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. “Collaborative Management of Chronic Illness.”. Annals of Internal Medicine. 1997;127:1097–102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- Wagner EH, Glasgow RE, Davis C, Bonomi AE, Provost L, McCulloch D, Carver P, Sixta C. “Quality Improvement in Chronic Illness Care: A Collaborative Approach.”. Journal on Quality Improvement. 2001;27(2):63–80. doi: 10.1016/s1070-3241(01)27007-2. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. “Prediction of Coronary Heart Disease Using Risk Factor Categories.”. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. “Probability of Stroke: A Risk Profile from the Framingham Study.”. Stroke. 1991;22(3):312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Young MJ, Breddy JL, Veves A, Boulton AJM. “The Prediction of Diabetic Neuropathic Foot Ulceration Using Vibration Perception Thresholds.”. Diabetes Care. 1994;17(6):557–60. doi: 10.2337/diacare.17.6.557. [DOI] [PubMed] [Google Scholar]
- Younis N, Broadbent DM, Vora JP, Harding SP. “Incidence of Sight-Threatening Retinopathy in Patients with Type 2 Diabetes in the Liverpool Diabetic Eye Study: A Cohort Study.”. Lancet. 2003;361:195–200. doi: 10.1016/s0140-6736(03)12267-2. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y, Albert PS. “Models for Longitudinal Data: A Generalized Estimating Equation Approach.”. Biometrics. 2004;44(4):1049–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diabetes Complication Simulation Model.


