Abstract
With worldwide concern over the use of antibiotics in animal agriculture and their contribution to the spread of antibiotic resistance, alternatives to conventional antibiotics are needed. Previous research in our laboratories has shown that colicin E1 is effective against some Escherichia coli strains responsible for postweaning diarrhea (PWD) in vitro. In this study we examined the efficacy of the dietary inclusion of colicin E1 in preventing experimentally induced PWD caused by F18-positive enterotoxigenic E. coli in young pigs. Twenty-four weaned pigs (23 days of age), identified by genotyping to be susceptible to F18-positive E. coli infections, were individually housed and fed diets containing 0, 11, or 16.5 mg colicin E1/kg diet. Two days after the start of the trial, all animals were orally inoculated with 1 × 109 CFU of each of two F18-positive E. coli strains isolated from pigs with PWD. The dietary inclusion of colicin E1 decreased the incidence and severity of PWD caused by F18-positive enterotoxigenic E. coli and improved the growth performance of the piglets. Additionally, the reduced incidence of PWD due to dietary colicin E1, lowered the levels of expression of the genes for interleukin 1β and tumor necrosis factor beta in ileal tissues from these animals. The dietary inclusion of colicin E1 may be an effective alternative to conventional antibiotics in the diets of weaning pigs for the prevention of PWD caused by F18-positive enterotoxigenic E. coli.
Postweaning diarrhea (PWD) is a serious threat to the economic success of the swine industry both due to losses as a result of mortalities and due to the reduced growth performance of surviving pigs. It is estimated that 50% of piglet mortality due to diarrhea is attributable to the causative agent of PWD, enterotoxigenic Escherichia coli (ETEC) (18). The ETEC strains most commonly associated with PWD possess either the F4 or the F18 fimibrial type (11, 39). As a result of the significant impact that F18-positive ETEC and other bacterial infections can have on pig production, prophylactic antibiotics are frequently included in the diets of young pigs in an attempt to prevent ETEC colonization and the resulting PWD. An estimated 78% of large swine farms in the United States include subtherapeutic concentrations of antibiotics in the diets for young pigs (36). Despite the use of antibiotic prophylaxis, 40.7% of these farms reported the occurrence of diarrhea caused by E. coli infections (36). The lack of the effective prevention of PWD with the use of prophylactic antibiotics is not surprising because of the frequency and spectrum of antibiotic resistance seen among ETEC strains (7, 23, 25). It is expected that antibiotic resistance will further increase among these strains, based on the overall increase in resistance to antibiotics by ETEC strains over the last 20 years (25).
With worldwide concern over the use of prophylactic antibiotics in animal agriculture and its contribution to the spread of antibiotic resistance (12, 34, 38), the development of alternatives to conventional antibiotics is urgently needed to protect swine from these E. coli infections. Public concerns surrounding the antibiotic resistance issue led to the elimination of prophylactic antibiotic use in animal agriculture in Denmark (20, 34). This cessation of the use of prophylactic antibiotics in pig production caused increases in the rate of PWD and a 30% increase in piglet mortality (34). These infections led to a large increase in veterinarian-prescribed antibiotic use in Denmark's swine industry (35). The switch from growth-promoting or prophylactic antibiotic usage to veterinarian-prescribed therapeutic usage resulted in only a very modest reduction, if any, in total antibiotic usage in Denmark's swine industry (35). In order to realize a true reduction in antibiotic use in animal agriculture, effective alternative therapies must be developed.
A potential alternative to conventional antibiotics that holds a great deal of promise are colicins. Colicins are a class of bacteriocins produced by and effective against E. coli and closely related bacteria (14). Pore-forming colicins, such as colicin E1, bind to their target bacteria and kill them by disrupting the ionic gradient of the cell (14). These proteins are particularly attractive for use as an alternative to conventional antibiotics for the control of E. coli-caused PWD for several reasons. We have previously shown them to be effective against ETEC strains isolated from pigs with PWD in vitro (33), and other work has demonstrated that colicin E1 is effective against a wide range of E. coli strains (22, 27, 30). They are also not related to any antibiotics that are currently being used in human medicine. Additionally, colicins would not be absorbed intact by the animals, thereby eliminating concerns over the presence of antibiotic residues in meat, and colicins could be effective at concentrations low enough to not significantly alter the nutrient density of the diet. The objective of this study was to determine the efficacy of the dietary inclusion of colicin E1 in preventing PWD due to F18-positive ETEC.
MATERIALS AND METHODS
Colicin production.
Colicin E1 was produced and purified to homogeneity by the method of Stahl et al. (33). Briefly, a colicin E1-producing strain of E. coli was grown in LB medium, and colicin production was induced by the addition of mitomycin C (EMD Biosciences, San Diego, CA) to the medium. The colicin E1 was purified from the cell-free supernatant by ion-exchange chromatography, first by using DEAE cellulose (Sigma-Aldrich, St. Louis, MO) and then by further purifying the protein with Q Sepharose (GE Healthcare, Piscataway, NJ). The purity of the colicin E1 used in this study has been visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and is estimated to be over 95% pure (30).
ETEC challenge strains.
Escherichia coli F18-producing strains 2144 (O147:NM, where NM indicates nonmotile) and S1191 (O139) were used as challenge strains. Strain S1191 was isolated from a pig with edema disease, produces heat-stable toxins (ST) STa and STb and Shiga toxin 2e, and is chloramphenicol resistant. Strain 2144 was a field isolate that was made nalidixic acid resistant and produces the toxins STa and STb. Both strains were grown overnight as pure cultures in LB medium at 37°C with shaking. They were then individually diluted to an optical density at 600 nm (OD600) of 0.1 in fresh LB medium and allowed to grow to an OD600 of ≈1. The cultures were then centrifuged at 4,000 × g for 10 min at 4°C. The bacterial pellets were resuspended in 20% dextrose and 5% nonfat dry milk. The challenge dose consisted of an equal amount of each strain and was determined by serial dilution and plating to provide a total 2 × 109 CFU/0.5 ml oral dose.
Animals.
All of our protocols involving animals were approved by the Institutional Animal Care and Use Committee of Iowa State University. The 24 barrows (castrated pigs) used were obtained from the Iowa State University Swine Nutrition Farm and were determined to be susceptible to F18-positive E. coli infections based on the restriction fragment length polymorphism test described by Frydendahl et al. (15). Briefly, genomic DNA was purified (DNeasy kit; QIAGEN, Valencia, CA) from pig tail clippings, and primers (forward primer, TTGGGAACCAGATGGGACAGTATG; reverse primer, CCCGCCAAGGAGCGTGCCTGTCTA) were used to amplify a 162-bp section of the 1,2α-fucosyltransferase enzyme gene (ECF18R) by PCR (26). The 162-bp PCR product was then digested with HhaI (New England Biolabs, Ipswich, MD), and the polymorphism was determined by size comparison on a 3% agarose gel.
Pigs were weaned at 17 days of age and allowed to adjust to solid food (TechStart 17-25; Kent Feeds, Muscatine, IA). At 21 days of age, the pigs (n = 8) were allotted into treatment groups on the basis of their body weights and were transferred to individual pens. The pigs were given 2 days to adjust to individual housing before the experimental diets were fed. The basal diet for all of the experimental diets was corn and soy based and contained no animal products (26% crude protein, 3.51 kcal/kg of diet). This diet met or exceeded the nutrient requirements of these pigs, based on the 1998 recommendations of the National Research Council (29). Either 0 (control), 11, or 16.5 mg of purified colicin E1 (supplied at 10 mg/ml in 10 mM Tris, pH 7.6) was added per kg to the basal diet (TestDiet; Purina, Richmond, IN), and the diets were then pelleted at low temperature. The pelleted rations were provided to the pigs twice daily at a rate which exceeded the consumption for each animal (approximately 500 g/day). The unconsumed feed was weighed daily and feed intake was determined.
After the animals received the experimental diets for 2 days, all animals were orally inoculated with the two F18-positive ETEC strains and their fecal scores were recorded. Fecal scores (0, dry, hard, and well-formed feces; 1, soft but formed feces; 2, pasty feces green or brown in color; 3, viscous feces light in color, episodic; 4, fluid feces light in color, episodic; 5, watery feces, continuous) were determined twice daily after the bacterial challenge. Fecal samples were obtained 2 days prior to the ETEC challenge and daily after the challenge by inserting a 10-μl fecal loop (Fisher Scientific, Pittsburgh, PA) into the rectum. These samples were immediately diluted in 5 ml of sterile phosphate-buffered saline. Serial 10-fold dilutions (up to 1:100,000) were plated onto MacConkey (MAC; Difco, Franklin Lakes, NJ), MAC-chloramphenicol, and MAC-nalidixic acid agar plates for CFU determination. Our limit of detection for the fecal samples was 10,000 CFU/g. Four days postchallenge, all pigs were euthanized by captive bolt, and tissue samples were collected. Ileal sections (approximately 10 cm each) were collected from each pig for RNA extraction and E. coli enumeration. Additionally, rectal and cecal contents were collected for E. coli enumeration.
Gene expression.
Isolation of RNA from the ileum was performed by using a whole ileal homogenate and an RNeasy kit (QIAGEN). Genomic DNA was eliminated from the extracted total RNA by using the DNA-free kit from Ambion (Austin, TX), according to the manufacturer's instructions. The RNA was reverse transcribed with Superscript III reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA), and the RNA was removed from the resulting cDNA by incubation with E. coli RNase H (Invitrogen), according to the manufacturer's instructions. The cDNA concentrations were determined by using a Quant-it kit (Invitrogen), and the cDNA samples were then stored at −80°C until analysis by real-time PCR. The levels of interferon (IFN), interleukin 10 (IL-10), IL-8, tumor necrosis factor alpha (TNF-α), tumor necrosis factor beta (TNF-β), IL-1β, and inducible nitric oxide synthase (iNOS) mRNA were determined semiquantitatively by real-time PCR with a the MyiQ single-color real-time PCR detection system and SYBR green (Bio-Rad Laboratories; Hercules, CA). The thermal cycling conditions included 45 cycles of 30 s of melting at 95°C, followed by 30 s of annealing and extension at 65°C. Following amplification, all samples were subjected to a melting curve analysis to ensure that only a single product was formed. Primer oligonucleotides (Table 1) were designed by using PrimerQuest software, available from Integrated DNA Technologies (Coralville, IA). All primer sets were validated to amplify only the sequence of interest and to do so in a linear fashion over a 2-log range of cDNA concentrations. The data for all samples were normalized to the cDNA concentration prior to statistical analysis.
TABLE 1.
Primer sequences for semiquantitative real-time PCR analysis of intestinal gene expression
| mRNA | Directiona | Primer sequence |
|---|---|---|
| iNOS | F | GGACGTACGAAGTGACCAACC |
| R | GAACGTACGAAGTGACCAACC | |
| IL-1β | F | TGAAGAAGAGCCCATCGTCCT TGA |
| R | TGCACAAAGCTCATGCAGAACACC | |
| TGF-βb | F | AGGCCGTACTGGCTCTTTACAACA |
| R | TTGGTTGCCGCTTTCCACCATTAG | |
| IL-10 | F | AAGACGTAATGCCGAAGGCAGAGA |
| R | TGCTAAAGGCACTCTTCACCTCCT | |
| IFN | F | ATGACTTCCAAACTGGCTGTTGCC |
| R | TATGCACTGCGATCGAAGTTCTGC | |
| IL-8 | F | ATGACTTCCAAACTGGCTGTTGCC |
| R | TATGCACTGGCATCGAAGTTCTGC | |
| TNF-α | F | GCCCACGTTGTAGCCAATGTCAAA |
| R | GTTGTCTTTCAGCTTCACGCCGTT | |
| TNF-β | F | AGATCAGCTGTCCAGACACACAGA |
| R | TAGAGCGAAGGCTCCAAAGAAGAC |
F, forward; R, reverse.
TGF-β, transforming growth factor β.
Bacterial enumeration.
The enumeration of E. coli from the ileal mucosa, cecal content, and rectal content samples was performed as follows. The samples were weighed, diluted 1:2 with buffered peptone water, and placed in a stomacher blender (Stomacher 80; Seward, Worthing, United Kingdom) for 30 s. The samples were then serially diluted over a 4-log range at a 1:200 starting dilution. Ten microliters of each dilution was plated in duplicate on MAC, MAC-chloramphenicol (30 μg/ml), and MAC-nalidixic acid (50 μg/ml) agar plates. The plates were incubated for 16 h at 37°C, and the countable dilution was then recorded. Our limit of detection for these samples was 1,000 CFU/g.
Statistical analysis.
Statistical analysis of the data was performed with Statistical Analysis Software, version 9.1 (SAS, Cary, NC), by the general linear models procedure for comparison of least-squares means. Treatment was considered a fixed effect, and for growth parameters, initial body weight was used as a covariate in the analysis. For bacterial enumeration data, when the counts were below our limit of detection, a value of 1 CFU/g less than our limit of detection was ascribed prior to analysis. Statistical significance was set at α equal to 0.05, and trends are discussed at an α value of 0.1.
RESULTS
Fecal scores.
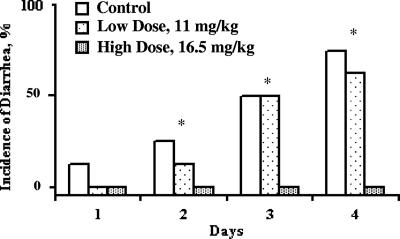
Prior to bacterial challenge, there was no indication of diarrhea or loose stools among any of the pigs. There were no differences in fecal scores between the treatment groups until 48 h postchallenge. At this point the group fed the control diet had a mean fecal score of 2.38, which tended to be higher (P < 0.06) than that for the group fed the 16.5-mg colicin E1/kg diet (mean score, 0.5) (Fig. 1). Over the entire study, the control group had a significantly higher (P < 0.05) average fecal score than the high-dose (16.5-mg/kg) colicin E1 group (2.1 and 0.59, respectively). The low-dose (11-mg/kg) colicin E1 group also had higher (P < 0.05) average fecal scores than the high-dose group, and these were not significantly different from those for the control pigs. These differences in group fecal scores were caused by the incidence of diarrhea (fecal scores, ≥4) in one, four, and five of the eight control group pigs 24, 48, and 72 h after ETEC inoculation, respectively. Among the pigs fed the low dose of colicin E1, no animals had diarrhea at 24 h postinoculation; however, three and four pigs had diarrhea at 48 and 72 h postchallenge, respectively. None of the pigs fed the high dose of colicin E1 had any incidence of diarrhea at any time during the experiment.
FIG. 1.
Effect of dietary inclusion of colicin E1 on incidence of diarrhea in weaning pigs following an F18-positive enteropathogenic E. coli challenge. Asterisks, significant differences (P < 0.05) between the high-dose group and both the low-dose and the control groups.
Growth performance.
The dietary inclusion of colicin E1 had a significant effect (P < 0.05) on the growth performance of the pigs in this study (Table 2). From the time of E. coli challenge until the completion of the study, the pigs fed the control diet gained an average of 380 g, while the pigs receiving the low and high doses of colicin E1 in their diets gained 540 and 940 g, respectively (Table 2). Although the animals fed the control diet gained the least body weight over the course of the study, they consumed significantly (P < 0.05) more feed than either of the groups fed diets containing colicin E1. The control group of animals averaged a total consumption of 1.54 kg of diet, while the pigs treated with the low and high doses of colicin E1 ate 1.22 kg and 1.44 kg, respectively. Although there were significant differences in both body weight gain and feed consumption, there was not a significant difference in feed conversion efficiency (body weight gain/feed intake) (P < 0.19) among any of the treatment groups. This was because over the length of the study two of the pigs in the control group lost weight (approximately 400 g each) and one pig gained virtually no weight (less than 10 g) while still consuming feed. This negative body weight gain resulted in a negative feed conversion efficiency, which resulted in tremendous variation in the feed conversion efficiency values for this group.
TABLE 2.
Effect of dietary inclusion of colicin E1 on growth performance of weaning pigsa
| Treatmentb | Wt gain (kg) | Feed intake (kg) | Feed efficiencyd |
|---|---|---|---|
| Control | 0.38*c (0.13) | 1.539† (0.544) | 27.01 (9.55) |
| Low dose | 0.54*,† (0.19) | 1.217* (0.424) | 2.83 (1.00) |
| High dose | 0.94† (0.33) | 1.442* (0.510) | 1.94 (0.69) |
The values presented are means (standard errors of the means).
The control, low-dose, and high-dose treatments contained 0, 11, and 16.5 mg colicin E1/kg of diet, respectively.
Values within a column not sharing a common symbol are significantly different (P < 0.05).
Feed efficiency is defined as feed intake/weight gain.
Bacterial enumeration. (i) Fecal cultures.
No colonies were isolated on the agar plates containing either chloramphenicol or nalidixic acid from any samples prior to the ETEC challenge. At 24 h postinoculation, the levels of both total coliforms and ETEC challenge strain 2144 recovered in the feces were significantly higher (P < 0.05) for the control animals than for both groups of colicin E1-fed pigs (Table 3). There were no significant differences between the groups in the recovery of ETEC strain S1191 at the first day postchallenge, but on the following day there was a reduction in the high-dose colicin E1 group compared to that in the controls (P < 0.05). By the last day of fecal sampling, the levels of strain S1191 had dropped below our detection limit for most of the animals, regardless of the dietary treatment (16/24 pigs), and there were no significant differences in the amount of strain 2144 recovered.
TABLE 3.
Effect of dietary inclusion of colicin E1 on E. coli levels recovered from the feces of weaning pigs
| Postchallenge time and treatmenta | No. of CFU/gb
|
||
|---|---|---|---|
| Total coliforms | E. coli 2144c | E. coli S1191d | |
| 1 day | |||
| Control | 1.1 × 109*e (4.6 × 108) | 1.7 × 108* (8.8 × 107) | 4.9 × 107 (4.3 × 107) |
| Low dose | 1.5 × 107† (5.6 × 106) | 1.2 × 106† (3.7 × 105) | 7.4 × 104 (2.3 × 104) |
| High dose | 1.3 × 107† (5.4 × 106) | 3.7 × 103† (2.5 × 106) | 4.8 × 104 (7.5 × 103) |
| 2 days | |||
| Control | 7.6 × 108 (2.9 × 108) | 1.6 × 108 (9.8 × 107) | 4.7 × 106 (2.7 × 106) |
| Low dose | 5.0 × 108 (2.9 × 108) | 9.1 × 107 (6.7 × 107) | 3.2 × 106 (3.1 × 106) |
| High dose | 6.0 × 108 (5.6 × 108) | 1.9 × 108 (1.9 × 108) | 2.4 × 105 (1.4 × 105) |
| 3 days | |||
| Control | 8.0 × 109 (5.3 × 109) | 6.3 × 109 (6.2 × 109) | 6.6 × 104 (2.6 × 104) |
| Low dose | 9.4 × 109 (7.3 × 109) | 2.9 × 109 (2.5 × 109) | 3.6 × 105 (3.6 × 105) |
| High dose | 2.3 × 108 (1.1 × 108) | 3.9 × 107 (3.1 × 107) | 7.5 × 105 (3.1 × 105) |
The control, low-dose, and high-dose treatments contained 0, 11, and 16.5 mg colicin E1/kg of diet, respectively.
The values presented are least-square means (standard errors of the means).
Escherichia coli strain 2144 is F18 positive, produces the toxins STa and STb, and was made nalidixic acid resistant.
Escherichia coli strain S1191 is F18 positive, produces the toxins STa and STb and Shiga toxin 2e, and is chloramphenicol resistant.
Values within a day and a column not sharing a common symbol are significantly different (P < 0.05).
(ii) Tissues.
In the ileum, both colicin E1-fed groups averaged lower levels (P < 0.05) of the S1191 challenge strain and coliforms compared to those in the controls (Table 4). There were no significant differences in the recovery of bacteria from the cecal samples among the groups. Strain S1191 was recovered from samples from only three of the eight animals in the high-dose colicin E1 group and was recovered from six and four of the eight animals in the control and the low-dose colicin E1 treatment groups, respectively. In the rectum, similar levels of total coliforms and strains 2144 and S1191 were recovered from the animals in all groups.
TABLE 4.
Dietary inclusion of colicin E1 affected bacterial recovery in the ileum of weaning pigs
| Treatmenta | No. of CFU/gb
|
||
|---|---|---|---|
| Total coliforms | E. coli 2144c | E. coli S1191d | |
| Control | 4.7 × 108*e (5.3 × 107) | 3.2 × 108 (1.8 × 106) | 2.7 × 105* (1.0 × 103) |
| Low dose | 1.1 × 108† (9.9 × 107) | 5.6 × 107 (5.3 × 107) | 1.0 × 103† (0) |
| High dose | 3.8 × 107† (1.7 × 107) | 2.7 × 106 (1.8 × 106) | 2.5 × 103† (1.0 × 103) |
The control, low-dose, and high-dose treatments contained 0, 11, and 16.5 mg colicin E1/kg of diet, respectively.
The values presented are least-square means (standard errors of the means).
Escherichia coli strain 2144 is F18 positive, produces the toxins STa and STb, and was made nalidixic acid resistant.
Escherichia coli strain S1191 is F18 positive, produces the toxins STa and STb and Shiga toxin 2e, and is chloramphenicol resistant.
Values within a column not sharing a common symbol are significantly different (P < 0.05).
Gene expression.
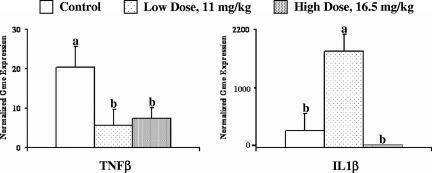
The concentration of TNF-β mRNA in ileal tissue was higher (P < 0.06) in the control animals than in either of the colicin E1-treated groups (Fig. 2). The amount of IL-1β mRNA was the highest in the group supplemented with the low dose of colicin E1 (sixfold higher [P < 0.01] than the amount in the controls), whereas the group supplemented with the high dose of colicin E1 had nearly undetectable levels of IL-1β mRNA (Fig. 2). The levels of expression of both TNF-α and iNOS tended (P < 0.1) to be greater in the control animals than in the animals receiving the diets with colicin E1 supplementation. There were no significant differences in the concentrations of gamma IFN (IFN-γ), IL-8, or IL-10 mRNA in the ileum among any of the groups.
FIG. 2.
Normalized expression of the genes for TNF-β and IL-1β in ileal tissue samples 4 days after oral F18-positive enteropathogenic E. coli challenge. Gene expression was determined by semiquantitative real-time PCR and was normalized to the cDNA concentration. The values presented are the least-square means, and the error bars represent the standard error of the mean. Values not sharing a common superscript are significantly different (P < 0.06).
DISCUSSION
We examined the efficacy of colicin E1 in preventing PWD caused by F18-producing E. coli because this disease has been estimated to be responsible for as much as 50% of the economic losses seen in the production of weaned pigs (19, 36). In herds with PWD, mortality of up to 2% (21, 37) can be seen among weaning pigs, but of greater economic significance is the morbidity and reduction in growth performance of the pigs that survive these infections. Although there is a need for alternatives to conventional antibiotics, the development of compounds that can be used to combat ETEC-associated PWD is beset with difficulties, including the availability of an adequate experimental model of ETEC infection. Establishing a good challenge model for this disease has proven to be difficult. Madec et al. (24) used four strains of ETEC and several different dosing methods in six trials with a total of 168 specific-pathogen-free piglets and was able to generate clinically significant, although transient, diarrhea in only 50% of the animals (24). The use of a viral coinfectious agent, such as transmissible gastroenteritis or rotavirus, that leads to immunosuppression and intestinal membrane disruption can also increase experimental ETEC infection rates (1, 9, 16, 28). While a cochallenge model can increase the rate of success of experimentally reproducing PWD, it also adds a potential confounding effect, particularly if the viral infection alone causes reduced growth performance. An alternative to the viral coinfection model that has also been shown to increase the success of an ETEC bacterial challenge is modification of the postweaning diet. The removal of all animal-based protein sources from the postweaning diet can increase the susceptibility of the pigs to ETEC infection due to a transient intestinal inflammatory response (2, 10). Diets designed to be fed to pigs immediately postweaning typically provide over 40% of the total protein in the diet as protein from animal sources, in part to help prevent this temporary inflammatory response. However, due to the higher cost of animal-based protein sources, there is a constant push to minimize the amount of animal protein included in pig diets. While both viral cochallenges and dietary alterations can increase infection rates in experimentally induced PWD models, identifying the genetic susceptibility of the animals may offer the most efficient way to increase infection rates in a challenge model. With the use of prescreening for an F18 receptor polymorphism, the rate of infection can be increased from 5.9% in pigs with the F18-resistant genotype to 87% in those that are genetically susceptible (15). In our study, we used only genetically susceptible animals, as well as a weaning diet that contained no animal protein. With this model we achieved a 75% infection rate in control animals, with no mortality after 4 days, with no use of viral coinfection, and with the use of an easily performed one-time oral challenge.
Although our high dose of colicin E1 (16.5 mg/kg of diet) was able to eliminate all clinical signs of PWD, our low dose of colicin E1 appeared to be able to slightly delay only the onset of PWD. The level of reduction of the challenge strains of E. coli that reached the ileum as a result of dietary colicin E1 inclusion may have been the determining factor in the development of clinical disease. Colicin-fed animals had significantly lower levels (P < 0.04) of fecal shedding of the strain 2144 challenge bacteria at 1 day postchallenge. While this suggests that the addition of colicin to the feed significantly reduced the amount of viable bacteria that reached the distal end of the small intestine, it also demonstrates that our colicin dose was not sufficient to completely eliminate the challenge strains. By the end of the study there were no significant differences in the fecal shedding of either challenge strain on the basis of colicin treatment. We would not anticipate that the feeding of colicin E1 would have an effect on reducing the colonization of the ETEC strains if they reached the ileum in a viable state, since colicin E1 is highly sensitive to proteolysis (3, 4, 6). It is likely that not all of the E. coli bacteria in our large challenge dose would be killed by the colicin E1 present in the digesta prior to the inactivation of the colicin E1 by proteolysis, but it appears that enough were eliminated as a result of high-dose colicin E1 supplementation to prevent disease. The gene expression data also suggest that fewer of the challenge bacteria were able to cause the inflammatory response leading to diarrhea in the pigs. This is supported by the lower levels of expression (P < 0.05) of IL-1β and TNF-β mRNA in the ileal tissues of pigs fed the high-dose colicin E1 diet compared with those in the control group. IL-1β is primarily secreted by macrophages and activates lymphocytes during an inflammatory response, and increases have been associated with E. coli toxin production (11, 17). While the concentration of IL-1β mRNA was significantly lower in the ileal tissue of the pigs fed the high dose of colicin E1 than in the ileal tissue of the control pigs, pigs in the low-colicin E1-dose group had mRNA levels that were over sixfold higher than those in the control group. At the time of tissue collection there was no longer any significant difference in fecal scores between the low-dose and the control groups, and this elevated expression may indicate a delay in the inflammatory response as a result of the challenge bacteria. This would be reasonable, since there appeared to be a delay in the onset of PWD in the pigs fed the low dose of colicin E1 compared with the time of onset in the control animals. An increase in IL-1β gene expression in the intestinal mucosa has been correlated with enteropathogenic E. coli challenge (16), although the associated increases in the expression of IL-6, IL-8, and IL-10 seen by Girard et al. (16) were not noted in our study. Regardless of the dose, colicin E1 in the diet caused reduced levels (P < 0.06) of expression of TNF-β. TNF-β, also known as lymphotoxin, is a primary effector of NO production and is associated with inflammatory responses related to T-cell recruitment (13). The lower levels of TNF-β and IL-1β in the ileal tissues of pigs fed the high dose of colicin E1 compared with those in the control animals suggest that colicin E1 was able to significantly reduce the bacterial load that initially reached the ileum in these animals, thereby reducing the inflammatory response to the ETEC challenge.
While other researchers have examined the efficacy of using colicin-producing bacterial cultures as probiotics for cattle in order to reduce E. coli O157 contamination (32), we are the first, to our knowledge, to examine a purified colicin as a feed component for the prevention of an ETEC infection. We have demonstrated with growth performance data, clinical indicators of PWD, and intestinal gene expression that the inclusion of colicin E1 can prevent experimentally induced PWD. The efficacy in preventing PWD at a level of dietary inclusion of 16.5 mg/kg of diet suggests that colicin E1 warrants further evaluation as a potential alternative to conventional antibiotics for use in weaning pig diets. This protein may also have significant implications for human food safety as well, since the efficacy of colicin E1 against many ETEC strains of concern for human food safety has been well documented (5, 8, 31).
Acknowledgments
This work was supported in part by the Biotechnology Research and Development Corporation (Peoria, IL).
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Benfield, D. A., D. H. Francis, J. P. McAdaragh, D. D. Johnson, M. E. Bergeland, K. Rossow, and R. Moore. 1988. Combined rotavirus and K99 Escherichia coli infection in gnotobiotic pigs. Am. J. Vet. Res. 49:330-337. [PubMed] [Google Scholar]
- 2.Bosi, P., L. Casini, A. Finamore, C. Cremokolini, G. Merialdi, P. Trevisi, F. Nobili, and E. Mengheri. 2004. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 82:1764-1772. [DOI] [PubMed] [Google Scholar]
- 3.Bowles, L. K., and J. Konisky. 1981. Cleavage of colicin Ia by the Escherichia coli K-12 outer membrane is not mediated by the colicin Ia receptor. J. Bacteriol. 145:668-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brey, R. N. 1982. Fragmentation of colicins A and E1 by cell surface proteases. J. Bacteriol. 149:306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaway, T. R., C. H. Stahl, T. S. Edrington, K. J. Genovese, L. M. Lincoln, R. C. Anderson, S. M. Lonergan, T. L. Poole, R. B. Harvey, and D. J. Nisbet. 2004. Colicin concentrations inhibit growth of Escherichia coli O157:H7 in vitro. J. Food Prot. 67:2603-2607. [DOI] [PubMed] [Google Scholar]
- 6.Cavard, D. 1990. Colicin cleavage by OmpT protease during both entry into and release from Eshcherichia coli cells. J. Bacteriol. 172:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, C., H. J. Ham, D. Kwon, J. Kim, D. S. Cheon, K. Min, W. S. Cho, H. K. Chung, T. Jung, K. Jung, and C. Chae. 2002. Antimicrobial susceptibility of pathogenic Escherichia coli isolated from pigs in Korea. J. Vet. Med. Sci. 64:71-73. [DOI] [PubMed] [Google Scholar]
- 8.Cole, K., M. B. Farnell, A. M. Donoghue, N. J. Stern, E. A. Svetoch, B. N. Eruslanov, L. I. Volodina, Y. N. Kovalev, V. V. Perelygin, E. V. Mitsevich, I. P. Mitsevich, V. P. Levchuk, V. D. Pokhilenko, V. N. Borzenkov, O. E. Svetoch, T. Y. Kudryavtseva, I. Reyes-Herrera, P. J. Blore, F. Solis de los Santos, and D. J. Donoghue. 2006. Bacteriocins reduce Campylobacter colonization and alter gut morphology in turkey poults. Poult. Sci. 85:1570-1575. [DOI] [PubMed] [Google Scholar]
- 9.Cox, E., E. Schrauwen, V. Cools, and A. Houvenaghel. 1991. Experimental induction of diarrhoea in newly-weaned piglets. Zentbl. Veterinarmed. A 38:418-426. [DOI] [PubMed] [Google Scholar]
- 10.Dreau, D., J. P. Lalles, V. Philouze-Rome, R. Toullec, and H. Salmon. 1994. Local and systemic immune responses to soybean protein ingestion in early-weaned pigs. J. Anim. Sci. 72:2090-2098. [DOI] [PubMed] [Google Scholar]
- 11.Fairbrother, J. M., E. Nadeau, and C. L. Gyles. 2005. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6:17-39. [DOI] [PubMed] [Google Scholar]
- 12.FDA 2003. Guidance for industry no. 152: evaluating the safety of antimicrobial new animal drugs with regard to their microbiological effects on bacteria of human health concern. CVM, FDA, DHHS, Washington, DC.
- 13.Ferrante, A., R. E. Staugas, B. Rowan-Kelly, S. Bresatz, L. M. Kumaratilake, C. M. Rzepczyk, and G. R. Adolf. 1990. Production of tumor necrosis factors alpha and beta by human mononuclear leukocytes stimulated with mitogens, bacteria, and malarial parasites. Infect. Immun. 58:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredericq, P. 1957. Colicins. Annu. Rev. Microbiol. 11:7-22. [DOI] [PubMed] [Google Scholar]
- 15.Frydendahl, K., T. Kare Jensen, J. Strodl Andersen, M. Fredholm, and G. Evans. 2003. Association between the porcine Escherichia coli F18 receptor genotype and phenotype and susceptibility to colonisation and postweaning diarrhoea caused by E. coli O138:F18. Vet. Microbiol. 93:39-51. [DOI] [PubMed] [Google Scholar]
- 16.Girard, F., I. Batisson, G. M. Frankel, J. Harel, and J. M. Fairbrother. 2005. Interaction of enteropathogenic and Shiga toxin-producing Escherichia coli and porcine intestinal mucosa: role of intimin and Tir in adherence. Infect. Immun. 73:6005-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyles, C. L. 2006. Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 85:E45-E62. [DOI] [PubMed] [Google Scholar]
- 18.Gyles, C. L. 1994. Escherichia coli verotoxin and other cytotoxins, p. 151-170. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Wallingford, United Kingdom.
- 19.Hani, H., A. Brandli, J. Nicolet, P. von Roll, H. Luginbuhl, and B. Horning. 1976. Occurrence and significance of swine diseases: analysis of dissected material (1971-1973). III. Pathology of the digestive tract. Schweiz. Arch. Tierheilkd. 118:13-30. (In German.) [PubMed] [Google Scholar]
- 20.Hayes, D. J., H. H. Jensen, L. Backstrom, and J. Fabiosa. 1999. Economic impact of a ban on the use of over-the-counter antibiotics in U.S. swine rations, p. 25-27. Iowa State University, Ames.
- 21.Jahn, S., and E. Uecker. 1997. Research on the economics of enterotoxaemia due to coliforms in pigs. Monatahefte. Veterinar. Medizin. 42:769-771. [Google Scholar]
- 22.Jordi, B. J., K. Boutaga, C. M. van Heeswijk, F. van Knapen, and L. J. Lipman. 2001. Sensitivity of Shiga toxin-producing Escherichia coli (STEC) strains for colicins under different experimental conditions. FEMS Microbiol. Lett. 204:329-334. [DOI] [PubMed] [Google Scholar]
- 23.Lanz, R., P. Kuhnert, and P. Boerlin. 2003. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 91:73-84. [DOI] [PubMed] [Google Scholar]
- 24.Madec, F., N. Bridoux, S. Bounaix, R. Cariolet, Y. Duval-Iflah, D. J. Hampson, and A. Jestin. 2000. Experimental models of porcine post-weaning colibacillosis and their relationship to post-weaning diarrhoea and digestive disorders as encountered in the field. Vet. Microbiol. 72:295-310. [DOI] [PubMed] [Google Scholar]
- 25.Maynard, C., S. Bekal, F. Sanschagrin, R. C. Levesque, R. Brousseau, L. Masson, S. Lariviere, and J. Harel. 2004. Heterogeneity among virulence and antimicrobial resistance gene profiles of extraintestinal Escherichia coli isolates of animal and human origin. J. Clin. Microbiol. 42:5444-5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijerink, E., S. Neuenschwander, R. Fries, A. Dinter, H. U. Bertschinger, G. Stranzinger, and P. Vogeli. 2000. A DNA polymorphism influencing alpha(1,2)fucosyltransferase activity of the pig FUT1 enzyme determines susceptibility of small intestinal epithelium to Escherichia coli F18 adhesion. Immunogenetics 52:129-136. [DOI] [PubMed] [Google Scholar]
- 27.Murinda, S. E., R. F. Roberts, and R. A. Wilson. 1996. Evaluation of colicins for inhibitory activity against diarrheagenic Escherichia coli strains, including serotype O157:H7. Appl. Environ. Microbiol. 62:3196-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy, B., L. H. Arp, H. W. Moon, and T. A. Casey. 1992. Colonization of the small intestine of weaned pigs by enterotoxigenic Escherichia coli that lack known colonization factors. Vet. Pathol. 29:239-246. [DOI] [PubMed] [Google Scholar]
- 29.National Research Council. 1998. Nutrient requirements of swine, 10th ed. National Academy Press, Washington, DC.
- 30.Patton, B. S., J. S. Dixon, S. M. Lonergan, S. A. Cutler, and C. H. Stahl. 2007. Inhibitory activity of Colicin E1 against Listeria monocytogenes. J. Food Prot. 70:1256-1262. [DOI] [PubMed] [Google Scholar]
- 31.Schamberger, G. P., and F. Diez-Gonzalez. 2002. Selection of recently isolated colicinogenic Escherichia coli strains inhibitory to Escherichia coli O157:H7. J. Food Prot. 65:1381-1387. [DOI] [PubMed] [Google Scholar]
- 32.Schamberger, G. P., R. L. Philips, J. L. Jacobs, and F. Diez-Gonzalez. 2004. Reduction of Escherichia coli O157:H7 populations in cattle by addition of colicin E7-producing E. coli to feed. Appl. Environ. Microbiol. 70:6053-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl, C. H., T. R. Callaway, L. M. Lincoln, S. M. Lonergan, and K. J. Genovese. 2004. Inhibitory activities of colicins against Escherichia coli strains responsible for postweaning diarrhea and edema disease in swine. Antimicrob. Agents Chemother. 48:3119-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein, H. H. 2002. Experience of feeding pigs without antibiotics: a European perspective. Anim. Biotechnol. 13:85-95. [DOI] [PubMed] [Google Scholar]
- 35.Svensmark, B., K. Nielsen, K. Dalsgaard, and P. Willeberg. 1989. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. III. Rotavirus infection. Acta Vet. Scand. 30:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.USDA. 2001. Part I: reference of swine health and management in the United States, 2000. Publication N338.0801. National Animal Health Monitoring System, Fort Collins, CO.
- 37.Wegmann, P. 1990. Pathology of swine—a portrait of economic loss in pig production in Switzerland. Proc. Int. Congr. Pig Vet. Soc. 11:295. [Google Scholar]
- 38.WHO. 2000. WHO global principles for the containment of antimicrobial resistance in animals intended for food: report of a WHO consultation. WHO/CDS/CSR/APH, Geneva, Switzerland.
- 39.Zhang, W., M. Zhao, L. Ruesch, A. Omot, and D. Francis. 2007. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet. Microbiol. 123:145-152. [DOI] [PubMed] [Google Scholar]