Abstract
Cyclic tetrapyrroles are among the most potent compounds with activity against transmissible spongiform encephalopathies (TSEs; or prion diseases). Here the effects of differential sulfonation and metal binding to cyclic tetrapyrroles were investigated. Their potencies in inhibiting disease-associated protease-resistant prion protein were compared in several types of TSE-infected cell cultures. In addition, prophylactic antiscrapie activities were determined in scrapie-infected mice. The activity of phthalocyanine was relatively insensitive to the number of peripheral sulfonate groups but varied with the type of metal bound at the center of the molecule. The tendency of the various phthalocyanine sulfonates to oligomerize (i.e., stack) correlated with anti-TSE activity. Notably, aluminum(III) phthalocyanine tetrasulfonate was both the poorest anti-TSE compound and the least prone to oligomerization in aqueous media. Similar comparisons of iron- and manganese-bound porphyrin sulfonates confirmed that stacking ability correlates with anti-TSE activity among cyclic tetrapyrroles.
The abnormal aggregation of protein monomers is commonly associated with the transmissible spongiform encephalopathies (TSEs) and over 20 other diseases, including type II diabetes and Alzheimer's, Parkinson's, and Huntington's diseases. The TSEs or prion diseases are infectious neurodegenerative diseases of mammals that include bovine spongiform encephalopathy, chronic wasting disease of deer and elk, scrapie in sheep, and Creutzfeldt-Jacob disease in humans. The pathogenesis of TSEs involves the conversion of the normally protease-sensitive prion protein (PrP-sen or PrPC) to a protease-resistant amyloidogenic oligomer/multimer, called PrP-res or PrPSc (for a review, see reference 2). Although the full nature of TSE infectivity remains uncertain, PrP-res is a key, if not the sole, component.
Anti-TSE compounds are often potent inhibitors of PrP-res formation in cell cultures (5) and have strong prophylactic antiscrapie activities in vivo. Among the most potent classes of inhibitors are the cyclic tetrapyrroles, which include porphyrins and phthalocyanines (5, 10, 11, 17, 18). The known phthalocyanine inhibitors contain four sulfonic acid groups at the periphery of their large aromatic ring system and may have metal ions bound to the four central nitrogens (Fig. 1) (5). The type of metal can strongly influence the potency of PrP-res inhibition in vitro. The only phthalocyanine tested in animals, the metal-free tetrasulfonate (H2PcS4), substantially prolonged the lives of scrapie-infected mice (18). H2PcS4 can also block the binding of PrP-sen to PrP-res, an activity that might account for the anti-TSE mechanism of action of various cyclic tetrapyrroles (15).
FIG. 1.
Structures of H2PcS4; metal (unspecified) PcS4 (MPcS4); and Fe(III)TMPyPo.
In addition to its effects on PrP-res formation, cyclic tetrapyrroles can also block other types of disease-associated protein aggregation. For instance, H2PcS4 suppresses the formation of α-synuclein amyloid, a pathological factor in Parkinson's disease (13). Tetrasulfonated porphyrins inhibit the aggregation of insulin (16), and hemin analogs delay fibril formation by the A/β peptide associated with Alzheimer's disease (7). These observations raise the possibility that cyclic tetrapyrroles have similar mechanisms of action in slowing the formation of a variety of pathological protein aggregates.
The structural requirements for efficient anti-TSE cyclic tetrapyrroles and their influence on PrP-res formation remain unclear. The phthalocyanine tetrasulfonate (PcS4) inhibitors carry four negatively charged SO33− groups with the potential to bond electrostatically with complementary positive centers on PrP molecules. The presence of negative charges on the periphery is not a critical determinant of the anti-TSE activity of cyclic tetrapyrroles because an iron porphyrin {iron(III) tetra-(4-N-methylpyridyl)porphine [Fe(III)TMPyPo]} (Fig. 1) and a tetra-anilinium porphyrin, each with four positively charged peripheral groups, are also active both in vivo and in vitro (5, 11, 18). Moreover, the often marked differences in in vitro anti-PrP-res activities among the various metal-PcS4 complexes do not correlate with several variables in the chemical properties of metals bound to cyclic tetrapyrroles, i.e., residual charge, affinity for axial ligands, and preferred stereochemistry. However, a metal-sensitive property of cyclic tetrapyrroles that may be relevant to inhibition is the ability to oligomerize via various types of π stacking (1, 6, 21).
In the present study, the effects of metal occupancy and the extent of sulfonation on the anti-TSE activities of cyclic tetrapyrroles were investigated in vivo and in vitro. The results correlated with the influence of the structure and the dissolving medium on cyclic tetrapyrrole oligomerization and π stacking. These findings support the idea that oligomerization is important in the anti-TSE mechanism of cyclic tetrapyrroles and related PrP-res inhibitors.
MATERIALS AND METHODS
Phthalocyanine sulfonates.
Phthalocyanine sulfonates were obtained from Frontier Science (Logan, UT) or Midcentury Chemicals (Posen, IL) as mixtures of regioisomers in undefined relative amounts. For example, with PcS1, which has only one sulfonate, isomers have either a 3-sulfonate or a 4-sulfonate. For PcS2, with two sulfonates, sulfonation may also occur on adjacent or opposite rings. The absence of defined locations for the sulfonates of the PcS4 structures in Fig. 1 reflects the large number of regioisomers possible for a tetrasulfonate. To minimize possible ambiguities by use of different preparations for a given phthalocyanine sulfonate, the same preparation was used for cell culture, mouse, and spectral studies.
Cell culture PrP-res inhibition assays.
In vitro assays for inhibition of PrP-res formation and the determination of the effective concentrations giving 50% inhibition (EC50s) of PrP-res formation were performed in N2a murine neuroblastoma cells chronically infected with the RML (20) or 22L (8) scrapie strains or in a rabbit epithelial cell line (Rov9) that expresses ovine PrP-sen and that is infected with sheep scrapie (22) by either Western blotting or dot blotting (9, 12).
Scrapie infection and phthalocyanine treatments.
Transgenic mice overexpressing hamster PrP-sen (Tg7 mice) have been described previously (19). Tg7 mice are highly susceptible to infection with hamster scrapie strain 263K and thus represent a relatively rapid assay system for scrapie disease inhibition (18). Brains from hamsters infected with scrapie strain 263K were Dounce homogenized in 0.32 M sucrose, the cellular debris was removed by low-speed centrifugation, and the supernatant was stored as a 10% (wt/vol) brain homogenate at −80°C. Prior to infection the homogenate was thawed, briefly sonicated, and diluted 1:10 in phosphate-buffered saline (PBS) containing 2% fetal bovine serum (1% [wt/vol] brain homogenate); and 0.05 ml was used to infect weanling Tg7 mice intraperitoneally (i.p.) (17). The homogenate used for i.p. administration had an i.p. titer of 1 × 104.6 50% infectious doses per 0.05 ml in Tg7 mice. Immediately following infection, the mice were treated i.p. with 0.05 ml of the individual phthalocyanine compounds. All of the compounds were dissolved in either water or saline, with the exception of H2PcS1, which was sufficiently soluble only in dimethyl sulfoxide (DMSO). DMSO alone does not have significant anti-TSE activity (17). The concentrations of the compounds used were as follows: H2PcS4, H2PcS2 and H2PcS1, 5 mg/ml; Ni(II)PcS4, V(IV)-OPcS4, and Al(III)PcS4, 10 mg/ml; and Fe(III)PcS4, 40 mg/ml. Treatment was continued three times per week over a 4-week period (12 treatments in total) and then halted. Control mice were not treated with any compounds. In separate experiments, Tg7 mice were treated with Cu(II)PcS4, Fe(III)TSP, or Mn(III)TSP dissolved in PBS to evaluate their antiscrapie activities (see Table 2). These mice were inoculated with 0.05 ml of 1% scrapie strain 263K-infected brain homogenate either i.p. or intracerebrally (i.c.). All mice were monitored and euthanized when they exhibited clear signs of early scrapie infection, including tremors, ataxia, and somnolence. Where necessary, diagnoses of scrapie were confirmed by the detection of PrP-res in the brains of infected animals by using Western blot analysis.
TABLE 2.
Effects of different cyclic tetrapyrrole treatments on scrapie strain 263K incubation periods in Tg7 mice
| Compound | Dose | Dosing regimena | Scrapie infection route | Incubation periods (days) | Mean ± SD |
|---|---|---|---|---|---|
| None | None | i.p. | 85, 76, 93, 91, 83, 83, 85, 97 | 86.6 ± 6.7 | |
| Cu(II)PcS4 | 25 mg/kg, i.p. | M, W, F for 6 wk starting 2 wk prior to inoculation | i.p. | 335, 354, 353, 365, 396, 294, 378, 332 | 350.9 ± 31.2 |
| Cu(II)PcS4 | 25 mg/kg, i.p. | M, W, F until death starting 50 days after inoculation | i.p. | 98, 97, 83, 91, 87, 83, 87, 82, 83 | 87.9 ± 6.2 |
| Noneb | 50 μl PBS, i.c. | Days 14, 21, 28, 35, 42 | i.c. | 42, 42, 44, 44, 45, 45, 46, 46, 46, 47, 47, 48, 51, 52, 53 | 46.5 ± 3.3 |
| Cu(II)PcS4 | 50 μl 0.5 mg/ml, i.c. | M, W, F for 3 wk starting 2 wk after inoculationc | i.c. | 51, 42, 51, 58, 79, 48, 45, 48 | 52.8 ± 11.6 |
| Cu(II)PcS4 | 50 μl 0.5 mg/ml, i.c. | Days 14, 21, 28, 35, 42 | i.c. | 41, 47, 46, 47, 44, 47, 45, 45 | 45.3 ± 2.1 |
| Fe(III)TSPb | 50 μl 0.5 mM, i.c. | Days 14, 21, 28, 35, 42 | i.c. | 53, 54, 58, 59, 59, 61, 62, 63, 63, 64, 65, 65, 71 | 61.3 ± 4.8 |
| Mn(III)TSP | 50 μl 0.5 mM, i.c. | Days 14, 21, 28, 35, 42 | i.c. | 46, 47, 48, 48, 48, 49, 50, 51 | 48.4 ± 1.6 |
M, Monday; W, Wednesday; F, Friday.
Combined data from two separate but identically conducted experiments.
Treatments were stopped at 3 weeks due to observed toxicity.
Spectral methods.
UV and visible spectra were collected by using an OLIS (Online Instruments) conversion of a Cary 16 spectrophotometer.
RESULTS
Sulfonation and metal effects on inhibition of PrP-res accumulation in vitro.
The relative abilities of differently sulfonated and metal-bound phthalocyanines to inhibit PrP-res accumulation were compared by using a murine neuroblastoma cell line (N2a) chronically infected with either the RML or 22L strain of scrapie (8, 20) and the Rov9 cell line infected with sheep scrapie (12, 22). After the cells were seeded at a low density, a series of concentrations of phthalocyanine was added to the culture medium. The cells were grown to near confluence, harvested, and analyzed for PrP-res by using immunoblotting procedures, as described previously (8). The EC50 of the inhibitor (the concentration of inhibitor that gave 50% of the PrP-res found in the controls) was estimated from semiquantitative analyses of the immunoblot signals (Table 1).
TABLE 1.
Phthalocyanine sulfonate inhibition of PrP-res formation in scrapie-infected cell cultures
| Compound | Mean EC50 ± SD (μM) (n = 3-6)
|
||
|---|---|---|---|
| Mouse scrapie strain 22L (N2a cells) | Mouse scrapie strain RML (N2a cells) | Sheep scrapie strain (Rov9 cells) | |
| H2PcS1 | 0.8 ± 0.2 | 0.7 ± 0.5 | NTa |
| H2PcS2 | 0.7 ± 0.1 | 0.4 ± 0.2 | NT |
| H2PcS4 | 1.1 ± 0.6 | 1.2 ± 0.6 | NT |
| Ni(II)PcS4 | 0.7 ± 0.0 | 0.2 ± 0.0 | 0.9 ± 1.5 |
| Fe(III)PcS4 | 0.7 ± 0.1 | 0.4 ± 0.2 | 4.5 ± 1.3 |
| Mn(III)PcS4 | 2.5 ± 1.2 | NT | 4.5 ± 1.4 |
| Cu(II)PcS4 | 3.0 ± 0.4 | NT | 5.0 ± 0.7 |
| V(IV)OpcS4 | 3.5 ± 1.2 | 2.3 ± 0.1 | 2.6 ± 1.5 |
| Zn(II)PcS4 | 6.1 ± 4.1 | NT | 3.7 ± 1.0 |
| Al(III)PcS4 | 10.0 ± 1.2 | >10 | >20 |
NT, not tested.
The extent of sulfonation of metal-free phthalocyanine had little effect on the EC50 values observed in a given infected cell type. The Ni(II) and Fe(III) complexes exhibited EC50 values similar to those for the metal-free compound. Other metal ions increased the EC50 values (i.e., reduced the anti-TSE potency of the phthalocyanine). The rank order of EC50s was, in general, H2PcS4, Ni(II)PcS4 < Cu(II)PcS4, V(IV)OPcS4, Zn(II)PcS4, Mn(III)PcS4 ≪ Al(III)PcS4. Fe(III)PcS4 was among the most potent in the murine cells, while it was of intermediate potency in the Rov9 cells.
Sulfonation and metal effects on in vivo antiscrapie activities of cyclic tetrapyrroles.
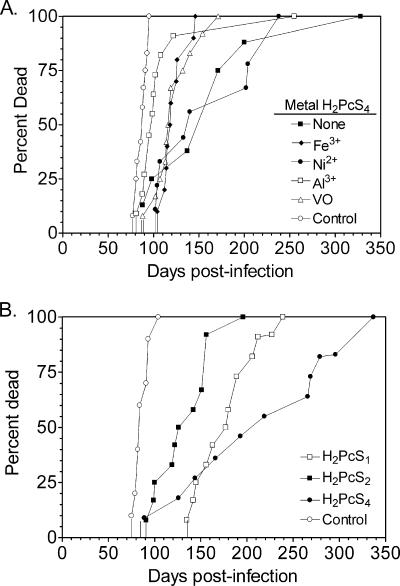
To assess the abilities of the various sulfonated and metal-bound phthalocyanines to affect the progression of TSE disease in vivo, the compounds were tested in a rodent model of scrapie. Tg7 mice, which overexpress hamster PrP-sen and which are highly susceptible to hamster scrapie (19), were infected i.p. with hamster scrapie strain 263K. Starting at the time of infection, the mice were treated i.p. with the different phthalocyanines three times a week for 4 weeks, and disease incubation times were monitored. As shown in Fig. 2A, the nickel-bound and metal-free compounds significantly delayed disease incubation times compared to the times for the untreated controls (P < 0.01 by one-way analysis of variance [ANOVA] with Dunnett's posttest), with the metal-free phthalocyanine doing so at half or less of the doses of the metal-bound compounds. While none of the other metal-bound phthalocyanines delayed disease significantly (P > 0.05), in the majority of animals the V(IV)OPcS4 and Fe(III) PcS4 compounds appeared to delay disease at least 2 weeks longer than Al(III)PcS4. The fact that the metal-free and Ni(II) compounds were most effective in vivo while Al(III)PcS4 was least effective correlated with their relative abilities to inhibit PrP-res formation in vitro.
FIG. 2.
Effect of prophylactic treatment with differentially metal-bound (A) and sulfonated (B) phthalocyanines on scrapie incubation periods in mice. Tg7 mice were infected i.p. with the 263K scrapie strain. “Control” designates mock-treated animals. Starting at the time of infection, mice were treated i.p. with the different compounds three times a week for 4 weeks. Comparisons of the relative efficacies of H2PcS1 and H2PcS4 (0.25 mg/dose) by Dunnett's, Tukey's, or Bonferroni's multiple-comparison tests showed no significant difference between the two compounds (P > 0.05), while analysis by the Newman-Keuls test showed only a marginal difference (P < 0.05). In some cases, the relative efficacies were not directly comparable because the phthalocyanine doses varied as follows: Ni(II)PcS4, V(IV)OPcS4 and Al(III)PcS4, 0.5 mg; Fe(III)PcS4, 2 mg.
As one of the more effective in vitro PrP-res inhibitors, Cu(II)PcS4 was tested against scrapie strain 263K in Tg7 mice by using several testing regimens (Table 2). As a prophylactic treatment, Cu(II)PcS4 administered i.p. for 4 weeks following i.p. scrapie challenge delayed the time to disease onset by about fourfold. However, as a postexposure treatment, it was ineffective against an established i.p. infection when it was administered i.p. or an established i.c. infection when it was administered i.c.
The differently sulfonated phthalocyanines were also tested in Tg7 mice (Fig. 2B). In vivo, H2PcS1 and H2PcS4 significantly delayed the disease incubation times compared with the times for the untreated controls (P < 0.01 by one-way ANOVA with Dunnett's posttest), whereas H2PcS2 had a marginally significant beneficial effect (P < 0.05). It should be noted that due to relative insolubility in aqueous media, H2PcS1 was dissolved in DMSO prior to inoculation, possibly affecting its relative activity. However, given that H2PcS1 and H2PcS4 exhibited similar effects in vivo (P > 0.05 or P < 0.05 by multiple one-way ANOVA tests; see the legend to Fig. 2B), these data are consistent with the observations in vitro and provide evidence that the number of sulfonates does not strongly influence anti-TSE activity.
Fe(III) complexed with meso-tetra(4-sulfonylphenyl)porphyrin [Fe(III)TSP] can substantially (P < 0.01 versus the results for the untreated animals) improve survival times either when it is administered i.p. prior to an i.p. scrapie inoculation or when it is administered i.c. beginning ∼2 weeks after an i.c. scrapie inoculation (11) (Table 2). However, Mn(III)TSP proved ineffective in the same test.
Self-association tendencies of phthalocyanine sulfonates.
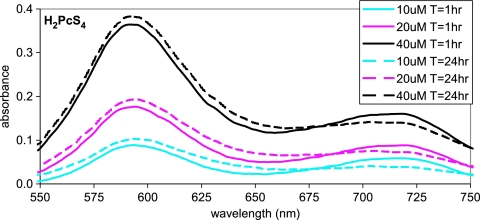
To study the possibility that the self-association of the phthalocyanines correlates with anti-TSE activity, relative aggregation tendencies were compared by using visible spectroscopy. The spectra of various sulfonates in graded DMSO-PBS mixtures were determined. The ability of DMSO to stabilize these compounds as monomeric species and the ability of aqueous media to promote their aggregation are well established (21, 24). Spectra can vary widely with changes in aggregation state and, hence, with mixtures of PBS and DMSO solvents. Aggregates characteristically exhibit much broader, less intense band maxima than the monomers from which they are formed (21). Depending on the geometric arrangement of the molecules in the aggregate, wavelength shifts toward the blue or red may be seen compared with the absorbance maximum of the monomer. For example, such solvent effects are shown for H2PcS1 in PBS, DMSO, and mixtures of these solvents (Fig. 3). The H2PcS1 spectrum in PBS exhibited a band at ∼590 nm for one type of aggregate that was blue shifted and less intense compared with the spectrum of the monomer in pure DMSO. Another aggregate type yielded spectra with a broad low-intensity band that was red shifted beyond 700 nm compared to the spectrum of the monomer (Fig. 3). Similar bands near 590 nm and 720 nm were observed for H2PcS4 in PBS. These bands were affected modestly by both concentration and the time that they were allowed to stand after solution preparation (Fig. 4). Blue-shifted species are described as being of the H type, wherein cyclic tetrapyrroles are oriented face to face, whereas aggregates with red shifts are described as being of the J type due to an edge-to-edge or slipped face-to-face orientation (21). Thus, the spectra in Fig. 4 indicate that H2PcS4 formed more H-type aggregate than J-type aggregate at all concentrations, but did so to a greater extent at the higher concentrations. At each concentration, slow conversions from the J type to the H type were evident. However, the spectra of solutions of H2PcS4 in DMSO exhibited no significant changes at comparable concentrations or times of standing (data not shown).
FIG. 3.
Effect of solvent polarity on visible absorbance spectra of H2PcS1. H2PcS1 solutions (50 μM) were prepared with the designated percentage of DMSO. The balance of the solvent was PBS (10 mM sodium phosphate, pH 6.9, 130 mM sodium chloride). The spectra of DMSO and PBS alone gave no significant absorbance in this spectral window (data not shown).
FIG. 4.
Absorbance spectra of H2PcS4 in PBS at the designated concentrations and times (T) after solution preparation.
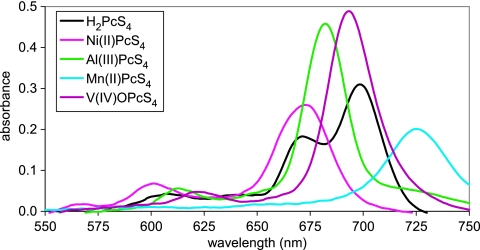
Relative to the similarities of the spectra of the differently sulfonated metal-free phthalocyanines, the spectra obtained for PcS4 molecules with different metals varied widely. Such differences are evident for the Ni(II), Mn(III), V(IV)O, and Al(III) complexes in the spectra in DMSO (Fig. 5). As with H2PcS4, when the solvent was changed from DMSO to PBS, the Ni(II), Mn(III), and V(IV)O complexes gave striking spectral changes that were indicative of self-association in PBS by the criteria described above (Fig. 6). In contrast, the Al(III)PcS4 spectra exhibited a major band near 670 nm and a much less intense band near 600 nm under all solvent conditions (Fig. 6), which are characteristic of the spectra for the soluble monomer. The solvent-induced subtle red shifts found with Al(III)PcS4 on going from PBS to DMSO may be attributed solely to differences in solvation. Because monomeric Al(III)PcS4 is a much less potent PrP-res inhibitor than the other much more aggregation-prone phthalocyanines, these results suggest that self-association is important in the inhibition of PrP-res formation.
FIG. 5.
Absorbance spectra of H2PcS4 and its Ni(II), V(IV)O, Mn(III), and Al(III) complexes at 5 μg/ml in DMSO.
FIG. 6.
Absorbance spectra of H2PcS4 and its Ni(II), V(IV)O, Mn(III), and Al(III) complexes in PBS, DMSO, and PBS-DMSO mixtures. The Al(III) complex was used at a concentration of 10 μM, and the others were used at concentrations of 5 μM. Data for a higher concentration of Al(III)PcS4 are shown to provide stronger evidence of its relative lack of a tendency to self-associate, because the oligomerization of molecules is more likely at higher concentrations.
Induction of phthalocyanine sulfonate aggregation by a cationic cyclic tetrapyrrole.
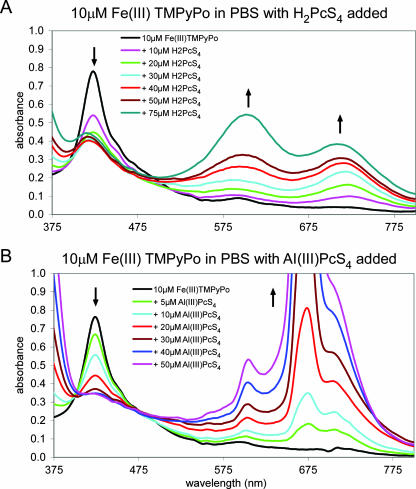
To further investigate aggregation behavior, Fe(III)TMPyPo (Fig. 1) was incorporated into phthalocyanine sulfonate solutions as another spectral probe and a potential nidus for the assembly of phthalocyanine oligomers. Fe(III)TMPyPo is a cationic cyclic tetrapyrrole that is capable of forming ion pairs and, possibly, π-stacked oligomers with anionic phthalocyanine sulfonates (14). Spectra for solutions in PBS containing a constant concentration of Fe(III)TMPyPo but different levels of H2PcS4 or Al(III)PcS4 were obtained (Fig. 7). With increasing phthalocyanine concentrations, the Fe(III)TMPyPo Soret band near 425 nm, which is in a region of low absorbance by the phthalocyanines, lost intensity as the absorbance from 575 nm to 800 nm increased. Although a detailed elucidation of the spectrum and composition of each individual heteroaggregate present was not attempted, the changes in isobestic points (points of intersection of spectral curves) observed in the spectra at 1:1 and 2:1 phthalocyanine/porphyrin ratios indicate the formation of heterodimers and heterotrimers with each of the phthalocyanines. More importantly, heteroaggregates with even higher phthalocyanine/porphyrin ratios were indicated by further changes in the isobestic point with increasing concentrations of H2PcS4 but not with increasing concentrations of Al(III)PcS4. Observations similar to those made for H2PcS4 were found for Ni(II)PcS4 (data not shown). In DMSO, which monomerizes phthalocyanine sulfonates, Fe(III)TMPyPo promoted the higher-order aggregation of both Ni(II)PcS4 and H2PcS4 (data not shown). No interaction with Al(III)PcS4 was evident even at high concentrations of Al(III)PcS4 relative to those of Fe(III)TMPyPo (data not shown). These results are consistent with Al(III)PcS4 having substantially less of a propensity to form higher-order aggregates than H2PcS4 or Ni(II)PcS4, even when it is “seeded” by a cationic cyclic tetrapyrrole.
FIG. 7.
Absorbance spectra of solutions of Fe(III)TMPyPo with the designated concentrations of H2PcS4 (A) and Al(III)PcS4 (B). The arrows denote directions of absorbance changes as concentrations of added H2PcS4 or Al(III)PcS4 increased.
DISCUSSION
Previous studies have shown that H2PcS4 inhibits PrP-res formation and has strong prophylactic activities in vivo (5, 17, 18). Furthermore, in vitro studies have shown that differential metal binding by PcS4 can affect its inhibition of PrP-res formation (5). Here, we provide evidence that the metal-dependent tendency of various cyclic tetrapyrroles to self-associate correlates with these activities in vivo and in vitro but that the degree of sulfonation of PcS4 is not as critical a determinant of activity.
Our attempts to correlate the in vitro and in vivo effects are complicated by the fact that different strains and host species were used in the different experimental systems. Species and strain effects have been known to be influential in the activities of some types of inhibitors (12) and may partially explain some of the current observations. Accordingly, one should be cautious about drawing mechanistic parallels between the in vitro and the in vivo systems. Nonetheless, a common observation in each of the various experimental systems is that active cyclic tetrapyrroles have a propensity to self-associate or stack, while those that are least active are much less prone to self-association.
A better understanding of the structure-activity relationships of cyclic tetrapyrroles may help to identify more effective anti-TSE compounds. Increasing the number of negatively charged sulfonate groups on phthalocyanines enhances their solubility in aqueous media and influences their distribution in vivo (1, 6, 21). However, the fact that H2PcS1, H2PcS2, and H2PcS4 each showed strong anti-TSE activities in vitro and in vivo indicates that the number of sulfonate groups bearing negative charges at the periphery of metal-free phthalocyanine macrocycles is not a critical determinant of anti-TSE activity. This finding suggests that electrostatic bonding interactions between negative sulfonates and positive centers on a target binding site on PrP contribute much less to the strength of the binding than do other types of bonding, such as π-π interactions involving the phthalocyanine aromatic system. The similarity of the UV-visible absorption spectra of the different metal-free phthalocyanine sulfonates is consistent with the fact that the number of sulfonates has little effect on the π-electron systems of these highly aromatic cyclic tetrapyrroles. Furthermore, the spectral data provided evidence that the tendencies to form stacked aggregates in PBS are similar for these metal-free phthalocyanines. Thus, one property that the three differentially sulfonated phthalocyanines have in common is an ability to enter into π bonding of the type required for stacking.
The substantial influence of different metals on the in vivo and in vitro anti-TSE activities of PcS4 compounds raises the question of how metal ion occupancy affects the behavior of this molecule. Several well-established differences in coordination chemistry among the metals in the phthalocyanine preparations appeared not to be critical determinants of activity. As noted above, one such variable is the residual positive charge at the metal. The displacement of two protons from the central nitrogens of H2PcS4 leaves two negative charges to neutralize two positive charges associated with an incoming metal ion (Fig. 1). As a result, there is no residual charge on the metal in the Ni2+, Cu2+, and Zn2+ complexes of PcS4 and one positive charge with the Fe3+, Mn3+, or Al3+ complexes. With V(IV)-OPcS4, wherein V4+ is bonded to an oxygen atom, there is no charge remaining on the vanadium. The results obtained suggest that the residual charge on the metal (Table 1) is not a critical determinant of inhibitor efficacy. Another variable is the preferred coordination stereochemistry among these metals. Ni(II) and Cu(II) prefer square planar complexes, with the metal atom being coplanar with the four nitrogens to which it is bonded. Fe(III), Al(III), and V(IV) each prefer square pyramidal structures, wherein the metal lies outside the plane defined by the four nitrogens. However, if two axial ligands bind to Fe(III), one on each side of the macrocycle, the iron atom can assume coplanarity with the four nitrogens. A third variable not obviously essential for anti-TSE activity is a marked difference in affinities of the metal for axial ligands among the metal PcS4s; Ni(II) and Cu(II) are expected to bind to ligands much less avidly than the other metals.
One property of cyclic tetrapyrroles that does appear to correlate with anti-PrP-res activity is their tendency to form stacked oligomers. Of the cyclic tetrapyrroles tested, Al(III)-PcS4 has much less of a tendency to self-associate and is by far the least effective PrP-res inhibitor in vivo and in cell culture. Previous studies have also shown that Al(III)PcS4 is a much weaker inhibitor of PrP-res formation in cell-free conversion reactions than H2PcS4 and Fe(III)PcS4 (5). Additional support for a relationship between PrP-res inhibition and aggregation tendency is found in studies of Mn(III) and Fe(III) complexes of TSP, another cyclic tetrapyrrole with four sulfonic acid groups on the periphery of the molecule (5). The previous study showed the Mn(III)TSP is a poor inhibitor in cell cultures compared to Fe(III)TSP. Furthermore, as shown in Table 2, Fe(III)TSP exhibits therapeutic activity in mice, whereas under the same conditions Mn(III)TSP does not. Others have shown that Mn(III)TSP is essentially monomeric in aqueous media at physiological pH, whereas Fe(III)TSP exists predominantly as dimers or larger aggregates (23). Furthermore, we have seen less inhibition of PrP-res accumulation in vitro by Mn(III) than Fe(III) complexes of deuteroporphyrinIX-2,4-disulfonate, a sulfonated cyclic tetrapyrrole structure related to hemin (data not shown). Moreover, spectral analyses in graded PBS-DMSO were consistent with the Mn(III) deuteroporphyrinIX-2,4-disulfonate having less of a tendency to oligomerize than the Fe(III) complex. Thus, analyses of several types of cyclic sulfonated tetrapyrroles suggest the importance of oligomerization in anti-TSE activity.
The spectra of mixtures of different PcS4s with Fe(III)TMPyPo indicated the formation of heteroaggregates between a positively charged molecule and one or more negative phthalocyanines. With H2PcS4 and Ni(II)PcS4, evidence for the stacking of many phthalocyanines per porphyrin was observed. Such stacking occurred to a greater extent in PBS than in DMSO. In contrast, Al(III)PcS4 showed no tendency to form heteroaggregates in DMSO and in PBS formed heterodimers and heterotrimers [possibly sandwiches with Fe(III)TMPyPo in the middle] but not higher-order aggregates. In the sense that Fe(III)TMPyPo can serve as a nidus for the stacking of the inhibitory PcS4 molecules, it may be acting in a manner analogous to the binding site on PrP that mediates the anti-TSE activity of cyclic tetrapyrroles. H2PcS4 has been shown to inhibit the conversion interaction between PrP-sen and PrP-res directly, apparently by binding to one or both forms of PrP (4, 5). Anti-TSE activity may depend on the ability of multiple cyclic tetrapyrroles to bind to PrP as a preformed aggregate or to stack sequentially onto a bound monomer. In this case, Al(III)PcS4 molecules may bind individually to PrP but have little tendency to attract additional Al(III)PcS4s, accounting for its low anti-TSE activity. Such relationships are consistent with a recently proposed general mechanism of anti-TSE activity (3).
The solvent effects on phthalocyanine associations observed in these studies, as well as other reports (21), provide evidence that differences within tissue microenvironments may modify the equilibrium constants and the kinetics of formation and dissociation of aggregates. A specific geometric arrangement that would be required for an aggregate to possess activity cannot be reliably proposed based solely on the present evidence. However, the further application of UV-visible and other spectral techniques may allow the clarification of the structure and bonding interactions of inhibitory cyclic tetrapyrrole oligomers when they are bound to PrP. Related future studies in which individual phthalocyanine sulfonate regioisomers are used may help to refine more sharply the structural requirements for maximal anti-TSE activity.
The present data are consistent with those from previous studies (17, 18) in showing that certain phthalocyanine sulfonates and other anti-TSE cyclic tetrapyrroles appear to be well tolerated by rodents receiving long-term dosing regimens. These observations remain consistent with the possibility that the development of effective pre- and postexposure prophylaxic treatments against a variety of TSE/prion diseases may be possible with cyclic tetrapyrroles or related compounds.
Acknowledgments
This work partly funded by the Intramural Research Program of the NIH, NIAID, and U.S. DoD Prion interagency transfer NP020114.
We thank Valerie Sim, Henry Onwubiko, and Richard Race for critical reading of the manuscript; Anita Mora for graphics assistance; and Ed Schreckendgust for animal handling.
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Boyle, R. W., and D. Dolphin. 1996. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 64:469-485. [DOI] [PubMed] [Google Scholar]
- 2.Caughey, B., and G. S. Baron. 2006. Prions and their partners in crime. Nature 443:803-810. [DOI] [PubMed] [Google Scholar]
- 3.Caughey, B., W. S. Caughey, D. A. Kocisko, K. S. Lee, J. R. Silveira, and J. D. Morrey. 2006. Prions and transmissible spongiform encephalopathy (TSE) chemotherapeutics: a common mechanism for anti-TSE compounds? Acc. Chem. Res. 39:646-653. [DOI] [PubMed] [Google Scholar]
- 4.Caughey, B., L. D. Raymond, G. J. Raymond, L. Maxson, J. Silveira, and G. S. Baron. 2003. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 77:5499-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95:12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gantchev, T. G., R. Ouellet, and J. E. van Lier. 1999. Binding interactions and conformational changes induced by sulfonated aluminum phthalocyanines in human serum albumin. Arch. Biochem. Biophys. 366:21-30. [DOI] [PubMed] [Google Scholar]
- 7.Howlett, D., P. Cutler, S. Heales, and P. Camilleri. 1997. Hemin and related porphyrins inhibit beta-amyloid aggregation. FEBS Lett. 417:249-251. [DOI] [PubMed] [Google Scholar]
- 8.Kocisko, D. A., G. S. Baron, R. Rubenstein, J. Chen, S. Kuizon, and B. Caughey. 2003. New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J. Virol. 77:10288-10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocisko, D. A., and B. Caughey. 2006. Searching for anti-prion compounds: cell-based high-throughput in vitro assays and animal testing strategies. Methods Enzymol. 412:223-234. [DOI] [PubMed] [Google Scholar]
- 10.Kocisko, D. A., B. Caughey, J. D. Morrey, and R. E. Race. 2006. Enhanced antiscrapie effect using combination drug treatment. Antimicrob. Agents Chemother. 50:3447-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocisko, D. A., W. S. Caughey, R. E. Race, G. Roper, B. Caughey, and J. D. Morrey. 2006. A porphyrin increases survival time of mice after intracerebral prion infection. Antimicrob. Agents Chemother. 50:759-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocisko, D. A., A. L. Engel, K. Harbuck, K. M. Arnold, E. A. Olsen, L. D. Raymond, D. Vilette, and B. Caughey. 2005. Comparison of protease-resistant prion protein inhibitors in cell cultures infected with two strains of mouse and sheep scrapie. Neurosci. Lett. 388:106-111. [DOI] [PubMed] [Google Scholar]
- 13.Lee, E. N., H. J. Cho, C. H. Lee, D. Lee, K. C. Chung, and S. R. Paik. 2004. Phthalocyanine tetrasulfonates affect the amyloid formation and cytotoxicity of alpha-synuclein. Biochemistry 43:3704-3715. [DOI] [PubMed] [Google Scholar]
- 14.Lipskier, J. F., and T. H. Tran-Thi. 1993. Supramolecular assemblies of porphyrins and phthalocyanines bearing oppositely charged substituents. First evidence of heterotrimer formation. Inorg. Chem. 32:722-731. [Google Scholar]
- 15.Maxson, L., C. Wong, L. M. Herrmann, B. Caughey, and G. S. Baron. 2003. A solid-phase assay for identification of modulators of prion protein interactions. Anal. Biochem. 323:54-64. [DOI] [PubMed] [Google Scholar]
- 16.Pasternack, R. F., E. J. Gibbs, S. Sibley, L. Woodard, P. Hutchinson, J. Genereux, and K. Kristian. 2006. Formation kinetics of insulin-based amyloid gels and the effect of added metalloporphyrins. Biophys. J. 90:1033-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Priola, S. A., A. Raines, and W. Caughey. 2003. Prophylactic and therapeutic effects of phthalocyanine tetrasulfonate in scrapie-infected mice. J. Infect. Dis. 188:699-705. [DOI] [PubMed] [Google Scholar]
- 18.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine anti-scrapie compounds. Science 287:1503-1506. [DOI] [PubMed] [Google Scholar]
- 19.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Race, R. E., B. Caughey, K. Graham, D. Ernst, and B. Chesebro. 1988. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J. Virol. 62:2845-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snow, A. W. 2003. Phthalocyanine aggregation, p. 129-176. In K. M. Kadish, K. M. Smith, and R. Guilard (ed.), The porphyrin handbook, vol. 17. Elsevier Science, New York, NY. [Google Scholar]
- 22.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yushmanov, V. E., H. Imasato, T. T. Tominaga, and M. Tabak. 1996. H NMR and electronic absorption spectroscopy of paramagnetic water-soluble meso-tetraarylsubstituted cationic and anionic metalloporphyrins. J. Inorg. Biochem. 61:233-250. [DOI] [PubMed] [Google Scholar]
- 24.Zelina, J. P., C. K. Njue, J. F. Rusling, G. N. Kamau, M. Masila, and J. Kibuqu. 1999. Influence of surfactant-based microheterogeneous fluids on aggregation of copper phthalocyanine tetrasulfonate. J. Porphyrins Phthalocyanines 3:188-195. [Google Scholar]