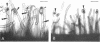Abstract
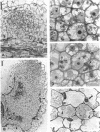
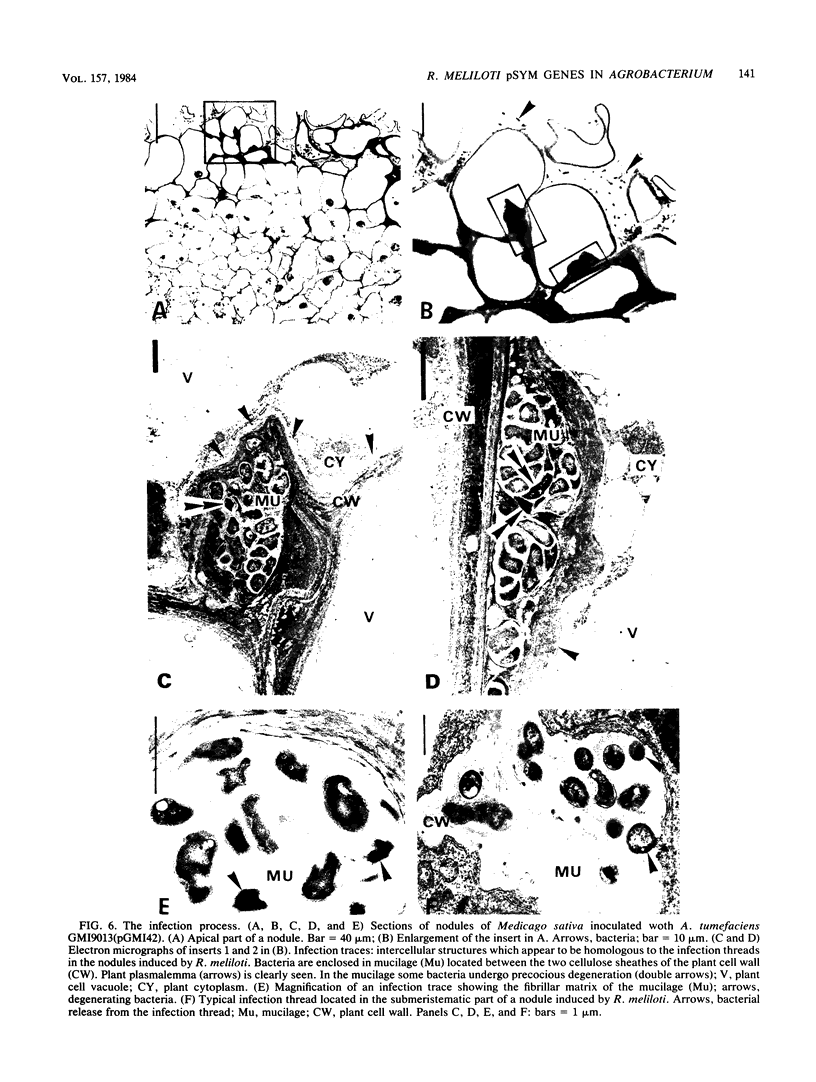
The pSym megaplasmid of Rhizobium meliloti 2011 mobilized by plasmid RP4, or plasmid pGMI42, an RP4-prime derivative which carries a 290-kilobase pSym fragment including nitrogenase and nod genes, was introduced into Agrobacterium tumefaciens. The resulting transconjugants induced root deformations specifically on the homologous hosts Medicago sativa and Melilotus alba and not on the heterologous hosts Trifolium pratense and Trifolium repens. The root deformations were shown to be genuine nodules by physiological and cytological studies. Thus, host specificity nodulation genes are located on the pSym megaplasmid. Host nodulation specificity did not seem to require recognition at the root hair level since no infection threads could be detected in the root hairs. Cytological observations indicated that bacteria penetrated only the superficial layers of the host root tissue by an atypical infection process. The submeristematic zone and the central tissue of the nodules were bacteria free. Thus, nodule organogenesis was probably triggered from a distance by the bacteria. Agrobacterium transconjugants carrying pSym induced the formation of more numerous and larger nodules than those carrying the RP4-prime plasmid pGMI42, suggesting that some genes influencing nodule organogenesis are located in a pSym region(s) outside that which has been cloned into pGMI42.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boucher C., Bergeron B., De Bertalmio M. B., Dénarié J. Introduction of bacteriophage Mu into Pseudomonas solanacearum and Rhizobium meliloti using the R factor RP4. J Gen Microbiol. 1977 Jan;98(1):253–263. doi: 10.1099/00221287-98-1-253. [DOI] [PubMed] [Google Scholar]
- Cami B., Kourilsky P. Screening of cloned recombinant DNA in bacteria by in situ colony hybridization. Nucleic Acids Res. 1978 Jul;5(7):2381–2390. doi: 10.1093/nar/5.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Forrai T., Vincze E., Bánfalvi Z., Kiss G. B., Randhawa G. S., Kondorosi A. Localization of symbiotic mutations in Rhizobium meliloti. J Bacteriol. 1983 Feb;153(2):635–643. doi: 10.1128/jb.153.2.635-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Long S. R., Bang M., Haskins N., Ausubel F. M. Structural studies of alfalfa roots infected with nodulation mutants of Rhizobium meliloti. J Bacteriol. 1982 Jul;151(1):411–419. doi: 10.1128/jb.151.1.411-419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., Snijdewint F. G., Schilperoort R. A. Identification of the Sym plasmid of Rhizobium leguminosarum strain 1001 and its transfer to and expression in other rhizobia and Agrobacterium tumefaciens. Plasmid. 1982 Jul;8(1):73–82. doi: 10.1016/0147-619x(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Huber J. D., Parker F., Odland G. F. A basic fuchsin and alkalinized methylene blue rapid stain for epoxy-embedded tissue. Stain Technol. 1968 Mar;43(2):83–87. doi: 10.3109/10520296809115048. [DOI] [PubMed] [Google Scholar]
- Julliot J. S., Boistard P. Use of RP4-prime plasmids constructed in vitro to promote a polarized transfer of the chromosome in Escherichia coli and Rhizobium meliloti. Mol Gen Genet. 1979 Jun 20;173(3):289–298. doi: 10.1007/BF00268639. [DOI] [PubMed] [Google Scholar]
- Latreille J., Barlogie B., Dosik G., Johnston D. A., Drewinko B., Alexanian R. Cellular DNA content as a marker of human multiple myeloma. Blood. 1980 Mar;55(3):403–408. [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Signer E. R. Genetic mapping of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1977 May;74(5):2076–2078. doi: 10.1073/pnas.74.5.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Leps W. T., Brill W. J. Agglutinin from Alfalfa Necessary for Binding and Nodulation by Rhizobium meliloti. Science. 1981 Sep 25;213(4515):1513–1515. doi: 10.1126/science.213.4515.1513. [DOI] [PubMed] [Google Scholar]
- Prakash R. K., Schilperoort R. A., Nuti M. P. Large plasmids of fast-growing rhizobia: homology studies and location of structural nitrogen fixation (nif) genes. J Bacteriol. 1981 Mar;145(3):1129–1136. doi: 10.1128/jb.145.3.1129-1136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Singh R. K., Singh H. N. Isolation and preliminary characterization of mutants of the cyanobacterium Nostoc muscorum resistant to growth inhibition by methylamine. Mol Gen Genet. 1981;184(2):334–336. doi: 10.1007/BF00272927. [DOI] [PubMed] [Google Scholar]