Abstract
The recommended treatments for Mycobacterium avium complex (MAC) infectious disease are combination regimens of clarithromycin (CLR) or azithromycin with ethambutol and rifamycin. However, these chemotherapy regimens are sometimes unsuccessful. Recently developed antimicrobial agents, such as newer fluoroquinolones (FQs) containing C-8 methoxy quinolone (moxifloxacin [MXF] and gatifloxacin [GAT]), are expected to be novel antimycobacterial agents. Here, we evaluated the in vitro and in vivo antimycobacterial activities of three FQs (MXF, GAT, and levofloxacin) and CLR against clinically isolated MAC strains. Subsequently, the in vitro and in vivo synergic activities of FQ-CLR combinations against MAC strains were investigated. CLR and the individual FQs alone showed promising activity against MAC strains in vitro, and the bacterial counts in organs (lungs, liver, and spleen) of MAC-infected mice treated with single agents were significantly reduced compared to control mice. CLR showed the best anti-MAC effect in vivo. When the three FQs were individually combined with CLR in vitro, mild antagonism was observed for 53 to 57% of the tested isolates. Moreover, mice were infected with MAC strains showing mild antagonism for FQ-CLR combinations in vitro, and the anti-MAC effects of the FQ-CLR combinations were evaluated by counting the viable bacteria in their organs and by histopathological examination after 28 days of treatment. Several FQ-CLR combinations exhibited bacterial counts in organs significantly higher than those in mice treated with CLR alone. Our results indicate that the activity of CLR is occasionally attenuated by combination with an FQ both in vitro and in vivo and that this effect seems to be MAC strain dependent. Careful combination chemotherapy using these agents against MAC infectious disease may be required.
Mycobacterium avium complex (MAC) infectious disease occurs on a global scale, but it is endemic to certain northern temperate regions (9). In Japan, pulmonary MAC infectious disease is the most common nontuberculous mycobacterial infection, and a recent increase in the incidence of MAC disease has been observed (12, 13). In 1997, the American Thoracic Society guidelines for MAC infectious disease in the absence of HIV infection recommended a regimen of a macrolide (clarithromycin [CLR] or azithromycin) combined with a rifamycin (rifampin or rifabutin) and ethambutol for 10 to 12 months after bacteria can no longer be detected in the sputum (2). However, this treatment is sometimes unsuccessful.
Recently developed antimicrobial agents, such as fluoroquinolones (FQs), are expected to have antimycobacterial effects (10, 17). FQs have advanced the treatment of nontuberculous mycobacterial infections (17). In fact, some FQs show activity against MAC and other mycobacteria in vitro (10, 14, 17, 19). However, their roles in the treatment of MAC infectious disease remain to be determined. On the other hand, CLR is currently known to be the most effective agent against MAC, both in vitro and in vivo, and is used in chemotherapy against MAC infectious disease worldwide. Based on the anti-MAC activities of such agents, it is very important to clarify how newer FQs interact with CLR against different MAC strains, especially in vivo, in order to predict their clinical utility.
In the present study, we evaluated in vitro and in vivo anti-MAC activities of novel FQs, such as moxifloxacin (MXF) and gatifloxacin (GAT), relative to that of CLR and determined the combined activities of various FQs with CLR against different MAC strains in vitro and in vivo in order to establish the clinical utility of such combination therapy.
(Parts of this work were presented at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC] in Washington, DC, December 2005, and the 46th ICAAC in San Francisco, CA, September 2006, sponsored by the American Society for Microbiology.)
MATERIALS AND METHODS
Microorganisms.
A total of 78 MAC strains, including two reference strains (M. avium JATA51-01 and Mycobacterium intracellulare JATA52-01, derived from M. avium ATCC 25291 and M. intracellulare ATCC 13950, respectively) purchased from the Japan Anti-Tuberculosis Association (Tokyo, Japan) were used in this study. The remaining 76 strains were clinically isolated from patients diagnosed as having MAC infection based on the criteria of the Mycobacteriosis Research Group of the Japanese National Chest Hospitals (20) (similar to the American Thoracic Society criteria). All isolates were confirmed using Amplicor mycobacterium (Hoffmann-La Roche, Basel, Switzerland) or DDH mycobacteria (Kyokuto Pharmaceutical Industrial Co., Tokyo, Japan). Bacterial colonies were grown on Difco Middlebrook 7H10 agar plates (Becton Dickinson, Rutherford, NJ) containing 10% oleic acid-albumin-dextrose complex (pH 7.4; Becton Dickinson) and 0.2% glycerol for 21 days. Subsequently, the bacteria were suspended in Difco Middlebrook 7H9 broth medium (Becton Dickinson) supplemented with 10% oleic acid-albumin-dextrose complex, 0.05% Tween 80, and 0.2% glycerol, followed by subculture at 37°C for 7 days. Bacterial suspensions were stored at −80°C until they were used.
Antimicrobial agents.
MXF (Bayer Health Care, Tokyo, Japan), GAT (Kyorin Pharmaceutical Co., Tokyo, Japan), levofloxacin (LVX) (Daiichi Pharmaceutical Co., Tokyo, Japan), and CLR (Taisho Toyama Pharmaceutical Co., Tokyo, Japan) were used in this study. Initially, CLR was dissolved in methanol, GAT was dissolved in 0.05 N NaOH, and MXF and LVX were dissolved in distilled water to create stock solutions (2.56 mg/ml). The stock solutions were stored at −80°C until they were used. From the stock solutions, working solutions were prepared by dilution with 7H9 broth medium.
Drug susceptibility testing.
The MICs of the three FQs and CLR for the 78 MAC strains were determined by broth microdilution assays using 7H9 broth medium. Briefly, serial twofold dilutions of each drug solution (ranging from 0.25 to 256 μg/ml) were prepared with 7H9 broth medium. Subsequently, 0.1-ml aliquots of the diluted solutions were dispensed into the wells of a 96-well microplate, followed by inoculation of 1.0 × 105 CFU/well of each MAC strain suspended in 7H9 broth medium. Thus, the final drug concentrations ranged from 0.125 to 128 μg/ml. When successful, growth in drug-free wells was observed after 14 days of incubation at 37°C. The MIC was defined as the lowest concentration of drug that completely inhibited bacterial growth. Each drug susceptibility test was performed in triplicate.
Determination of combination activity in vitro.
The combination activities of each FQ with CLR against different MAC strains were studied by employing the checkerboard titration technique with 7H9 broth medium (7). Checkerboard titration was performed in 96-well microplates with a final volume of 200 μl. The MICs of all the antibiotics were predetermined before the checkerboard titration was performed. In the serial twofold dilution scheme used in the checkerboard titration, the first antibiotic, in 50-μl volumes, was diluted twofold in the microplates to give concentrations equal to four times that of the final drug concentration for testing. The second antibiotic was diluted twofold with broth at concentrations equal to four times those of the final drug concentrations used in testing and was then added to the wells in 50-μl volumes. The first row of wells contained only 50 μl of one drug, and the first column of wells contained only 50 μl of the other drug, together with 50 μl of 7H9 broth medium, and was subsequently inoculated with 1.0 × 105 CFU/well of each MAC strain suspended in 100 μl 7H9 broth medium. The drug concentrations of the checkerboard ranged from 0.125 to 8 μg/ml for each of the FQs, except LVX (0.25 to 16 μg/ml), and from 0.125 to 128 μg/ml for CLR. The tested MAC strains were selected if their MICs for all four drugs fell within the concentration range used for the checkerboard titration in this study. The data were interpreted by calculating the fractional inhibitory concentration (FIC) index as follows: FIC = (MICdrug A combination/MICdrug A alone) + (MICdrug B combination/MICdrug B alone). In this study, the FIC index was basically interpreted as follows: a FIC of ≤0.5, synergism; a FIC of >0.5 but ≤2.0, indifference; a FIC of >2.0, antagonism (8, 11, 15). In addition, antagonism was determined to be more strict, with FICs of >2.0 and >4.0. Each experiment evaluating combined activity was carried out three times.
Mouse model of MAC infection.
Female C57BL/6J mice (8 weeks old) were purchased from Charles River Laboratories Japan (Kanagawa, Japan). The mice were intravenously infected with 1.0 × 107 to 3.0 × 107 CFU of M. avium JATA51-01 in 100 μl saline suspension (4, 18), and mortality was observed for 130 days after infection. In total, 10 mice per study were used for the determination of mortality, and each study was performed twice. Additional mice were sacrificed on days 7, 14, 21, 28, 35, 56, 84, and 112 after infection in order to evaluate bacterial numbers and histopathology in the lungs, liver, and spleen in the absence of drug treatment. The target organs of the mice were aseptically removed, and part of each organ was individually homogenized with 1 ml sterile saline using a tissue homogenizer. The homogenized suspensions were serially diluted in 7H9 broth medium and plated on 7H10 agar plates for quantification of viable bacteria. In addition, tissues from the remaining part of each target organ were fixed with formalin, sectioned, and stained with hematoxylin and eosin. Each experiment was performed using 10 mice from the mortality experiment and 5 from each sacrifice group. The study was carried out twice.
Therapeutic evaluation of MAC-infected mice.
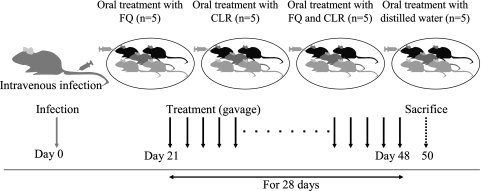
Mice were infected with MAC strains N016, N018, and N084, as well as the two reference strains, by injection of 100 μl bacterial suspension containing 1.0 × 107 to 3.0 × 107 CFU of each MAC through the tail vein (4, 18). After 21 days, treatment with antimicrobial agents was initiated. The mice were treated with one of the three FQs alone (MXF, 100 mg/kg of body weight/day; GAT, 100 mg/kg/day; or LVX, 200 mg/kg/day), CLR alone (200 mg/kg/day), or an FQ-CLR combination, with both agents at the same doses described above. The dose of each antibiotic was based on that in previous studies (1, 3-5). Each therapeutic study group included five mice (Fig. 1). Agents were administered daily by gavage using a round-headed needle and a syringe for 28 days. A control group of mice received distilled water over the same period instead of antibiotics. The mice were sacrificed 48 h after the completion of therapy in order to prevent any carry-over effects of the drugs (Fig. 1). Bacterial counts in the lungs, liver, and spleen were determined using the method described above, and histopathological examination was performed using hematoxylin- and eosin-stained tissue sections. For histopathological examination, the numbers of granulomas were determined in 30 randomly selected fields (×100 magnification) in the lung tissue specimens from three mice. The category of granuloma formation was conventionally defined by the diameter as follows: small, diameter 0 to 249 μm; medium, diameter 250 to 499 μm; and large, diameter >500 μm. Therapeutic and histopathological examinations were performed twice. This study was conducted in accordance with animal experimentation guidelines and approved by the Institutional Animal Care and Use Committee of Nagasaki University (approval no. 0409090382).
FIG. 1.
Schematic representation of our study of MAC-infected mice. The mice were infected intravenously with 1.0 × 107 to 3.0 × 107 CFU of MAC strain N016, N018, or N084 or one of two reference strains. After 21 days, treatment was initiated with CLR alone (200 mg/kg/day), FQ alone (MXF, 100 mg/kg/day; GAT, 100 mg/kg/day; or LVX, 200 mg/kg/day), or FQ-CLR combinations at the same doses described above. Control mice were treated with distilled water. All agents were administered daily by gavage for 28 days. Viable bacterial counts in organs and histopathological examination were carried out 2 days after completion of treatment.
Statistical analysis.
Statistical analysis was performed using StatView (Hulinks, Tokyo, Japan). The significance of bacterial counts in the target organs was assessed by Fisher's protected least significant difference test after analysis of variance and was set at a P value of <0.05.
RESULTS
In vitro activities of FQs and CLR.
The MIC90 values for the three FQs and CLR for the 76 clinical isolates, as well as the two reference strains, are shown in Table 1. MXF and GAT showed promising activity against MAC in vitro, with MIC90 values of 2 and 4 μg/ml, respectively, while CLR had an MIC90 of 64 μg/ml. The MICs for the same agents against different MAC strains used in the subsequent in vivo evaluation are also shown in Table 1. The in vitro interactions of the individual FQs combined with CLR are summarized in Table 2. In total, 43 of the 76 MAC strains were evaluated for the combined effects of MXF and CLR, while 42 of the 76 MAC strains were used to study the combined effects of GAT or LVX and CLR (the MIC ranges of strains used in the combination study were as follows: MXF, 0.5 to 2 μg/ml; GAT, 0.5 to 2 μg/ml; LVX, 0.5 to 8 μg/ml; and CLR, 0.5 to 4 μg/ml).
TABLE 1.
In vitro antimicrobial activities of FQs and CLR against 76 clinically isolated MAC strains and 2 reference MAC strains
| MAC clinical isolate (n = 76) | MIC (μg/ml)b
|
|||
|---|---|---|---|---|
| MXF | GAT | LVX | CLR | |
| M. avium JATA51-01 | 2 | 2 | 4 | 2 |
| M. intracellulare JATA52-01 | 0.5 | 1 | 4 | 0.5 |
| MAC N016a | 0.5 | 0.5 | 2 | 2 |
| MAC N018a | 0.5 | 1 | 4 | 0.5 |
| MAC N084a | 0.5 | 1 | 8 | 0.5 |
Strain used in subsequent in vivo experiments.
Ranges were ≤0.125 to 8, ≤0.125 to 8, ≤0.125 to 64, and ≤0.125 to >128, and MIC90s were 2, 4, 16, and 64 μg/ml for MXF, GAT, LVX, and CLR, respectively.
TABLE 2.
In vitro antimicrobial activities of FQ-CLR combinations against clinically isolated MAC strains
| FQ-CLR | No. (%) of isolates showing interactiona:
|
||
|---|---|---|---|
| Synergism | Indifference | Antagonism | |
| MXF-CLR (n = 43) | 1 (2) | 19 (45) | 23 (53) [1 (2)] |
| GAT-CLR (n = 42) | 1 (2) | 17 (41) | 24 (57) [1 (2)] |
| LVX-CLR (n = 42) | 0 (0) | 18 (43) | 24 (57) [0 (0)] |
Synergism, FIC ≤ 0.5; indifference, 0.5 < FIC ≤ 2.0; antagonism, FIC > 2.0 [FIC > 4.0].
When antagonism was defined as a FIC of >4.0, 1 (N046) of 43 strains for MFX-CLR, 1 (N002) of 42 strains for GAT-CLR, and 0 of 42 strains for LVX-CLR showed antagonism, and most strains showed an indifferent interaction for all combinations. On the other hand, when antagonism was defined as a FIC of >2.0, antagonism was observed in 23 of 43 strains (53%) for MXF-CLR. Similarly, when GAT was combined with CLR, antagonism was observed in 24 of 42 strains (57%), while the combination of LVX with CLR had reduced effects in 24 of 42 strains (57%). In addition, a total of 19 strains showed antagonism (FIC > 2.0) for all three FQ-CLR combinations. Thus, when the FIC was greater than 2.0, antagonism was observed in 53 to 57% of MAC clinical isolates for combined treatment with an FQ and CLR. In contrast, synergism was observed in one strain each for MXF-CLR and GAT-CLR, and indifference was observed in ∼45% of all strains treated with FQ-CLR combinations (Table 2). To summarize, the in vitro effects of FQ-CLR combinations against certain MAC strains (FIC values for MXF-CLR, GAT-CLR, and LVX-CLR, respectively) were as follows: indifference, M. avium JATA51-01 (1.5, 2.0, and 2.0) and N016 (1.25, 1.5, and 1.5); antagonism (>2.0), M. intracellulare JATA52-01 (3.0, 3.0 and 2.5), N018 (3.0, 3.0 and 3.0), and N084 (3.0, 3.0 and 4.0). These strains were used in subsequent in vivo experiments.
MAC-infected mice.
Although some mice infected with M. avium JATA51-01 had died by 20 days after inoculation, almost all the mice survived for more than 100 days postinfection. However, a number of mice died after 110 days, and all succumbed within 130 days (data not shown). Variable bacterial counts were observed in the lungs, livers, and spleens of mice at 7, 14, 21, 28, 35, 56, 84, and 112 days after inoculation. Just after infection, an increase in viable bacterial counts was observed, and the number of viable bacteria in the lungs reached 4.31 ± 0.07 (log10 CFU/organ; mean ± standard error of the mean) and also reached 7.35 ± 0.05 in the liver and 6.41 ± 0.14 in the spleen 7 days after infection. After 28 days, the counts in the liver and spleen reached 9.81 ± 0.08 and 9.19 ± 0.09, respectively; however, viable bacterial counts reached a plateau after 56 days (10.93 ± 0.15 in the liver and 10.30 ± 0.30 in the spleen) and continued until day 112 (11.29 ± 0.03 in the liver and 10.54 ± 0.07 in the spleen). On the other hand, the bacterial counts in the lungs kept increasing until the end of the observation period and were as follows: 6.77 ± 0.09 at day 28, 8.78 ± 0.18 at day 56, and 10.68 ± 0.10 at day 112.
Histopathological examination of organs from infected mice revealed progressive infiltration of inflammatory cells 21 days after inoculation and the development of granulomatous lesions after 28 days of infection. The development of inflammation and granuloma formation paralleled an increase in the number of viable bacteria. Within the lungs, severe inflammation was observed after 56 days of infection, and it was difficult to identify normal alveolar tissue after 84 days of infection. Similarly, development of granulomatous lesions in the liver and spleen was confirmed after 35 days of infection, and there was little normal tissue at 112 days after infection (data not shown).
Treatment of MAC-infected mice.
Chemotherapy with the three FQs and CLR was started after 21 days of infection and administered daily for 28 days in order to evaluate the effects of treatment on disseminated MAC infection in mice. The MICs of the three FQs and CLR against various MAC strains are shown in Table 1. All experiments were performed in duplicate. Although there was a small difference in the numbers of viable bacteria, similar results were obtained from the first and second experiments. All mice that received CLR alone, and most of the mice that received one of the FQs, showed significantly reduced bacterial counts in all of the organs examined (P < 0.05) relative to control mice (Table 3). However, some treatments with one of the FQs did not show this effect. For example, similar bacterial counts were obtained in lung tissues of mice treated with GAT or LVX alone compared with control mice after infection with M. avium JATA51-01. Furthermore, similar bacterial counts were found in the lung tissues and spleens of mice treated with GAT or LVX compared with control mice after infection with M. intracellulare JATA52-01. These findings were also observed for each of the three FQs in the lungs and for GAT in the liver following infection with MAC N084. Furthermore, the bacterial counts in the organs examined were significantly lower in mice treated with CLR alone than in those treated with one of the FQs (Table 3).
TABLE 3.
In vivo activities of MXF, GAT, LVX, and CLR alone and FQ-CLR combinations against MAC-infected mice
| Strain | Agent | Log10 CFU/organa
|
||
|---|---|---|---|---|
| Lungs | Liver | Spleen | ||
| M. avium JATA51-01 (indifferent) | Control | 9.16 ± 0.06c | 10.15 ± 0.07 | 9.58 ± 0.08 |
| MXF | 8.83 ± 0.18b | 9.68 ± 0.05b,c | 9.18 ± 0.08b,c | |
| GAT | 8.90 ± 0.14c | 9.91 ± 0.05b,c | 9.18 ± 0.08b,c | |
| LVX | 9.01 ± 0.09c | 9.92 ± 0.07b,c | 9.38 ± 0.13b,c | |
| CLR | 7.10 ± 0.08b | 8.12 ± 0.11b | 7.10 ± 0.09b | |
| MXF + CLR | 7.48 ± 0.10b | 8.13 ± 0.09b | 7.41 ± 0.04b | |
| GAT + CLR | 7.10 ± 0.18b | 8.08 ± 0.07b | 7.27 ± 0.12b | |
| LVX + CLR | 7.10 ± 0.19b | 8.12 ± 0.06b | 6.97 ± 0.09b | |
| M. intracellulare JATA52-01 (antagonistic) | Control | 5.51 ± 0.12 | 5.51 ± 0.06 | 4.60 ± 0.16 |
| MXF | 4.83 ± 0.17b,c | 4.76 ± 0.12b,c | 4.15 ± 0.10b,c | |
| GAT | 5.32 ± 0.16c | 5.15 ± 0.06b,c | 4.41 ± 0.11c | |
| LVX | 5.47 ± 0.08c | 5.16 ± 0.11b,c | 4.48 ± 0.12c | |
| CLR | 3.17 ± 0.07b | 3.20 ± 0.04b | 3.16 ± 0.12b | |
| MXF + CLR | 3.23 ± 0.08b | 3.59 ± 0.05b,c | 3.42 ± 0.08b | |
| GAT + CLR | 3.43 ± 0.02b | 3.65 ± 0.06b,c | 3.46 ± 0.06b,c | |
| LVX + CLR | 3.36 ± 0.07b | 3.39 ± 0.05b | 3.32 ± 0.05b | |
| MAC N016 (indifferent) | Control | 6.61 ± 0.15 | 7.64 ± 0.07 | 7.61 ± 0.10 |
| MXF | 5.35 ± 0.10b,c | 6.53 ± 0.08b,c | 6.60 ± 0.14b,c | |
| GAT | 5.48 ± 0.11b,c | 6.60 ± 0.11b,c | 6.81 ± 0.09b,c | |
| LVX | 5.48 ± 0.09b,c | 6.52 ± 0.10b,c | 6.72 ± 0.16b,c | |
| CLR | 3.94 ± 0.21b | 5.91 ± 0.02b | 5.36 ± 0.11b | |
| MXF + CLR | 4.17 ± 0.11b | 5.78 ± 0.03b | 5.57 ± 0.06b | |
| GAT + CLR | 3.96 ± 0.06b | 5.72 ± 0.06b | 5.35 ± 0.11b | |
| LVX + CLR | 4.25 ± 0.21b | 5.86 ± 0.21b | 5.57 ± 0.06b | |
| MAC N018 (antagonistic) | Control | 6.40 ± 0.05 | 7.63 ± 0.06 | 7.40 ± 0.04 |
| MXF | 5.79 ± 0.06b,c | 7.09 ± 0.05b,c | 6.60 ± 0.08b,c | |
| GAT | 5.96 ± 0.08b,c | 7.21 ± 0.06b,c | 6.75 ± 0.10b,c | |
| LVX | 6.01 ± 0.04b,c | 7.40 ± 0.05b,c | 6.82 ± 0.04b,c | |
| CLR | 2.18 ± 0.09b | 5.03 ± 0.08b | 4.06 ± 0.10b | |
| MXF + CLR | 3.05 ± 0.18b,c | 5.37 ± 0.07b,c | 4.26 ± 0.14b | |
| GAT + CLR | 3.11 ± 0.12b,c | 5.25 ± 0.09b,c | 4.34 ± 0.13b | |
| LVX + CLR | 2.37 ± 0.13b | 5.39 ± 0.05b,c | 4.32 ± 0.09b,c | |
| MAC N084 (antagonistic) | Control | 8.13 ± 0.16 | 8.17 ± 0.07 | 8.04 ± 0.11 |
| MXF | 7.90 ± 0.06c | 7.78 ± 0.07b,c | 7.39 ± 0.10b,c | |
| GAT | 8.14 ± 0.07c | 8.00 ± 0.08c | 7.60 ± 0.08b,c | |
| LVX | 7.82 ± 0.07c | 7.77 ± 0.09b,c | 7.58 ± 0.13b,c | |
| CLR | 5.28 ± 0.11b | 4.58 ± 0.06b | 4.30 ± 0.11b | |
| MXF + CLR | 5.68 ± 0.08b,c | 5.47 ± 0.15b,c | 4.90 ± 0.09b,c | |
| GAT + CLR | 5.54 ± 0.08b,c | 5.29 ± 0.11b | 4.72 ± 0.12b,c | |
| LVX + CLR | 5.24 ± 0.08b | 5.08 ± 0.15b,c | 4.60 ± 0.12b,c | |
Activities of CLR (200 mg/kg/day), MXF (100 mg/kg/day), GAT (100 mg/kg/day), LVX (200 mg/kg/day), and FQ-CLR combinations against MAC-infected mice. Viable bacterial counts were determined in the lungs, liver, and spleen 48 h after completion of therapy. A total of five mice were examined per group, and each value represents the mean ± standard error of the mean. Significant differences (P < 0.05) in viable bacterial numbers between treatments with each FQ alone and with FQ-CLR combinations (e.g., MXF versus MXF-CLR) were obtained for all target organs. The experiment was performed twice, and the data shown are from the second experiment. Similar results and statistical tendencies were obtained from the first experiment.
P < 0.05 versus control mice as evaluated by Fisher's protected least significant difference test after analysis of variance.
P < 0.05 versus CLR alone as evaluated by Fisher's protected least significant difference test after analysis of variance.
The therapeutic effects of CLR alone and each of the FQ-CLR combination treatments against MAC-infected mice are also shown in Table 3. Mice infected with M. avium JATA51-01 and MAC N016, which showed indifferent effects for each FQ-CLR combination in vitro, did not exhibit significant differences in the bacterial counts in the organs examined compared to those treated with CLR alone or a FQ-CLR combination. On the other hand, in mice infected with MAC strains that showed antagonistic effects (FIC > 2.0) with FQ-CLR combinations in vitro (M. intracellulare JATA52-01 and MAC N018 and N084 strains), significant differences in the bacterial counts in organs were confirmed between treatment with CLR alone and some FQ-CLR combinations (e.g., CLR versus MXF-CLR or GAT-CLR in the livers and CLR versus GAT-CLR in the spleens of M. intracellulare JATA52-01-infected mice and CLR versus MXF-CLR or GAT-CLR in the lungs and CLR versus each of the three FQ-CLR combinations in the livers of MAC N018-infected mice; P < 0.05). Regarding histopathological examination, MAC N084-infected mice exhibited small but significantly different bacterial counts in their lung tissues after treatment with CLR alone, MXF-CLR, and GAT-CLR (Table 3), while lung histopathology revealed more severe inflammation and a larger number of granulomas (average numbers: small granulomas, 30 and 39; medium, 18 and 19; and large, 1 and 2) after treatment with MXF-CLR and GAT-CLR than after treatment with CLR alone (average numbers: small granulomas, 36; medium, 4; and large, 0). On the other hand, no clear pathological differences were observed between mice treated with CLR alone and with FQ-CLR combinations following infection with M. avium JATA51-01 and MAC N016 (data not shown).
Finally, no mice in any of the treatment or control groups died during the whole period of chemotherapy.
DISCUSSION
FQs, especially those containing the C-8 methoxy group, such as MXF and GAT, are expected to show activity against MAC infectious disease, since a number of reports have indicated that they are active against Mycobacterium tuberculosis (1, 16). However, there is only limited evidence regarding the clinical efficacy of FQs against MAC infectious disease. Nevertheless, FQs are sometimes used as second-line agents against MAC infectious disease because no alternative antimycobacterial agents are currently available.
In the present study, FQs demonstrated activities equivalent to that of CLR against various MAC strains in vitro. Indeed, other researchers have reported that MXF and GAT are active against MAC in vitro (17), as well as in cultured macrophages (19) and mice (3). In general, in vitro MICs do not reflect the therapeutic efficacy against MAC infections (2, 6). However, the therapeutic effect of CLR can be predicted by its in vitro MIC (2, 6). Regarding this issue, although the FQs used in the present study showed promising in vitro activities against MAC, the individual FQs alone exhibited lower efficacy than CLR alone for the treatment of MAC infectious disease in mice. Our results indicate that it may be difficult to predict the in vivo therapeutic effects of FQs based on their in vitro MICs. However, a number of other factors may also be important. For example, differences in the pharmacokinetic and pharmacodynamic properties of FQs and macrolide antibiotics in mice, as well as differences in their daily doses and therapeutic durations, may influence the results. Further research is required to identify these factors.
Multidrug combination chemotherapy is usually used for the treatment of mycobacterial diseases, and CLR is a key agent against MAC infectious disease. Thus, we also determined the in vitro and in vivo activities of each of the FQs in combination with CLR against various MAC strains in order to explore the clinical utility of these combination regimens against MAC infectious disease. In our study of the combined effects of FQs and CLR in vitro, a few strains showed antagonism, with FICs of >4.0, while mild antagonism, with FICs of ≤4.0 and >2.0 was seen for more than half the MAC clinical isolates examined (conventionally, we used “mild antagonism” as the term for FICs of ≤4.0 and >2.0). All the MAC strains used in this study were isolated in Japan, and the frequencies of such strains in other countries are of great interest, since they may show geographical differences. Regarding the antagonistic effects between FQs and CLR, this phenomenon has previously been reported by Tomioka et al., who showed that CLR decreases the activities of GAT and LVX against MAC strains in vitro (19). In addition, protein synthesis inhibitors, such as CLR, interfere with the lethal antibacterial activities of FQs (19). Moreover, we had concerns about whether such mild antagonistic effects of FQ-CLR combinations in vitro could be confirmed by in vivo experiments. With this background, we carried out therapeutic experiments using MAC strains that showed mild antagonism against all FQ-CLR combinations. Although most of the FQ-CLR combinations did not show significantly altered antibacterial effects in organs compared to treatment with CLR alone, several combinations (e.g., MXF-CLR and GAT-CLR against MAC N084) showed significantly greater numbers of viable bacteria in organs than treatment with CLR alone. In addition, comparison of the effects of individual FQs alone and FQ-CLR combination regimens revealed increased bacterial counts in mice treated with individual FQs. These results suggest that FQs containing the C-8 methoxy group can attenuate the anti-MAC activity of CLR. However, the anti-MAC activity of FQ-CLR combination treatments remains superior to that of FQs alone in vivo. Furthermore, the attenuation of CLR activity by combined therapy with an FQ was found to depend on the MAC strain under examination. Accordingly, the mild antagonistic effect of FQ-CLR combination therapy in vitro could also be confirmed in vivo.
In the present study, an intravenously infected mouse model was used in order to ensure that the bacteria were present in the target organs and to stabilize the bacterial counts in each organ. In this regard, our mouse model can be considered to be similar to disseminated MAC infection. However, our results are not directly applicable to human disseminated or lung MAC infections. In this regard, our data are limited, and further studies are required (e.g., using other infection route models, longer treatment durations, or different treatment start points). Additionally, we did not evaluate the in vivo efficacy of FQ-CLR treatment against MAC strains for which the FQ-CLR combinations showed synergistic effects in vitro, and such experiments should be carried out in the near future. However, we consider that our results concerning the mild antagonistic effects of FQ-CLR combinations in vivo provide important information for the treatment of refractory MAC infectious disease, such as in failure of first-line chemotherapy or disseminated MAC infectious disease, and therefore report these data as an initial study.
Finally, current chemotherapy regimens against mycobacterial infectious disease, including those caused by MAC, usually involve combinations of three or four agents. Thus, further studies are also required to evaluate how the use of three or four agents may influence the anti-MAC activities of combined FQ-CLR regimens in vivo. However, given the importance of our finding that >50% of the clinical MAC isolates in the present study were less responsive to combined FQ-CLR regimens than to CLR alone, such combination therapy against MAC infections should be performed very carefully in clinical situations. In addition, the strain-dependent antagonism of FQ-CLR combinations should also be considered, if treatment with a particular combination fails against MAC infections.
Acknowledgments
We thank Taisho Pharmaceutical Co., Kyorin Pharmaceutical Co., Bayer Health Care, and Daiichi Pharmaceutical Co. for kindly providing the antimicrobial agents used in this study. We also thank Koich Taura and all the members of our affiliated hospitals for kindly providing the clinical isolates of M. avium complex strains, as well as all members of our laboratory for their thoughtful discussions.
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Alvirez-Freites, E. J., J. L. Carter, and M. H. Cynamon. 2002. In vitro and in vivo activities of gatifloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Petrofsky, P. Aralar, M. Wu, and L. S. Young. 2001. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium. Antimicrob. Agents Chemother. 45:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Petrofsky, and L. S. Young. 1994. Clarithromycin, dapsone, and a combination of both used to treat or prevent disseminated Mycobacterium avium infection in beige mice. Antimicrob. Agents Chemother. 38:2717-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Wu, L. Barbara- Burnham, and L. S. Young. 1996. Activities of bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob. Agents Chemother. 40:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field, S. K., D. Fisher, and R. L. Cowie. 2004. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest 126:566-581. [DOI] [PubMed] [Google Scholar]
- 7.Gradelski, E., B. Kolek, D. P. Bonner, L. Valera, B. Minassian, and J. Fung-Tomc. 2001. Activity of gatifloxacin and ciprofloxacin in combination with other antimicrobial agents. Int. J. Antimicrob. Agents 17:103-107. [DOI] [PubMed] [Google Scholar]
- 8.Hewlett, P. S. 1969. Measurement of the potencies of drug mixtures. Biometrics 25:477-487. [PubMed] [Google Scholar]
- 9.Inderlied, C. B., C. A. Kemper, and L. E. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs, M. R. 2004. Fluoroquinolones as chemotherapeutics against mycobacterial infections. Curr. Pharm. Des. 10:3213-3220. [DOI] [PubMed] [Google Scholar]
- 11.Katou, K., A. Nakamura, T. Kato, K. Tonegawa, T. Kutsuna, T. Niwa, H. Morita, and M. Itoh. 2005. Combined effects of panipenem and aminoglycosides on methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Chemotherapy 51:387-391. [DOI] [PubMed] [Google Scholar]
- 12.Kobashi, Y., and T. Matsushima. 2004. Comparison of clinical features in patients with pulmonary Mycobacterium avium complex (MAC) disease treated before and after proposal for guidelines. J. Infect. Chemother. 10:25-30. [DOI] [PubMed] [Google Scholar]
- 13.Kobashi, Y., and T. Matsushima. 2003. The effect of combined therapy according to the guidelines for the treatment of Mycobacterium avium complex pulmonary disease. Intern. Med. 42:670-675. [DOI] [PubMed] [Google Scholar]
- 14.Leysen, D. C., A. Haemers, and S. R. Pattyn. 1989. Mycobacteria and the new quinolones. Antimicrob. Agents Chemother. 33:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perichon, B., and P. Courvalin. 2006. Synergism between β-lactams and glycopeptides against VanA-type methicillin-resistant Staphylococcus aureus and heterologous expression of the vanA operon. Antimicrob. Agents Chemother. 50:3622-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez, J. C., M. Ruiz, M. Lopez, and G. Royo. 2002. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 20:464-467. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez Diaz, J. C., M. Lopez, M. Ruiz, and G. Royo. 2003. In vitro activity of new fluoroquinolones and linezolid against non-tuberculous mycobacteria. Int. J. Antimicrob. Agents 21:585-588. [DOI] [PubMed] [Google Scholar]
- 18.Struillou, L., Y. Cohen, N. Lounis, G. Bertrand, J. Grosset, J. L. Vilde, J. J. Pocidalo, and C. Perronne. 1995. Activities of roxithromycin against Mycobacterium avium infections in human macrophages and C57BL/6 mice. Antimicrob. Agents Chemother. 39:878-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomioka, H., C. Sano, K. Sato, and T. Shimizu. 2002. Antimicrobial activities of clarithromycin, gatifloxacin and sitafloxacin, in combination with various antimycobacterial drugs against extracellular and intramacrophage Mycobacterium avium complex. Int. J. Antimicrob. Agents 19:139-145. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamura, M., N. Kita, H. Shimoide, S. Nagasawa, H. Arakawa, A. Kuze, A. Shinoda, N. Takasawa, H. Kamimura, R. Wada, et al. 1985. Studies on lung disease due to non-tuberculous mycobacteria in Japan (report of the year 1983 of the Mycobacteriosis Research Group of the Japanese National Chest Hospitals). Kekkaku 60:299-308. [PubMed] [Google Scholar]