Abstract
Rifampin is a cornerstone of modern antituberculosis therapy. However, rifampin's half-life of 3 h is believed to limit its utility for intermittent therapy, so new congeners with long half-lives are being developed. Using an in vitro pharmacokinetic-pharmacodynamic model of tuberculosis, we examined the relationships between rifampin exposure, microbial killing of log-phase-growth Mycobacterium tuberculosis, and suppression of resistance. Rifampin's microbial killing was linked to the area under the concentration-time curve-to-MIC ratio. The suppression of resistance was associated with the free peak concentration (Cmax)-to-MIC ratio and not the duration that the rifampin concentration was above MIC. Rifampin prevented resistance to itself at a free Cmax/MIC ratio of ≥175. The postantibiotic effect duration was ≥5.2 days and was most closely related to the Cmax/MIC ratio (r2 = 0.96). To explain rifampin's concentration-dependent effect, we examined the kinetics of rifampin entry into M. tuberculosis. Rifampin achieved concentration-dependent intracellular steady-state concentrations within 15 min. Our results suggest that doses of rifampin higher than those currently employed would optimize the effect of rifampin, if patients could tolerate them. Another major implication is that in the design of new rifampin congeners for intermittent therapy, the important properties may include (i) the efficient entry of the rifamycin into M. tuberculosis, (ii) the achievement of a free Cmax/MIC of >175 that can be tolerated by patients, and (iii) a long postantibiotic effect duration.
Tuberculosis (TB) is arguably the most important infectious disease to have confronted humankind. Currently, Mycobacterium tuberculosis infects 2 billion of the 6 billion people worldwide (50). The discovery of rifampin 40 years ago (31) was revolutionary in allowing the creation of potent combination drug regimens against this ancient nemesis. Rifampin is the backbone of modern anti-TB chemotherapy by virtue of being active against M. tuberculosis in exponential growth phase as well as possessing activity against nonreplicating persistent bacilli. Major limitations to rifampin use are believed to include its short half-life (t1/2), which allows cycles of M. tuberculosis regrowth and resistance emergence.
It has been the belief for almost half a century that if a drug such as rifampin was given once a week in the initial phase of therapy, its short serum t1/2 of 3 h would allow short periods of microbial killing followed by regrowth between doses (33, 37). The inference is that the length of time that rifampin is above a certain threshold concentration is the most important index associated with M. tuberculosis killing and the prevention of resistance. Thus, one of the strategic goals of the World Health Organization, the pharmaceutical industry, and research scientists has been to develop rifampin congeners with long t1/2s, such as rifapentine, which would make intermittent dosing more effective (3, 9, 24, 26).
It is widely believed that no one anti-TB drug can prevent resistance to itself (4, 32, 37). Prevention of the emergence of drug resistance is currently defined as “the ability of a drug to prevent selection of mutants resistant to companion drug” (37, p. 53). Thus, the strategy that was adopted to reduce the emergence of resistance was multidrug therapy. This strategy was first successfully employed more than 50 years ago in a randomized clinical trial comparing streptomycin, para-aminosalicylic acid, and the streptomycin-para-aminosalicylic acid combination (2). The explanation is that the likelihood of occurrence of chromosomal mutants resistant to two or more different drugs is very small (32). However, the occurrence of drug resistance is often multifaceted and may include not only chromosomal mutations but also induction or the presence of efflux pumps; the poor absorption of certain drugs in patients, which leads to effective monotherapy; and even drug antagonism of the component drugs in combination therapy (7, 16, 21, 38, 45). In the present study, we reexamined if the underlying assumptions (i) that rifampin cannot suppress resistance to itself and (ii) that intermittent dosing leads to the regrowth of resistant M. tuberculosis isolates when rifampin concentrations fall below the MIC are, indeed, true.
(The data were presented, in part, at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, September 2003, Chicago, IL, abstr. A-1156 [21a].)
MATERIALS AND METHODS
Organism.
M. tuberculosis H37Ra (ATCC 25177; American Type Culture Collection, Manassas, VA) was used throughout the studies. Stock cultures of M. tuberculosis were stored at −80°C in Middlebrook 7H9 broth and 10% glycerol (Becton Dickinson, Sparks, MD). For each study, the bacterial stock was thawed and incubated at 37°C for 4 days in Middlebrook 7H9 broth (herein termed broth) under shaking conditions and 5% CO2 to facilitate exponential-phase growth prior to inoculation of the organisms into our in vitro pharmacodynamic (PD) system.
Drug.
Rifampin (lot number 37H0769; potency, 95%) was purchased from Sigma-Aldrich (St. Louis, MO). The drug was dissolved in dimethyl sulfoxide and was then serially diluted in broth to the desired final drug concentration. The final dimethyl sulfoxide concentration had no effect on M. tuberculosis growth.
Silicone oil.
For studies of rifampin intracellular concentrations, two types of oil, 99% dioctyl phthalate and poly(dimethylsiloxane-co-methyl-phenylsiloxane) 550, were purchased from Sigma-Aldrich and were mixed at 70% to 30%, by volume, respectively.
Determination of MIC and mutation frequency.
MICs were determined as described by the Clinical and Laboratory Standards Institute (6). The mutation frequency was determined by placing 1 ml each of 109 CFU/ml of M. tuberculosis onto the surface of 10 Middlebrook 7H10 agar (herein termed agar) plates that had been supplemented with 1 mg/liter of rifampin. This concentration of rifampin was chosen because it is the “critical concentration” of rifampin, defined as the lowest concentration of rifampin that inhibits ≥95% of the wild-type strains of M. tuberculosis that have not been exposed to rifampin therapy but that does not inhibit resistant strains isolated from patients not responding to rifampin therapy (6). The M. tuberculosis density was validated by quantitative cultures on non-drug-containing agar that were incubated at 37°C. The colonies were counted after 3 weeks and then weekly thereafter until there were no changes in number of colonies. These experiments were conducted twice.
In vitro PD model of TB.
Our in vitro PD model of TB uses the hollow fiber system (HFS) and has been described in detail in the past (18-21). In each study, the HFSs (Spectrum Laboratory Systems, Los Angeles, CA) were maintained under 5% CO2 and at 37°C throughout the experiments and were preconditioned with rifampin for 3 days prior to M. tuberculosis inoculation. Ten milliliters of 106 CFU/ml M. tuberculosis cultures on day 4 of log-phase growth was inoculated into the peripheral compartment of each hollow fiber cartridge. These bacilli in the peripheral compartment continued in log-phase growth and were sampled via syringe ports. The peripheral compartment of each HFS is separated from the central compartment by semipermeable hollow fibers. The semipermeable hollow fibers have pore sizes ≤30 kDa, which is large enough to allow nutrients, drugs, and bacterial metabolites to freely transverse into and out of the peripheral compartment but too small for the M. tuberculosis cells to leave the peripheral compartment. Fresh broth was circulated in the central compartment and used broth was circulated out of the central compartment by the use of Masterflex peristaltic pumps (Cole-Parmer Instrument Company, Vernon Hills, IL) to simulate a drug t1/2 of 3 h (1, 27, 35). Rifampin was dosed into the central compartment, starting 1 h after log-phase-growth M. tuberculosis had been inoculated into the peripheral compartment. The rifampin diffused to and from the peripheral compartment by first-order kinetics. In each study, the rifampin concentrations attained in the HFS were validated by sampling the central compartment at 1, 2, 4, 8, 11, 17, 20, 24, 25, 27, 30, 46, and 48 h after the first dose.
We simulated the pharmacokinetics (PKs) of rifampin achieved in patients, as described in the work by other scientists (1, 27, 35). Since it is the free or non-protein-bound fraction of a drug that is pharmacologically active (8, 28), all rifampin drug exposures in the experiments were expressed as free drug exposures. The free serum rifampin concentration was 20% of the total rifampin concentration (27, 49). Rifampin had a serum t1/2 of 3 h. The standard oral rifampin dose of 600 mg a day achieved a free peak concentration (fCmax) of 2 mg/liter and a free area under the concentration-time curve (fAUC) from 0 h to infinity of 16 mg·h/liter, which, given the t1/2 of 3 h, is virtually similar to the fAUC from 0 to 24 h (fAUC0-24). The AUC for 1 week (168 h) with daily dosing translates to 112 mg·h/liter. For the purposes of our experiments, we assumed that the rifampin PKs in patients were linear (1, 27), so that the peak concentration (Cmax) increases proportionately with the dose, such that a dose of 2,100 mg twice a week will have an fCmax of 7 mg/liter and a dose of 4,200 mg once a week will achieve an fCmax of 14 mg/liter. However, since the t1/2 is the same regardless of the dose, the doses of 600 mg daily, 2,100 mg twice a week, and 4,200 mg once a week will also achieve the same fAUC from 0 to 168 h (AUC0-168), which is 112 mg·h/liter. These exposures (fCmax, fAUC) and t1/2 were used in the experiments described below.
In order to determine the relationship between rifampin exposure and microbial killing, dose-effect studies were performed. Rifampin was administered to eight HFSs every 24 h with an intent to achieve AUC0-24 values between 0 and 54 mg·h/liter (corresponding to oral rifampin doses of 0 to 2,050 mg daily). These exposures were based on the results of a pilot study (data not shown). The peripheral compartment was sampled daily for 7 days to obtain the bacterial contents. Each sample was washed twice with saline to prevent drug carryover, serially diluted, and plated on agar for quantitative cultures, as described before (18, 19, 21).
Next, we determined the effect of dose scheduling on the microbial killing of the total M. tuberculosis population. M. tuberculosis cells in the peripheral compartments of four HFSs were exposed to the exposures mimicking serum rifampin concentration-time profiles for human oral doses of (i) 600 mg each day for 7 days, (ii) 2,100 mg twice weekly on day 0 and day 3.5, (iii) 4,200 mg given on day 1, and (iv) a control. Therefore, all rifampin-treated HFSs received a cumulative weekly AUC0-168 of 122 mg·h/liter. The bacterial contents of the peripheral compartment of each HFS were sampled daily for 7 days, and bacillary densities were determined as described above.
Next, we determined the effect of different rifampin doses and dose schedules on the size of the rifampin-resistant M. tuberculosis population. On the basis of the results of a pilot study, log-phase growth M. tuberculosis in each of the HFS were exposed to rifampin doses in the experimental scheme shown in Table 1. A single rifampin dose was administered at the start of the 7-day experiment to achieve one of the following AUC0-168s: 14, 28, or 56 mg·h/liter. In parallel, each of these rifampin doses was divided by seven and was then administered to three HFSs to achieve AUC0-24 values of 2, 4, or 8 mg·h/liter daily for 7 days. A further two HFSs were treated with daily rifampin doses for an AUC0-24 of 0 or 1 mg·h/liter for 7 days. The central compartment was sampled to validate the rifampin concentrations. The peripheral compartment was sampled for M. tuberculosis cultures on days 0, 3, and 7. The bacterial suspension was washed twice, serially diluted, and plated on agar to determine the size of the total M. tuberculosis population. In addition, the bacterial suspension was plated on agar supplemented with 1 mg/liter of rifampin (6) to determine the size of the rifampin-resistant M. tuberculosis population. The agar plates were incubated, and the colonies were counted as described above. Ten representative colonies from each treatment regimen were obtained from the drug-containing agar for DNA sequencing. DNA extraction and molecular analysis were performed by methods that we have published previously (42). The gene target was an 81-bp region in the genes encoding DNA-dependent RNA polymerase gene, or rpoB.
TABLE 1.
Dosing scheme in experiments to determine effect of rifampin dose scheduling on Mycobacterium tuberculosis resistance
| Rifampin dosing scheme | Drug exposures achieved in the following HFS:
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| AUC0-24 (mg·h/liter) in HFSs treated with rifampin daily (n = 5) | 0 | 1 | 2 | 4 | 8 |
| AUC0-168 (mg·h/liter) in HFSs treated with rifampin once a wk (n = 3) | 14 | 28 | 56 | ||
| Cumulative AUC (mg·h/liter) for 7 days | 0 | 7 | 14 | 28 | 56 |
To determine the PK-PD index best explanatory of the emergence of resistance (the percentage of the dosing interval above the MIC [TMIC], Cmax/MIC, or AUC/MIC), we examined scatter plots of the size of the resistant population versus the drug exposures achieved. On the basis of the scatter plots, we chose a nonlinear regression model and calculated the coefficient of determination for the exposure versus the size of the rifampin-resistant population using Prism software (GraphPad Software Inc., San Diego, CA). The relationship between the rifampin concentration and the changes in the susceptible and resistant M. tuberculosis subpopulations over time were also described by using a mathematical model that we have described in the past (19).
PAE of rifampin.
M. tuberculosis was incubated in broth for 4 days. Then, 106-CFU/ml cultures were exposed to one of the following rifampin concentrations: 14 mg/liter for 1/2 h (AUC = 7 mg·h/liter), 7 mg/liter for 1 h (AUC = 7 mg·h/liter), 7 mg/liter for 2 h (AUC = 14 mg·h/liter), and 2 mg/liter for 7 h (AUC = 14 mg·h/liter). After the exposure to rifampin, the cultures (including the controls) were washed twice by centrifugation at 2,000 × g for 10 min, the supernatant was discarded, and the pellet was suspended in 100 ml of saline. After the second centrifugation, the pellet was suspended in 100 ml of fresh broth and incubated in Erlenmeyer flasks under shaking conditions at 37°C in 5% CO2. The cultures were sampled every other day for quantitative cultures on agar. The postantibiotic effect (PAE) was calculated by subtracting the time that the control cultures took to grow 1 log10 CFU/ml from the time that the rifampin-exposed cultures took to grow 1 log10 CFU/ml.
Determination of relationship between rifampin exposure and steady-state intracellular concentration.
The following experiment was performed in triplicate. Ninety milliliters of sterile Middlebrook 7H9 broth was poured into eight Erlenmeyer flasks. Four of the flasks were incubated overnight at 0°C, while four flasks were incubated at 37°C. Two hours prior to rifampin exposure, 10 ml of 107-CFU/ml M. tuberculosis cultures on day 4 of log-phase growth was added to each Erlenmeyer flask to make a final bacterial density of 106 CFU/ml. At time zero, rifampin was added to the flasks (to four flasks at 0°C and four flasks at 37°C) and vigorously mixed, to make final rifampin concentrations of either 0, 2, 7, or 14 mg/liter. Just prior to the addition of rifampin and at 180 min, 1 ml of the cultures was obtained for determination of the protein concentration, and another 1 ml was used for quantitative cultures. For the purpose of measuring the rifampin concentration inside the bacteria, 1 ml of sample was obtained from each flask at 0, 5, 15, 30, and 60 min after addition of rifampin. Each sample was immediately added to a microcentrifuge tube that had been prefilled with 300 μl of the silicone oil mixture and centrifuged at a relative centrifugal force of 16,100 for 5 min. A pellet formed at the tip of the tube and was separated from the drug-containing medium by the oil. After centrifugation, the samples were immediately frozen overnight at −20°C. On the following day, a microtube cutter was used to remove the tip of the tube, and measurement of the rifampin concentration in the pellet at the tip was performed, as described below. Since it has been argued that at 0°C the fluidity of the cell membranes of bacteria is reduced, which prevents the transport of chemicals into the cell (36, 48), the rifampin concentration measured at 0°C represents nonspecific binding to the bacterial cell wall (48). Therefore, to calculate intracellular rifampin concentrations, the concentrations measured at 0°C were subtracted from those measured at 37°C (36, 48).
An estimate of the bacterial cell volume was made by determining the protein content of each microbial cell (17, 39). In order to determine the protein concentration, a 1-ml sample was centrifuged in oil as described above. Immediately after centrifugation, the broth and oil were carefully aspirated from the tubes. One milliliter of 0.1-mm-diameter glass beads (Biospec Products Inc., Bartlesville, OK) was added to the pellet, after which 1 ml of distilled water was added. The M. tuberculosis cells were then mechanically disrupted in a Mini-Beadbeater (Biospec Products Inc.) for 5 min and then spun at a relative centrifugal force of 16,100 for 10 min. The protein concentration was determined from the supernatant by using a bicinchoninic acid protein assay kit (Sigma, St. Louis, MO).
Measurement of rifampin concentration.
After cleanup on Waters Oasis HLB solid-phase extraction medium, the samples were subjected to liquid chromatography and mass spectrometry on an Alltima HP C18, 3-μm column (50 by 2.1 mm) with a mobile phase consisting of 58% 25 mM ammonium acetate and 42% acetonitrile at a flow rate of 0.3 ml/min. Rifampin was monitored at an m/z 823.3, the [M + H]1+ ion. The internal standard, rifamycin SV, was monitored at m/z 720.3, the [M + Na]1+ ion. The assay was linear over a 2,000-fold range of 0.01 to 20 μg/ml (r2 > 0.99). The average intraday and interday coefficients of variation were 5.64% and 5.87%, respectively, with all coefficients of variation and variances in accuracy of <±20%. The assay bias was −2.7%.
RESULTS
MIC and mutation frequency.
Our M. tuberculosis isolate had an MIC of 0.03 mg/liter. The proportion of mutants resistant to ≥1 mg/liter rifampin was 1 mutant in 1.55 × 108 bacteria.
Rifampin microbial killing and prevention of resistance in the in vitro PD model of tuberculosis.
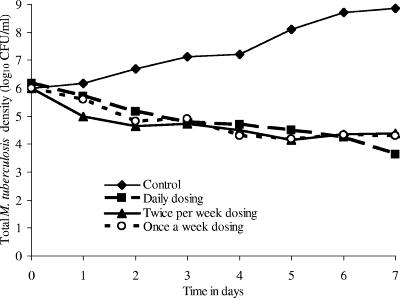
The dose-effect study revealed that at the end of 7 days of therapy, maximal microbial killing was achieved by a rifampin daily AUC0-24/MIC of 24.14, which translates to an AUC0-168/MIC ratio of 169. For exposures mimicking the standard dose of 600 mg a day, the microbial killing at the end of 7 days compared to that at the baseline was 1.95 CFU/ml, or 0.28 CFU/ml/day. The dose-scheduling study, in which we mimicked administration of rifampin at 600 mg daily for 7 days, rifampin at 2,100 mg on days 0 and 3.5, and rifampin at 4,200 mg as a single dose on day 0, revealed that regardless of the dose schedule the declines in microbial density were similar in all three treatment groups (Fig. 1). This would suggest that rifampin microbial killing is associated with the AUC/MIC. In addition, the once-a-week dose resulted in a persistent microbial density decline for more than 4 days after the rifampin concentrations declined below the MIC for M. tuberculosis, so that there was no regrowth for the entire 1-week dosing interval. This suggests that rifampin has a long PAE.
FIG. 1.
Response of Mycobacterium tuberculosis to rifampin administered in three dose schedules. The level of microbial killing was the same for all three dosing schemes.
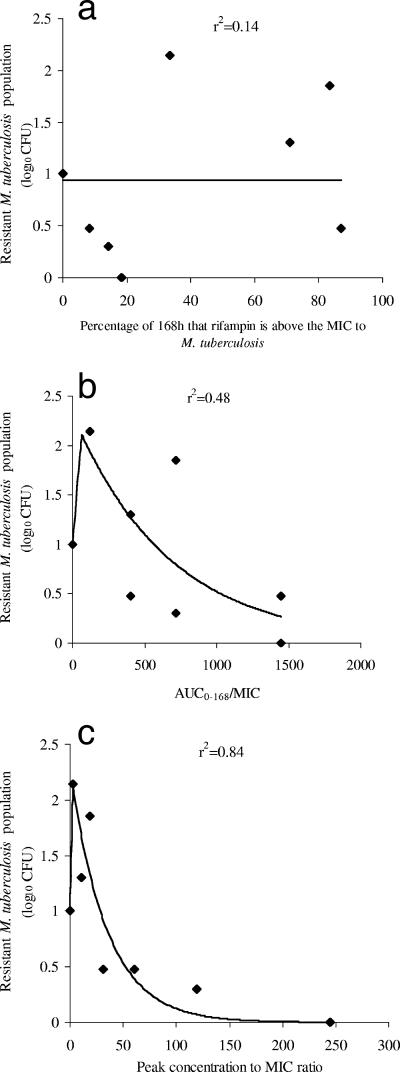
We examined the effect of dose scheduling on the rifampin-resistant subpopulation. Several doses either were administered as a single dose on day 0 or were divided into seven equal portions and administered daily. The effect of this dose scheduling on the rifampin-resistant subpopulation size is shown in Fig. 2. The TMIC was the least explanatory of the size of the resistant subpopulation (r2 = 0.14), while the AUC0-168/MIC ratio was somewhat explanatory (r2 = 0.48). However, the Cmax/MIC ratio was the best associated with the size of the rifampin-resistant subpopulation (r2 = 0.84) (P < 0.05). Rifampin Cmax/MIC ratios between 2.24 and 17.0 amplified for the emergence of the resistant population. Beyond a ratio of 17.0, the resistant population declined progressively with an increase in the Cmax/MIC ratio until the total suppression of resistance was seen at ratios of between 117 and 242.
FIG. 2.
Relationship between the size of the resistant M. tuberculosis subpopulation and the rifampin exposure.
The relationship between the rifampin concentration and changes in the rifampin-susceptible and rifampin-resistant subpopulations with time were simultaneously comodeled, and the solutions are the population parameter estimates shown in Table 2. Using these solutions, we calculated the breakpoint exposure for the suppression of rifampin resistance as a Cmax/MIC ratio of 175. This means that beyond an fCmax/MIC of 175, it is possible for rifampin to suppress resistance to itself.
TABLE 2.
Population-median parameter estimates of PD model
| Parametera | Estimate | SD |
|---|---|---|
| Clearance rate (liters/h) | 99.418 | 2.426 |
| Volume of central compartment (liters) | 430.888 | 0.858 |
| KgmaxS [log10 (CFU/ml)/h (10−3)] | 9.756 | 0.508 |
| C50gS (mg/liter) | 1.932 | 0.585 |
| HgS | 0.361 | 0.120 |
| KgmaxR [log10 (CFU/ml)/h (10−3)] | 6.195 | 1.657 |
| C50gR (mg/liter) | 2.019 | 1.575 |
| HgR | 4.542 | 4.508 |
| KkmaxS [log10 (CFU/ml)/h] | 15.829 | 0.763 |
| C50kS (mg/liter) | 7.652 | 0.146 |
| HkS | 1.388 | 0.965 |
| KkmaxR [log10 (CFU/ml)/h] | 29.379 | 0.184 |
| C50kR (mg/liter) | 22.405 | 0.233 |
| HkR (10−1) | 45.326 | 0.009 |
| POPMAX (CFU/ml) | 2.023 × 109 | 7.102 × 108 |
| Total population (NS + NR) (CFU/ml) | 1.027 × 106 | 2.018 × 104 |
| Resistant population (NR) (CFU/ml [10−1]) | 13.12 | 0.004 |
Kgmax, rate constant for maximum bacterial growth; Kkmax, rate constant for maximal bacterial kill; C50k, the drug concentration needed to achieve a 50% maximal killing rate; C50g, the drug concentration needed to achieve a 50% effect on the maximum growth rate; Hk, sigmoidicity constant for microbial killing; Hg, sigmoidicity constant for drug effect on microbial growth; N, number of bacilli. The parameters are provided for the sensitive (S) and resistant (R) subpopulations. POPMAX, estimated maximal size of the bacterial density in control HFSs after bacterial growth enters stationary phase.
We also sequenced some rifampin-resistant isolates from the subtherapeutic treatment arms. The rifampin-resistant isolates were two types of mutants; one had a mutation at codon 526 (CAC-His→CGC-Arg) in the rpoB gene, and the other had a mutation at codon 531 (TCG-Ser→TTG-Leu) in the rpoB gene.
PAE of rifampin.
In our studies of the rifampin dose versus microbial killing, growth inhibition persisted long after the rifampin concentration had declined to below the MIC for the M. tuberculosis isolate, consistent with a long PAE. To confirm this, we measured the rifampin PAE by standard assays. In the first study, the M. tuberculosis cultures exhibited PAEs of 5.2 days after exposure to 2 mg/liter of rifampin for 7 h, 12.0 days after exposure to 7 mg/liter for 1 h, 12.9 days after exposure to 7 mg/liter for 2 h, and 19.3 days after exposure to 14 mg/liter for 1/2 h (Table 3). The PAE increased linearly with the Cmax/MIC ratios (r2 = 0.96) and not the exposure time. In the repeat experiment, the PAE durations were even longer, but the pattern was the same. The PAE durations were 5.7 days after exposure to 2 mg/liter of rifampin for 7 h, 23.7 days after exposure to either 7 mg/liter for 2 h or 7 mg/liter for 1 h, and >25 days after exposure to 14 mg/liter for 1/2 h. Similar to the first study, the PAE was the most strongly associated with the Cmax/MIC.
TABLE 3.
Relationship between PD indices and PAE
| Rifampin concn (mg/liter) or parameter | Exposure duration (h) | AUC/MIC | Cmax/MIC | PAE (days) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 2 | 7 | 448.72 | 64.10 | 5.20 |
| 7 | 1 | 224.36 | 224.36 | 12.00 |
| 7 | 2.00 | 448.72 | 224.36 | 12.90 |
| 14 | 0.50 | 224.36 | 448.72 | 19.30 |
| R | −0.24 | 0.33 | 0.98 | |
| r2 | 0.06 | 0.11 | 0.96 |
Rifampin exposure and steady-state intracellular concentration.
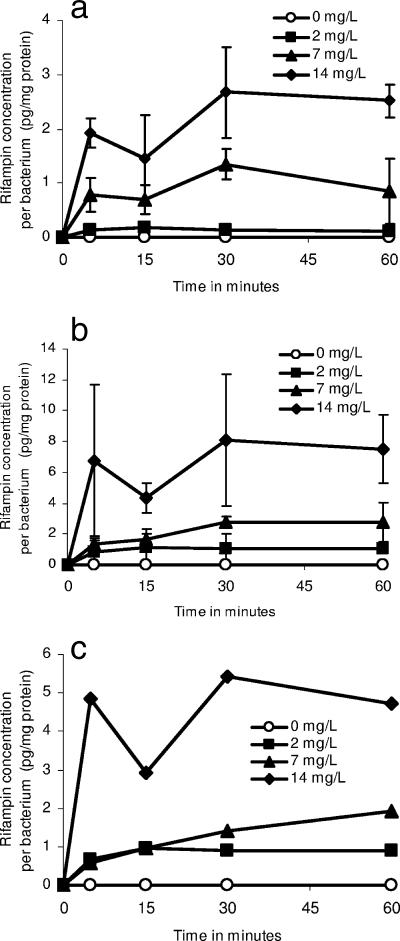
Since very short exposures to 14 mg/liter of free rifampin mediated prolonged killing and a long PAE and since Cmax correlates the best with resistance suppression, we believed that it was imperative to examine if rifampin uptake into bacilli could explain some of these effects. When M. tuberculosis cultures in Erlenmeyer flasks were exposed to rifampin in a pilot study performed over 30 min, intracellular steady-state concentrations were achieved within 15 min of incubation and were dependent on the concentration in the incubation medium. The results of the main study, which was performed over 60 min, are shown in Fig. 3. Similar to the pilot study, the intracellular steady-state concentrations increased with an increase in the concentration of rifampin in the medium and not with the exposure duration (after 15 min).
FIG. 3.
Intracellular rifampin concentration in Mycobacterium tuberculosis exposed to various rifampin concentrations. M. tuberculosis cultures were exposed to rifampin at (a) 0°C and (b) 37°C. (c) Intracellular rifampin concentrations, which were calculated by subtracting the concentrations attained at 37°C from those attained at 0°C.
DISCUSSION
The emergence of drug resistance in the clinical arena is more than enough reason to question the wisdom of uncoupling the microbial killing (bactericidal activity) by an anti-TB drug from its ability to suppress resistance to itself. We have recently learned, in the case of moxifloxacin, ciprofloxacin, and isoniazid, that some exposures associated with the optimal killing of M. tuberculosis may be the most likely to amplify the emergence of drug-resistant subpopulations (18-21). While it is true that this was in the context of monotherapy and not combination therapy, we know from the results of PK-PD studies with other organisms that the PK-PD index associated with a particular drug's optimal effect in monotherapy in the hollow fiber is the same as that even in combination therapy (13). Therefore, the optimization of dosing in monotherapy will likely optimize combination therapy regimens.
In our in vitro PD model, the microbial killing by the exposure that simulated rifampin at 600 mg a day was a monoexponential decline of 0.28 CFU/ml/day. This is virtually the same as the early bactericidal activity of 0.23 ± 0.14 CFU/ml/day seen with this rifampin dose in patients (41), which means that the results of our in vitro model have clinical relevance. The microbial killing by rifampin was linked to the AUC/MIC ratio in our in vitro model. This result is similar to that of a recent murine TB study by Jayaram et al. (25), confirming the validity of our in vitro findings. In another recent study, mice were treated with rifampin at 10 mg/kg of body weight daily or with rifapentine at 10, 15, or 20 mg/kg twice a week concurrently with isoniazid and pyrazinamide for 6 weeks, following 2 weeks of standard therapy (38). The bactericidal and sterilizing activities were superior with the higher doses of rifapentine. The authors attributed this to higher AUC0-168/MIC ratios and TMIC achieved with the higher rifapentine doses. In contrast, murine (43) and guinea pig (33) studies from three to four decades ago demonstrated the microbiological and survival superiority of once-weekly rifampin compared to that of the same cumulative dose given daily for up to 6 weeks. One would infer from the findings of those studies that rifampin's effect was associated with the Cmax/MIC ratio. We theorize that in these older animal experiments, the longer durations of therapy provided enough rounds of bacillary replication, even with the long doubling time of M. tuberculosis, to allow the substantial amplification of the mutant population and more clearly unmask the effect of the Cmax/MIC ratios.
Uniquely, in our in vitro PD model of TB, we measured the size of the rifampin-resistant population. With subtherapeutic concentrations, M. tuberculosis developed rifampin resistance due to mutations at either codon 526 (CAC-His→CGC-Arg) or codon 531 (TCG-Ser→TTG-Leu) of the rpoB gene. These are the two mutations associated with rifampin resistance in 86% of clinical isolates (42). We did not examine the isolates for efflux pumps, which have also been associated with rifampin resistance (22, 40, 44). Resistance emergence and suppression were best explained by the Cmax/MIC ratios and less so by the AUC/MIC ratio. We speculate that what may initially appear as AUC/MIC-associated microbial killing for the 7-day dosing period in our in vitro system can be explained by the Cmax/MIC modified by a long PAE. In fact, it is likely that rifamycins as a class have Cmax/MIC-linked antimicrobial effects. For example, we recently demonstrated with the mouse thigh model of Staphylococcus aureus infection that the PK-PD index linked to the effect of the rifamycin rifalazil is also Cmax/MIC (30). The effect of Cmax/MIC on microbial killing could be demonstrated in a mere 96 h of therapy because of the faster rate of replication of S. aureus per hour, which leads to >100-fold replications in the 96-h dosing interval, so that the higher Cmax/MIC-driven killing in this context would be considered a reflection of success in both microbial killing and suppression of the emergence of resistance.
Our studies with the in vitro PD model of tuberculosis demonstrated that despite rifampin's short t1/2, its long PAE prevented regrowth during the entire 1-week dosing interval, so that cycles of killing and regrowth were not encountered. Standard PAE assays confirmed the long duration of rifampin's PAE and that the PAE was the most closely associated with Cmax/MIC. On the other hand, when two exposures of 7 mg/liter with different durations of exposure were examined, they had virtually identical PAE durations, demonstrating that the duration of exposure had a minimal relationship to the PAE. In the past, other researchers have demonstrated that rifampin indeed has a long PAE. Chan et al. exposed M. tuberculosis to a rifampin Cmax/MIC of 32 and demonstrated a PAE of 2.8 days (5). This harmonizes well with our own result, in which a Cmax/MIC of 64.1 (approximately double the value of 32) had a PAE double the one demonstrated by Chan et al. (5). Indeed, a reexamination of a recent clinical trial in which several doses of rifapentine were administered to patients reveals that a 1.84 times increase in fCmax/MIC resulted in a 1.75 times increase in the “lag period” to regrowth (2 versus 3.5 days) after the decline of the serum rifapentine concentrations to below the MIC (41). Thus, for rifampin and other rifamycins, the PAE seems to be closely related to the Cmax/MIC. What this means is that whether one looks at microbial killing, resistance suppression, or PAE, the effect of rifampin is concentration dependent.
The concentration-dependent effects of rifampin may, in part, be explained by what appeared to be enhanced rifampin entry at higher concentrations in the bacillary milieu. The reasons are unclear, but the findings suggest a role for saturable efflux transporter proteins. Piddock et al. have demonstrated that rifampin accumulation in wild-type M. tuberculosis increases in the presence of reserpine, consistent with a low-level efflux pump (36). Higher concentrations in the microenvironment may be more able to overcome the effects of the pump. Once inside the cell, rifampin's macrocyclic ring acts by inhibition of an intracellular target, rpoB. Rifampin forms a very stable drug-enzyme complex with rpoB, a process that is complete within 10 min of incubation and that is not enhanced by a longer incubation (23, 46). We theorize that higher peak concentrations may enable the achievement of higher rifampin intracellular concentrations, making it more likely to inhibit poor-affinity rpoB rifampin binding sites in relatively resistant subpopulations.
Since all measures of microbial effect (microbial killing, resistance suppression, and PAE) are concentration dependent, it is the size of the rifampin dose rather than the time above the threshold which will have a major impact on rifampin's effect. When one examines the population PKs of patients treated with 600 mg of rifampin (35) and takes into account the fact that the rifampin MIC90 for clinical isolates is ≥0.125 mg/liter (29), then many patients are expected to achieve rifampin AUC/MIC and Cmax/MIC ratios associated with suboptimal microbial killing and resistance suppression. Therefore, higher doses of rifampin will likely improve both microbial killing and resistance suppression, as long as patients can tolerate them. This lends experimental PK-PD support to the calls for the use of higher doses by Peloquin (34) and Weiner and colleagues (47), who have studied the tolerability of higher rifamycin doses in TB patients. Another potential advantage would have included the ability to give the cumulative weekly dose of rifampin as a single dose without compromising its effect. However, these sorts of high intermittent doses of rifampin are currently not recommended because of the “flu-like” illness encountered in many patients (10, 15). This is probably due more to the intermittent nature of the high doses than the just high doses per se (34). However, the pharmacological lesson for rifamycin therapy is that this dosing strategy is likely to optimize the effect, if patients can tolerate the higher doses.
Our PK-PD studies also point toward a series of desirable properties of new rifampin congeners. In the early stages, one desirable property of a new rifamycin would include the ability to penetrate into M. tuberculosis cells within a few minutes and, once it is inside the bacterial cell, to be able to form a stable rifamycin-rpoB complex. In designing or screening for the new compounds, a target PK-PD index value may be an fCmax/MIC close to 175. Finally, doses that achieve this exposure should be well tolerated by patients. On the other hand, the current paradigm, which is to develop rifamycins with long t1/2s, may place unnecessary limitations on effective rifamycins that may happen to have short t1/2s. However, the strategy currently applied to suppress resistance emergence in clinical practice is to use combination therapy. With this strategy, optimizing the AUC/MIC, which may be easier to achieve than a fCmax/MIC of 175, could also reduce the rate of the of resistance emergence.
Our study has several limitations. First, we used M. tuberculosis H37Ra in our studies and not the more virulent strains. This may limit the generalizabilty of results. However, in prior studies with this isolate, the PK-PD findings have correlated with the clinical observations (18-21). Second, other M. tuberculosis strains such as M. tuberculosis H37Rv, as well as most clinical isolates, have higher MICs than the strain that we used, so that high doses of rifampin would need to be administered to achieve the AUC/MIC and Cmax/MIC ratios associated with an optimal effect. This is the reason for assessing the efficacy of higher rifampin doses in TB patients. Third, our studies were not designed to examine the sterilizing activity of rifampin. Therefore, PK-PD studies will need to be performed with nonreplicating persistent bacilli. Fourth, we would like to acknowledge that there are other strategies that have been used to study the effect of concentration on the emergence of resistance. For example, one approach is the time in the mutant suppression window hypothesis, in which minimizing the duration that drug concentrations are within the mutant suppression window reduces the chance that resistant mutants will arise (11, 12). The mutant selection window is the concentration range above the MIC of an antibiotic for the pathogen but below the concentration that prevents the selection of the first-step mutation that confers drug resistance. In our studies with isoniazid, the time in the mutant suppression window was found not to be linked to resistance emergence (20). Nevertheless, this strategy will need to be examined in the context of rifampin in the future. On the other hand, optimization of classic PK-PD indices and exposures, as in the present study, has already been successful in designing dosing strategies in mice and humans that have reduced the emergence of drug resistance in other bacteria (14), strongly suggesting the clinical relevance of our approach.
Acknowledgments
Financial support for the study was provided by Charitable Leadership Foundation, Clifton Park, NY.
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Acocella, G., G. Segre, R. Conti, V. Pagani, R. Pallanza, G. Perna, and P. Simone. 1984. Pharmacokinetic study on intravenous rifampicin in man. Pharmacol. Res. Commun. 16:723-736. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1952. Prevention of streptomycin resistance by combined chemotherapy; a Medical Research Council investigation. Br. Med. J. i:1157-1162. [PMC free article] [PubMed] [Google Scholar]
- 3.Arioli, V., M. Berti, G. Carniti, E. Randisi, E. Rossi, and R. Scotti. 1981. Antibacterial activity of DL 473, a new semisynthetic rifamycin derivative. J. Antibiot. (Tokyo) 34:1026-1032. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2003. Treatment of tuberculosis. Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:1-77. [Google Scholar]
- 5.Chan, C. Y., C. Au-Yeang, W. W. Yew, M. Hui, and A. F. Cheng. 2001. Postantibiotic effects of antituberculosis agents alone and in combination. Antimicrob. Agents Chemother. 45:3631-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2003. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic Actinomycetes; approved standard. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed]
- 7.Colangeli, R., D. Helb, S. Sridharan, J. Sun, M. Varma-Basil, M. H. Hazbon, R. Harbacheuski, N. J. Megjugorac, W. R. Jacobs, Jr., A. Holzenburg, J. C. Sacchettini, and D. Alland. 2005. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol. Microbiol. 55:1829-1840. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A., and C. M. Kunin. 1976. Significance of serum protein and tissue binding of antimicrobial agents. Annu. Rev. Med. 27:287-300. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson, J. M., and D. A. Mitchison. 1990. In vitro activities against mycobacteria of two long-acting rifamycins, FCE22807 and CGP40/469A (SPA-S-565). Tubercle 71:109-115. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson, J. M., D. A. Mitchison, S. K. Lee, Y. Y. Ong, M. G. O'Mahoney, D. J. Girling, and A. J. Nunn. 1977. Serum rifampicin concentration related to dose size and to the incidence of the ‘flu’ syndrome during intermittent rifampicin administration. J. Antimicrob. Chemother. 3:445-452. [DOI] [PubMed] [Google Scholar]
- 11.Dong, Y., X. Zhao, B. N. Kreiswirth, and K. Drlica. 2000. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drlica, K. 2003. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 52:11-17. [DOI] [PubMed] [Google Scholar]
- 13.Drusano, G. L., D. Z. D'Argenio, S. L. Preston, C. Barone, W. Symonds, S. LaFon, M. Rogers, W. Prince, A. Bye, and J. A. Bilello. 2000. Use of drug effect interaction modeling with Monte Carlo simulation to examine the impact of dosing interval on the projected antiviral activity of the combination of abacavir and amprenavir. Antimicrob. Agents Chemother. 44:1655-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drusano, G. L., A. Louie, M. Deziel, and T. Gumbo. 2006. The crisis of resistance: identifying drug exposures to suppress amplification of resistant mutant subpopulations. Clin. Infect. Dis. 42:525-532. [DOI] [PubMed] [Google Scholar]
- 15.Girling, D. J. 1973. Hong Kong Treatment Services-Royal Postgraduate Medical School-British Medical Research Council Co-operative study of rifampicin plus ethambutol in daily and intermittent regimens. Clinical observations on adverse reactions. Scand. J. Respir. Dis. Suppl. 84:119-124. [PubMed] [Google Scholar]
- 16.Grosset, J., C. Truffot-Pernot, C. Lacroix, and B. Ji. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerlava, P., V. Izac, and J. L. Tholozan. 1998. Comparison of different methods of cell lysis and protein measurements in Clostridium perfringens: application to the cell volume determination. Curr. Microbiol. 36:131-135. [DOI] [PubMed] [Google Scholar]
- 18.Gumbo, T., A. Louie, M. R. Deziel, and G. L. Drusano. 2005. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob. Agents Chemother. 49:3178-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 20.Gumbo, T., A. Louie, W. Liu, D. Brown, P. G. Ambrose, S. M. Bhavnani, and G. L. Drusano. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumbo, T., A. Louie, W. Liu, P. G. Ambrose, S. M. Bhavnani, D. Brown, and G. L. Drusano. 2007. Isoniazid's bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J. Infect. Dis. 195:194-201. [DOI] [PubMed] [Google Scholar]
- 21a.Gumbo, T., A. Louie, M. R. Deziel, and G. L. Drusano. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1156.
- 22.Gupta, A. K., D. S. Chauhan, K. Srivastava, R. Das, S. Batra, M. Mittal, P. Goswami, N. Singhal, V. D. Sharma, K. Venkatesan, S. E. Hasnain, and V. M. Katoch. 2006. Estimation of efflux mediated multi-drug resistance and its correlation with expression levels of two major efflux pumps in mycobacteria. J. Communicable Dis. 38:246-254. [PubMed] [Google Scholar]
- 23.Harshey, R. M., and T. Ramakrishnan. 1976. Purification and properties of DNA-dependent RNA polymerase from Mycobacterium tuberculosis H37RV. Biochim. Biophys. Acta 432:49-59. [DOI] [PubMed] [Google Scholar]
- 24.Hudson, A., T. Imamura, W. Gutteridge, T. Kanyok, and P. Nunn. 2003. The current anti-TB drug research and development pipeline. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, World Health Organization, Geneva, Switzerland.
- 25.Jayaram, R., S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharat, R. K. Shandil, E. Kantharaj, and V. Balasubramanian. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji, B., C. Truffot-Pernot, C. Lacroix, M. C. Raviglione, R. J. O'Brien, P. Olliaro, G. Roscigno, and J. Grosset. 1993. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am. Rev. Respir. Dis. 148:1541-1546. [DOI] [PubMed] [Google Scholar]
- 27.Kenny, M. T., and B. Strates. 1981. Metabolism and pharmacokinetics of the antibiotic rifampin. Drug Metab. Rev. 12:159-218. [DOI] [PubMed] [Google Scholar]
- 28.Kunin, C. M., W. A. Craig, M. Kornguth, and R. Monson. 1973. Influence of binding on the pharmacologic activity of antibiotics. Ann. N. Y. Acad. Sci. 226:214-224. [DOI] [PubMed] [Google Scholar]
- 29.Lee, C. N., and L. B. Heifets. 1987. Determination of minimal inhibitory concentrations of antituberculosis drugs by radiometric and conventional methods. Am. Rev. Respir. Dis. 136:349-352. [DOI] [PubMed] [Google Scholar]
- 30.Louie, A., W. Liu, M. Deziel, M. Drusano, L. Turner, T. Gumbo, and G. L. Drusano. 2004. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1297.
- 31.Maggi, N., C. R. Pasqualucci, R. Ballotta, and P. Sensi. 1966. Rifampicin: a new orally active rifamycin. Chemotherapy 11:285-292. [DOI] [PubMed] [Google Scholar]
- 32.Mitchison, D. A. 2004. Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Semin. Respir. Crit. Care Med. 25:307-315. [DOI] [PubMed] [Google Scholar]
- 33.Mitchison, D. A., and J. M. Dickinson. 1971. Laboratory aspects of intermittent drug therapy. Postgrad. Med. J. 47:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peloquin, C. 2003. What is the ‘right’ dose of rifampin? Int. J. Tuberc. Lung Dis. 7:3-5. [PubMed] [Google Scholar]
- 35.Peloquin, C. A., G. S. Jaresko, C. L. Yong, A. C. Keung, A. E. Bulpitt, and R. W. Jelliffe. 1997. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 41:2670-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piddock, L. J., K. J. Williams, and V. Ricci. 2000. Accumulation of rifampicin by Mycobacterium aurum, Mycobacterium smegmatis and Mycobacterium tuberculosis. J. Antimicrob. Chemother. 45:159-165. [DOI] [PubMed] [Google Scholar]
- 37.Rieder, H. L. 2002. Interventions for tuberculosis control and elimination. International Union against Tuberculosis and Lung Diseases, Paris, France.
- 38.Rosenthal, I. M., K. Williams, S. Tyagi, C. A. Peloquin, A. A. Vernon, W. R. Bishai, J. H. Grosset, and E. L. Nuermberger. 2006. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am. J. Respir. Crit. Care Med. 174:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rottenberg, H. 1965. The measurement of membrane potential and ΔpH in cells, organelles, and vesicles. Methods Enzymol. 64:547-559. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqi, N., R. Das, N. Pathak, S. Banerjee, N. Ahmed, V. M. Katoch, and S. E. Hasnain. 2004. Mycobacterium tuberculosis isolate with a distinct genomic identity overexpresses a tap-like efflux pump. Infection 32:109-111. [DOI] [PubMed] [Google Scholar]
- 41.Sirgel, F. A., P. B. Fourie, P. R. Donald, N. Padayatchi, R. Rustomjee, J. Levin, G. Roscigno, J. Norman, H. McIlleron, and D. A. Mitchison. 2005. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 172:128-135. [DOI] [PubMed] [Google Scholar]
- 42.Somoskovi, A., L. M. Parsons, and M. Salfinger. 2001. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir. Res. 2:164-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbist, L. 1969. Rifampicin activity “in vitro” and in established tuberculosis in mice. Acta Tuberc. Pneumol. Belg. 60:397-412. [PubMed] [Google Scholar]
- 44.Viveiros, M., and L. Amaral. 2001. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int. J. Antimicrob. Agents 17:225-228. [DOI] [PubMed] [Google Scholar]
- 45.Viveiros, M., I. Portugal, R. Bettencourt, T. C. Victor, A. M. Jordaan, C. Leandro, D. Ordway, and L. Amaral. 2002. Isoniazid-induced transient high-level resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2804-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wehrli, W., F. Knusel, K. Schmid, and M. Staehelin. 1968. Interaction of rifamycin with bacterial RNA polymerase. Proc. Natl. Acad. Sci. USA 61:667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiner, M., N. Bock, C. A. Peloquin, W. Peloquin, W. J. Burman, A. Khan, A. Vernon, Z. Zhao, S. Weis, T. R. Sterling, K. Hayden, and S. Goldberg. 2004. Pharmacokinetics of rifapentine at 600, 900, and 1,200 mg during once-weekly tuberculosis therapy. Am. J. Respir. Crit. Care Med. 169:1191-1197. [DOI] [PubMed] [Google Scholar]
- 48.Williams, K. J., and L. J. Piddock. 1998. Accumulation of rifampicin by Escherichia coli and Staphylococcus aureus. J. Antimicrob. Chemother. 42:597-603. [DOI] [PubMed] [Google Scholar]
- 49.Woo, J., W. Cheung, R. Chan, H. S. Chan, A. Cheng, and K. Chan. 1996. In vitro protein binding characteristics of isoniazid, rifampicin, and pyrazinamide to whole plasma, albumin, and alpha-1-acid glycoprotein. Clin. Biochem. 29:175-177. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. 2000. Tuberculosis. Fact sheet no. 104. World Health Organization, Geneva, Switzerland.