Abstract
We demonstrate using an in vitro pharmacodynamic model that the likelihood of selection of Escherichia coli mutants resistant to a fluoroquinolone was increased when the initial size of the bacterial population, exposed to fluoroquinolone concentrations within the mutant selection window, was increased.
Resistant bacteria selected under the pressure of fluoroquinolone exposure generally grow from a few spontaneously resistant mutants present before any treatment. When the bacterial load at the infectious site is greater than 109 to 1010 CFU, it can be assumed, if the spontaneous mutation rate is about 10−9 to 10−7 (6), that, before any antibiotic treatment, a small subpopulation of first-step resistant mutants already coexists with the larger susceptible wild-type population. The MIC allows the determination of the susceptibility of the predominant bacterial population, whereas the mutant prevention concentration (MPC) indicates the susceptibility of the small resistant subpopulation (2, 7, 9). The MIC and MPC define the bounds of the mutant selection window (MSW), a range of antibiotic concentrations favoring the selection of the first-step mutant subpopulation (9). Previous studies (5, 10) have indicated that the growth of this first-step mutant subpopulation was prevented when fluoroquinolone concentrations exceeded the MPC for more than 80% of the dosage interval, i.e., when time within the MSW (TMSW) was less than 20%. However, those studies tested only a single inoculum size, but the bacterial load increases during the time course of infections, and the likelihood of a mutant appearing may increase with inoculum size.
The aim of this study was to use marbofloxacin, a fluoroquinolone extensively used in veterinary medicine, to investigate the effect of a possible interaction between inoculum sizes (105, 107, and 109 CFU/ml) and various antibiotic exposures, characterized by different TMSWs (0%, 25%, and 100%), on the selection of Escherichia coli-resistant mutants.
The marbofloxacin MIC for Escherichia coli ATCC 25922 was determined by a microdilution technique and the MPC by a previously described method (1). The MIC and MPC were 0.008 and 0.256 μg/ml, respectively.
Bacteria suspended in Mueller-Hinton broth were exposed in vitro to marbofloxacin according to three monoexponential kinetic profiles to ensure TMSWs of 0%, 25%, and 100%, i.e., antibiotic concentrations above the MPC for 100%, 75%, and 0% of the total exposure time, respectively. The actual bacterial exposure to marbofloxacin was measured by the high-performance liquid chromatography method, and killing and regrowth of the bacterial population were assessed by counting the viable bacteria.
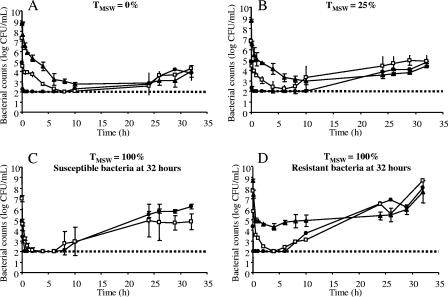
The bacterial counts without antibiotic, irrespective of the initial inoculum size, revealed similar exponential growth rates until the carrying capacity of the in vitro system was reached (about 109 CFU/ml). Figure 1A and B give the bacterial counts obtained from inoculum sizes of 105, 107, and 109 CFU/ml exposed to marbofloxacin, with TMSWs of 0% and 25%, respectively. The bacterial counts for experiments carried out with a TMSW of 100% are shown in Fig. 1C or D, depending on the susceptibility of the bacteria surviving at the end of the experiments. Whatever the initial inoculum size, all marbofloxacin regimens showed bactericidal activity during the first hours of exposure. Killing rates then declined with time until regrowth occurred, whatever the TMSW and inoculum size. The minimal counts of surviving bacteria in the central flask increased with inoculum size, although the limit of detection of 100 CFU/ml prevented comparison of the 105- and 107-CFU/ml inocula (Table 1). Bacterial counts after 32 h ranged from 104 to 2.106 CFU/ml, at which time most of the surviving bacteria were susceptible to a marbofloxacin concentration of 0.128 μg/ml, i.e., when they were not first-step mutants. The counts ranged from 5.107 to 6.108 CFU/ml when most of the surviving bacteria were resistant to this concentration, i.e., when they had the same phenotype as that of first-step mutants. The higher regrowth associated with resistant-bacterium selection may be explained by a higher growth rate or a lower rate of killing of resistant bacteria in the presence of marbofloxacin. A previously proposed pharmacodynamic parameter, called ABBC (3), was used to describe the marbofloxacin antimicrobial effect during the first 10 hours of exposure. ABBC describes the ratio of areas from 0 to 10 h delimited by time-kill curves in the absence and presence of marbofloxacin with the same inoculum sizes. Overall, inoculum size had no net effect on ABBC (Table 1) even if slightly lower ABBC values and higher minimal counts were obtained with a 109-CFU/ml inoculum exposed to a TMSW of 100%. Lower ABBCs (indicating a less efficacious action of the antibiotic) were observed only when bacteria resistant to marbofloxacin concentrations of 0.128 μg/ml were selected, suggesting that ABBC and resistant-mutant selection might be related. The shortcoming of our detection limit might explain why no relation between ABBC and resistance selection was observed for the 105- and 107-CFU/ml inocula. The relatively weak effect of inoculum size on the initial fluoroquinolone bactericidal activity observed in the present study is in agreement with a previous report on Escherichia coli exposure to ciprofloxacin or trovafloxacin in an in vitro pharmacodynamic model (4).
FIG. 1.
Observed viable counts of Escherichia coli ATCC 25922 following exposure of initial inoculum sizes of 105 (•), 107 (□), or 109 (▴) CFU/ml to concentrations of marbofloxacin inside the MSW for 0% (A), 25% (B), or 100% (C and D) of the time. For a TMSW of 100%, experiments in which surviving bacteria were mainly susceptible to a marbofloxacin concentration of 0.128 μg/ml are represented in panel C, and those in which surviving bacteria were mainly resistant to 0.128 μg/ml are shown in panel D. (A, B, and C) Each symbol represents the mean of results from two experiments. (D) The symbols • and □ represent results of a single experiment, and the symbol ▴ represents the mean from three experiments. Error bars show standard deviations. The dotted line indicates the lower limit of detection (2 log10 CFU/ml) used for bacterial quantification.
TABLE 1.
Resistance selection and bactericidal activity of marbofloxacin
| TMSW (%) | Inoculum size (CFU/ml) | Susceptibilitya | Minimal count (CFU/ml) | Final count (CFU/ml) | ABBC (log CFU/ml/h) |
|---|---|---|---|---|---|
| 100 | 105 | − | <100 | 2.106 | 53 |
| + | <100 | 1.108 | 51 | ||
| 107 | − | <100 | 7.104 | 60 | |
| + | <100 | 6.108 | 56 | ||
| 109 | + | 2.104 | 5.107 | 44 | |
| 25 | 105 | − | <100 | 6.104 | 54 |
| 107 | − | 150 | 8.104 | 56 | |
| 109 | − | 103 | 3.104 | 53 | |
| 0 | 105 | − | <100 | 7.103 | 54 |
| 107 | − | <100 | 2.104 | 57 | |
| 109 | − | 600 | 1.104 | 51 |
Susceptibility is assessed at the end of the experiments. The populations that were mainly resistant to a marbofloxacin concentration of 0.128 μg/ml (+) or mainly susceptible to a concentration of 0.128 μg/ml (−) are noted.
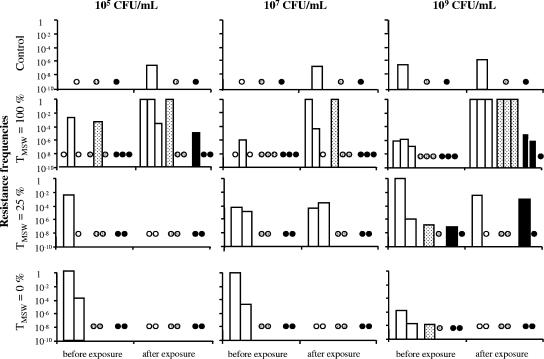
Moreover, to assess resistance selection, bacteria were grown in the presence of 0.016 (2× MIC), 0.128 (1 dilution before the MPC), and 0.256 (MPC) μg/ml marbofloxacin and counted before and 32 h after exposure to marbofloxacin. The frequencies of resistant bacteria were determined from the ratio of bacterial counts in the presence and absence of marbofloxacin. Before exposure to marbofloxacin, very few bacteria were resistant to a marbofloxacin concentration of 0.128 μg/ml, and resistance to 0.256 μg/ml was detected in only one initial inoculum of 109 CFU/ml (Fig. 2). At the end of the control experiments without antibiotic, no mutant resistant to a marbofloxacin concentration of 0.128 μg/ml was observed, whatever the inoculum size. In contrast, bacteria exposed to a TMSW of 100% became mostly resistant to 0.128 μg/ml in five out of nine experiments, as shown in Fig. 2. Most of these resistant bacteria were still susceptible to the MPC of marbofloxacin for Escherichia coli ATCC 25922 (0.256 μg/ml), suggesting that these resistant populations corresponded to those of the first-step mutants. The detection of first-step mutants when marbofloxacin concentrations were maintained within the MSW is in agreement with previous studies (5, 10). However, resistant mutants emerged systematically in all three experiments carried out with 109 CFU/ml, but only in one of three for the 105-CFU/ml inoculum and one of three for the 107-CFU/ml inoculum. We then calculated the area under the concentration-time curve (AUC)/MPC ratios (a pharmacokinetic/pharmacodynamic index obtained by dividing the AUC of marbofloxacin concentrations from 0 to 24 h by the MPC). The observed AUC/MPC values associated with the prevention of mutant selection irrespective of inoculum size were 44 to 54 h (Table 2). A value of 22 h was previously reported as sufficient to prevent the emergence of mutants resistant to ciprofloxacin in large inocula (1010 CFU) of susceptible Escherichia coli strains (8). However, in two-thirds of our experiments with inoculum sizes of 105 and 107 CFU/ml, an AUC/MPC of 9 to 12 h was sufficient to prevent the emergence of resistant mutants. These results support the hypothesis that breakpoint values of pharmacokinetic/pharmacodynamic indices, associated with the MPC and MSW concepts for preventing the emergence of resistant mutants, may depend on the size of the exposed bacterial population present at the infection site.
FIG. 2.
Frequencies of bacteria resistant to 0.016 μg/ml (white bars), 0.128 μg/ml (dotted bars), and 0.256 μg/ml (black bars) of marbofloxacin before and after exposure of initial inoculum sizes of 105, 107, and 109 CFU/ml to no antibiotic (one experiment per inoculum size) or to marbofloxacin concentrations within the MSW for 100% (three experiments per inoculum size), 25% (two experiments per inoculum size), and 0% (two experiments per inoculum size) of the time. White circles, dotted circles, and black circles indicate that no bacteria resistant to 0.016, 0.128, or 0.256 μg/ml of marbofloxacin, respectively, were detected.
TABLE 2.
Marbofloxacin pharmacokinetic parameters in relation to MIC, MPC, and MSWa
| Targeted TMSW (%) | T>MIC (%) | T>MPC (%) | AUC/MPC ratio (range) |
|---|---|---|---|
| 100 | 100 | 0 | 9-12 |
| 25 | 100 | 75 | 44-54 |
| 0 | 100 | 100 | 176-210 |
T>MIC, time that the marbofloxacin concentration was above the MIC; T>MPC, time that the marbofloxacin concentration was above the MPC.
In summary, our results confirmed that maintaining concentrations above the MPC prevents the emergence of resistance. However, the process of mutant selection within the MSW was not evenly linked to an underexposure to antibiotics but was influenced by the presence of mutants before any antibiotic treatment, a condition directly linked to the initial bacterial population size. The in vivo relevance of these in vitro results merits further investigation in animal models of infection to ensure the proper use of quinolones.
Acknowledgments
We thank Nathalie Arpaillange for technical assistance in bacteriology and Sylvie Puel and Charles-Adrien Richard for performing analytical assays.
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Blondeau, J. M., X. Zhao, G. Hansen, and K. Drlica. 2001. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong, Y., X. Zhao, B. N. Kreiswirth, and K. Drlica. 2000. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firsov, A. A., D. Savarino, M. Ruble, D. Gilbert, B. Manzano, A. A. Medeiros, and S. H. Zinner. 1996. Predictors of effect of ampicillin-sulbactam against TEM-1 β-lactamase-producing Escherichia coli in an in vitro dynamic model: enzyme activity versus MIC. Antimicrob. Agents Chemother. 40:734-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firsov, A. A., S. N. Vostrov, O. V. Kononenko, S. H. Zinner, and Y. A. Portnoy. 1999. Prediction of the effects of inoculum size on the antimicrobial action of trovafloxacin and ciprofloxacin against Staphylococcus aureus and Escherichia coli in an in vitro dynamic model. Antimicrob. Agents Chemother. 43:498-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firsov, A. A., S. N. Vostrov, I. Y. Lubenko, K. Drlica, Y. A. Portnoy, and S. H. Zinner. 2003. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1604-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komp Lindgren, P., Å. Karlsson, and D. Hughes. 2003. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob. Agents Chemother. 47:3222-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcusson, L. L., S. K. Olofsson, P. Komp Lindgren, O. Cars, and D. Hughes. 2005. Mutant prevention concentrations of ciprofloxacin for urinary tract infection isolates of Escherichia coli. J. Antimicrob. Chemother. 55:938-943. [DOI] [PubMed] [Google Scholar]
- 8.Olofsson, S. K., L. L. Marcusson, P. Komp Lindgren, D. Hughes, and O. Cars. 2006. Selection of ciprofloxacin resistance in Escherichia coli in an in vitro kinetic model: relation between drug exposure and mutant prevention concentration. J. Antimicrob. Chemother. 57:1116-1121. [DOI] [PubMed] [Google Scholar]
- 9.Zhao, X., and K. Drlica. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33(Suppl. 3):S147-S156. [DOI] [PubMed] [Google Scholar]
- 10.Zinner, S. H., I. Y. Lubenko, D. Gilbert, K. Simmons, X. Zhao, K. Drlica, and A. A. Firsov. 2003. Emergence of resistant Streptococcus pneumoniae in an in vitro dynamic model that simulates moxifloxacin concentrations inside and outside the mutant selection window: related changes in susceptibility, resistance frequency, and bacterial killing. J. Antimicrob. Chemother. 52:616-622. [DOI] [PubMed] [Google Scholar]