Abstract
The frozen version of live attenuated influenza vaccine (LAIV; FluMist) was compared with a newly licensed, refrigerated formulation, the cold-adapted influenza vaccine, trivalent (CAIV-T), for their immunogenicity, safety, and tolerability in healthy subjects 5 to 49 years of age. Eligible subjects were randomized 1:1 to receive CAIV-T or frozen LAIV. Subjects 5 to 8 years of age received two doses of vaccine 46 to 60 days apart; subjects 9 to 49 years of age received one dose of vaccine. Equivalent immunogenicities were defined as serum hemagglutination inhibition (HAI) geometric mean titer (GMT) ratios >0.5 and <2.0 for each of the three vaccine-specific strains. A total of 376 subjects 5 to 8 years of age and 566 subjects 9 to 49 years of age were evaluable. Postvaccination HAI GMT ratios were equivalent for CAIV-T and LAIV. The GMT ratios of CAIV-T/LAIV for the H1N1, H3N2, and B strains were 1.24, 1.02, and 1.00, respectively, for the 5- to 8-year-old age group and 1.14, 1.12, and 0.96, respectively, for the 9- to 49-year-old age group. Seroresponse/seroconversion rates (fourfold or greater rise) were similar in both age groups for each of the three vaccine strains. Within 28 days, the most frequent reactogenicity event in the CAIV-T and LAIV groups was runny nose/nasal congestion, which occurred at higher rates after dose 1 (44% and 42%, respectively) than after dose 2 (41% and 29%, respectively) in the 5- to 8-year-old group. Otherwise, the rates of adverse events (AEs) were similar between the treatment groups and the two age cohorts, with no serious AEs related to the study vaccines. The immunogenicities, reactogenicity events, and AEs were comparable for refrigerated CAIV-T and frozen LAIV.
Influenza virus infection is a significant cause of morbidity in people of all ages, including school-aged children and working adults. Each year in the United States, an estimated 17 million children younger than 18 years of age contract influenza (28), and hospitalization rates of 16 to 19 per 100,000 person-months have been reported in children 5 to 17 years of age (11). A significant proportion of influenza-related hospitalizations occur in previously healthy children (25). Likewise, up to 26% of adults 18 to 64 years of age contract influenza annually, and this has a significant effect on work absenteeism (7). Influenza and related complications impose a significant economic burden, with estimated annual direct and indirect costs in the United States being as high as $71 billion to $166 billion (17).
Annual vaccination with influenza vaccine is the most effective method of influenza prevention. Injectable trivalent inactivated influenza vaccine (TIV) has been used for many decades and has variable efficacies against homologous and antigenically drifted strains of influenza viruses, particularly in young children (10, 12, 13). In a recent review of five clinical trials, the pooled efficacy of TIVs was reported to be only 63% (95% confidence interval [CI], 45% to 70%) in children younger than 9 years of age (30). Furthermore, a lack of protection against frequently occurring antigenically drifted influenza virus strains presents a significant challenge to the universal application of TIVs (7).
Intranasally administered live attenuated influenza vaccines induce local and systemic antibodies and cellular immune responses to multiple influenza virus proteins and have the potential to elicit broader immunity against drifted influenza virus strains than TIVs do (3, 6, 19, 20, 27). Live attenuated influenza vaccine (LAIV; FluMist; MedImmune, Gaithersburg, MD) has been approved in the United States for use in healthy children and adults aged 2 to 49 years (FluMist, Influenza Virus Vaccine Live, Intranasal, 2007, MedImmune, Gaithersburg, MD). Studies with LAIV have shown significant efficacy in preventing influenza, including influenza caused by antigenically drifted strains (4, 22).
Originally licensed in the United States for healthy persons 5 to 49 years of age, the frozen formulation of LAIV was recently replaced with a refrigerated formulation, referred to as cold-adapted influenza vaccine, trivalent (CAIV-T). This new formulation is more convenient for the end user, no longer requiring freezer space and using refrigerator storage for the intranasal spray applicators. In addition, the volume delivered at vaccination has been reduced from 0.25 ml to 0.1 ml per nostril. In clinical trials with children aged 6 months to 17 years, CAIV-T demonstrated a significantly greater relative efficacy compared with those of TIVs in preventing culture-confirmed influenza (1, 2, 9). Our study was designed to compare the immunogenicities and safety of CAIV-T and LAIV in healthy children and adults 5 to 49 years of age.
MATERIALS AND METHODS
Vaccines.
Both LAIV and CAIV-T were supplied by MedImmune. Each dose was formulated to contain approximately 107 fluorescent focus units of each of three reassortant influenza virus strains representing the hemagglutinin and neuraminidase antigens of the A/New Caledonia/20/99 (H1N1), A/Panama/2007/99 (H3N2), and B/Hong Kong/330/01 influenza virus strains recommended by the World Health Organization to be covered in the 2003-2004 Northern Hemisphere influenza season (29). Each 0.5-ml dose of LAIV also included allantoic fluid and sucrose-phosphate-glutamic acid (SPG). Each 0.2-ml dose of CAIV-T was formulated with allantoic fluid, SPG, acid-hydrolyzed porcine gelatin, and arginine. CAIV-T and LAIV were filled into spray applicators and shipped to the study sites, where LAIV was stored at −15°C or below and CAIV-T was stored at 2°C to 8°C until just before intranasal administration. For LAIV and CAIV-T, 0.25 ml and 0.1 ml, respectively, were sprayed into each nostril.
Subjects.
We enrolled children and adults 5 to 49 years of age who were in good health, as confirmed by their medical histories and physical examinations. Exclusion criteria included serious chronic disease (including asthma or reactive airway disease); any metabolic disorder; known or suspected disease of the immune system, current receipt of immunosuppressive therapy, or the presence of an immunosuppressed or immunocompromised individual in the same household; receipt of any blood products within the previous 90 days through the study conclusion; previous receipt of any influenza vaccine (children 5 to 8 years of age only); for women, pregnancy, breast-feeding, or lactation; a documented history of hypersensitivity to egg, egg protein, or any other component of LAIV or CAIV-T; a history of Guillain-Barré syndrome; receipt of aspirin or aspirin-containing products within the 30 days before enrollment (children <18 years of age); receipt of any live vaccine within 30 days before enrollment or anticipated receipt within 30 days of study vaccination; receipt of any inactivated vaccine within 2 weeks before enrollment or anticipated receipt within 2 weeks of study vaccination; and participation in another investigational study or receipt of any investigational agent from 30 days before enrollment to the conclusion of the study.
Study design.
This prospective, phase III, randomized, double-blind, multicenter trial was conducted at 26 sites in the United States. Vaccine doses were administered between 23 July 2004 and 1 November 2004, with follow-up continuing through 13 May 2005. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for good clinical practice. The study was reviewed and approved by the institutional review board at each site. Written informed consent was obtained from the parent or legal guardian for subjects <18 years of age or from each subject if he or she was ≥18 years of age.
Subjects were randomized in a 1:1 ratio to receive CAIV-T or LAIV by using a computer-generated randomization schedule with a fixed block size of four, and each study site received vaccine packaged in complete blocks. Randomization was stratified by age (5 to 8 years and 9 to 49 years) to account for differences in baseline serostatus and because the vaccination schedule was age related. Subjects 5 to 8 years of age received two doses of LAIV or CAIV-T 46 to 60 days apart. Subjects 9 to 49 years of age received a single dose of study vaccine. Subjects, their parents or guardians, and the clinical site staff evaluating the subjects (including the investigators, study nurses, and coordinators) were blinded to the treatment group. However, because of obvious volume differences between the doses of CAIV-T and LAIV, the personnel administering study vaccine were unblinded to the treatment but did not participate in subject evaluation.
The study was conducted during the spring, after the 2003-2004 influenza season, and, thus, two of the three vaccine strains did not match the coverage recommendations for the following 2004-2005 influenza season. Subsequently, at the start of the 2004-2005 influenza season, all subjects were offered the updated influenza vaccine.
Study evaluations.
Immunogenicity was evaluated by measuring strain-specific serum hemagglutination inhibition (HAI) titers to each of the A/H1N1, A/H3N2, and B influenza virus strains contained in the vaccine. Serum samples were collected immediately before the first dose of study vaccine and 28 to 35 days after the last dose of study vaccine. Serum HAI testing was performed by MedImmune using standard laboratory assays with techniques that have been described previously (14, 15, 24).
Reactogenicity events (REs), adverse events (AEs), and the use of concomitant medicine were monitored by the subjects or their parents or guardians on daily worksheets for 28 days after each vaccine dose. REs were predefined as fever (temperatures, ≥100.0°F oral, ≥100.6°F rectal, or ≥99.6°F axillary), runny nose or nasal congestion, sore throat, cough, vomiting, headache, muscle ache, chills, decreased activity, or irritability. After unblinding of the treatments, the definition of fever was changed to >100.0°F to be consistent with the package insert for LAIV. An AE was defined as any adverse change from the study subject's baseline condition that occurred within 28 days postvaccination. Serious AEs (SAEs) were defined as events that resulted in death, were life-threatening, resulted in hospitalization or prolonged hospitalization, resulted in significant disability or incapacity, or were another important medical event that required intervention to prevent one of these outcomes. Significant new medical conditions (SNMCs) included newly diagnosed chronic medical conditions not considered by the investigator to be SAEs. The subjects were monitored for SAEs and SNMCs from the time of initial vaccination through the end of the study (for approximately 180 days after the final vaccination).
Study end points.
The primary efficacy end point was the immunogenicity of CAIV-T and LAIV, as measured by strain-specific serum HAI titers to each of the three influenza vaccine strains. Immunogenicity was considered equivalent when the 95% CIs for the ratios of the postvaccination strain-specific geometric mean titers (GMTs) for serum HAI in the CAIV-T group relative to those in the LAIV group were greater than 0.5 but less than 2.0 for each of the three influenza virus vaccine strains, regardless of the baseline serostatus. Secondary efficacy end points included the proportion of subjects who achieved strain-specific seroconversion (defined as a fourfold of greater increase in antibody titer compared with that at the baseline in subjects who were seronegative at the baseline), the proportion of subjects who achieved a strain-specific seroresponse (defined as a fourfold or greater increase in antibody titer compared with that at the baseline, regardless of baseline serostatus), and the proportion of subjects with a strain-specific HAI titer of ≥1:32 1 month after vaccination. Historically, a postvaccination titer of ≥1:32 has been accepted as a correlate for protection against influenza for individuals receiving TIVs (21). Subjects 5 to 8 years of age with baseline serum strain-specific HAI titers of ≤1:4 were considered seronegative, and those with baseline HAI titers of >1:4 were considered seropositive. Most people ≥9 years of age have been naturally exposed to the three major human influenza virus subtypes, and few are truly seronegative. Therefore, subjects 9 to 49 years of age with baseline strain-specific serum HAI titers ≤1:8 were considered serosusceptible rather than seronegative to indicate that despite measurable prevaccination antibody titers, these titers were low and not likely to be protective. Safety end points included the incidence of AEs and REs within 28 days after each study vaccination and SAEs and SNMCs from the time of enrollment through the completion of the study.
Statistical analysis.
Sample size calculations were based on assumed standard deviations (loge values) of 1.5 and 1.7 for the three influenza virus vaccine strains in the 5- to 8-year-old and 9- to 49-year-old cohorts, respectively, and a subject discontinuation rate of 10% in each group. Sample sizes of 195 (5- to 8-year-old cohort) and 250 (9- to 49-year-old cohort) per treatment group were estimated to provide at least a 95% power to exclude a twofold or greater difference in postvaccination GMTs for serum HAI determinations for all three influenza virus vaccine strains among all subjects, independent of baseline serostatus.
Three populations were defined: intent to treat (ITT; all randomized subjects for whom data were available), immunogenicity (all subjects who received required doses of study vaccination per protocol and had valid HAI assay results at all relevant time points), and safety (all subjects who received any dose of study vaccine).
Immunogenicity analyses were based on the immunogenicity population within each cohort. A two-level categorical stratification factor for serostatus was employed to control for the influence of this factor. CIs for each age cohort and vaccine strain were constructed by using a percentile-based bootstrap method (8). CAIV-T was declared to have immunogenicity equivalent to that of LAIV if the lower and upper bounds of the 95% CIs for the strain-specific GMT ratios (CAIV-T/LAIV) after the final dose were greater than 0.5 and less than 2.0. Secondary end points were evaluated by the use of two-sided 95% CIs for the proportions presented to allow population estimates within each treatment group.
Comparison of the rates of incidence of REs and AEs between CAIV-T recipients and LAIV recipients was performed by using Fisher's exact test. No adjustments were made for multiple comparisons.
RESULTS
Subjects.
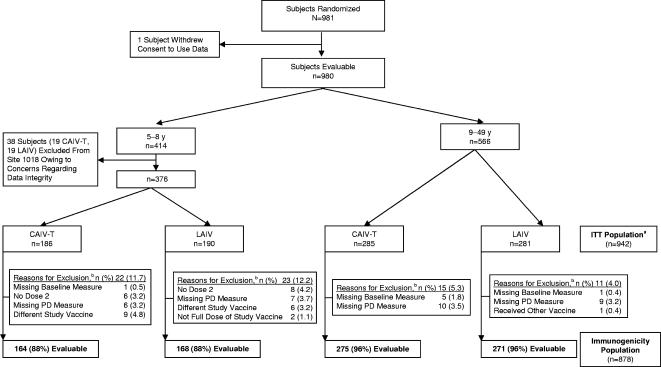
A total of 981 subjects were randomized. One subject withdrew consent to use his or her data, and data for 38 subjects at one site (19 subjects who received CAIV-T and 19 subjects who received LAIV, all of whom were ages 5 to 8 years) were excluded from the final analysis owing to concerns regarding data integrity. The ITT population therefore comprised 942 subjects, of whom 376 (186 subjects who received CAIV-T, 190 subjects who received LAIV) were in the two-dose group (ages 5 to 8 years) and 566 (285 subjects who received CAIV-T, 281 subjects who received LAIV) were in the one-dose group (ages 9 to 49 years). Figure 1 summarizes the flow of participants through the study. The demographic characteristics of the ITT population are summarized in Table 1. Baseline demographics were similar between treatment groups for both age cohorts.
FIG. 1.
Participant flow, including reasons for withdrawal and exclusion from the immunogenicity analysis. PD, postdose; a, all randomized subjects for whom data are available; b, the reasons for exclusion are not necessarily mutually exclusive.
TABLE 1.
Characteristics of study participants (ITT population)
| Characteristic | Two-dose group (ages 5 to 8 yr)
|
One-dose group (ages 9 to 49 yr)
|
||
|---|---|---|---|---|
| CAIV-T (n = 186) | LAIV (n = 190) | CAIV-T (n = 285) | LAIV (n = 281) | |
| Age (yr) | ||||
| Mean (SDa) | 6.4 (1.11) | 6.6 (1.11) | 26.3 (11.37) | 25.8 (11.62) |
| Median | 6 | 7 | 25 | 25 |
| Percentile (25th-75th) | 5-7 | 6-8 | 16-35 | 15-35 |
| Range | 5-8 | 4-8b | 9-49 | 9-49 |
| Sex (no. [%] male) | 100 (53.8) | 104 (54.7) | 122 (42.8) | 125 (44.5) |
| Race/ethnicity (no. [%] of subjects) | ||||
| White/non-Hispanic | 128 (68.8) | 126 (66.3) | 238 (83.5) | 232 (82.6) |
| Black | 24 (12.9) | 29 (15.3) | 24 (8.4) | 24 (8.5) |
| Hispanic | 30 (16.1) | 28 (14.7) | 17 (6.0) | 23 (8.2) |
| Asian | 0 (0.0) | 1 (0.5) | 2 (0.7) | 1 (0.4) |
| Other | 4 (2.2) | 6 (3.2) | 4 (1.4) | 1 (0.4) |
SD, standard deviation.
One subject in the two-dose group (ages 5 to 8 years) who received LAIV was 59 months old at the time of the dose 1 vaccination.
Strain-specific HAI titers.
The baseline distributions of the HAI titers for each of the three vaccine strains were similar for both age cohorts in the CAIV-T and LAIV treatment groups (Table 2). For subjects aged 5 to 8 years, the proportions of baseline seronegative subjects (HAI titers, ≤1:4) in the CAIV-T and LAIV groups were the highest for the influenza B virus strain (80% and 74%, respectively) and the lowest for the H3N2 strain (5% and 6%, respectively). For subjects aged 9 to 49 years, the proportions of baseline serosusceptible subjects (HAI titers, ≤1:8) in the CAIV-T and LAIV groups were similar for the influenza B virus (59% and 62%, respectively) and H1N1 (61% and 62%, respectively) strains and were the lowest for the H3N2 strain (19% and 23%, respectively). Baseline GMTs were higher against H3N2 than against the other influenza virus strains in both cohorts and treatment groups.
TABLE 2.
Baseline strain-specific HAI titer distributions
| Age group and HAI titera | No. (%) of subjects
|
|||||
|---|---|---|---|---|---|---|
| CAIV-T
|
LAIV
|
|||||
| H1N1 | H3N2 | B | H1N1 | H3N2 | B | |
| 5 to 8 yr | 176 | 176 | 176 | 182 | 182 | 182 |
| ≤1:4 | 61 (34.7) | 9 (5.1) | 141 (80.1) | 65 (35.7) | 11 (6.0) | 134 (73.6) |
| 1:8 | 15 (8.5) | 0 (0.0) | 16 (9.1) | 28 (15.4) | 3 (1.6) | 23 (12.6) |
| 1:16 | 44 (25.0) | 4 (2.3) | 12 (6.8) | 43 (23.6) | 6 (3.3) | 12 (6.6) |
| 1:32 | 33 (18.8) | 17 (9.7) | 5 (2.8) | 29 (15.9) | 19 (10.4) | 8 (4.4) |
| 1:64 | 14 (8.0) | 38 (21.6) | 2 (1.1) | 10 (5.5) | 39 (21.4) | 4 (2.2) |
| 1:128 | 5 (2.8) | 48 (27.3) | 0 (0.0) | 6 (3.3) | 55 (30.2) | 1 (0.5) |
| 1:256 | 3 (1.7) | 52 (29.5) | 0 (0.0) | 1 (0.5) | 41 (22.5) | 0 (0.0) |
| 1:512 | 1 (0.6) | 8 (4.5) | 0 (0.0) | 0 (0.0) | 8 (4.4) | 0 (0.0) |
| GMT | 11.01 | 97.16 | 3.17 | 9.14 | 82.29 | 3.65 |
| 9 to 49 yr | 280 | 280 | 280 | 279 | 279 | 279 |
| ≤1:8 | 170 (60.7) | 54 (19.3) | 166 (59.3) | 174 (62.4) | 64 (22.9) | 174 (62.4) |
| 1:16 | 27 (9.6) | 25 (8.9) | 41 (14.6) | 33 (11.8) | 28 (10.0) | 40 (14.3) |
| 1:32 | 29 (10.4) | 45 (16.1) | 33 (11.8) | 28 (10.0) | 44 (15.8) | 24 (8.6) |
| 1:64 | 25 (8.9) | 55 (19.6) | 23 (8.2) | 17 (6.1) | 46 (16.5) | 28 (10.0) |
| 1:128 | 14 (5.0) | 49 (17.5) | 10 (3.6) | 11 (3.9) | 49 (17.6) | 10 (3.6) |
| 1:256 | 9 (3.2) | 33 (11.8) | 7 (2.5) | 11 (3.9) | 30 (10.8) | 3 (1.1) |
| 1:512 | 5 (1.8) | 15 (5.4) | 0 (0.0) | 4 (1.4) | 15 (5.4) | 0 (0.0) |
| 1:1,024 | 1 (0.4) | 4 (1.4) | 0 (0.0) | 1 (0.4) | 3 (1.1) | 0 (0.0) |
| GMT | 9.26 | 45.93 | 8.64 | 8.22 | 40.62 | 7.88 |
Seronegative was defined for 5- to 8-year-old children as a baseline strain-specific HAI titer of ≤1:4; serosusceptible was defined for 9- to 49-year-old children and adults as a baseline strain-specific HAI titer of ≤1:8.
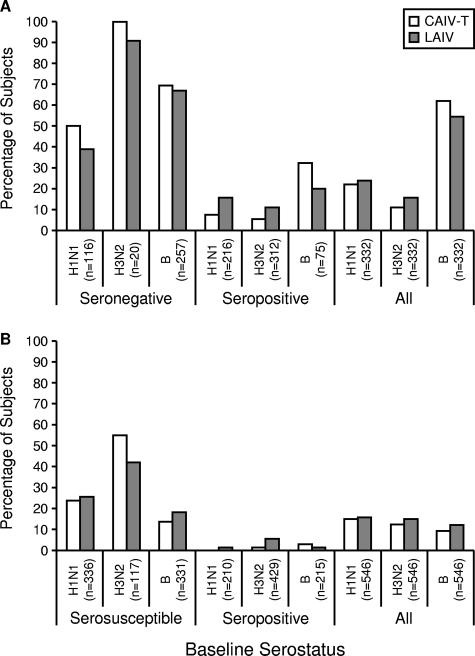
After vaccination, the ratios of the GMTs to the different influenza virus strains for CAIV-T versus those for LAIV ranged from 0.96 to 1.24 and showed equivalence for both age cohorts for each influenza virus strain in the vaccines (Table 3). For both age groups, the seroconversion/seroresponse rates to all three vaccine viruses were similar for CAIV-T and LAIV, regardless of baseline serostatus (Fig. 2). Baseline seronegative/serosusceptible subjects in both age groups exhibited the highest rates of seroconversion/seroresponse to each strain. In the two-dose group (5- to 8-year-old cohort), more than 90% of the subjects who were seronegative at the baseline seroconverted to positivity for the H3N2 strain. For the influenza B virus strain, seroconversion rates were similar between treatment groups (69% for CAIV-T, 67% for LAIV). Similar proportions of subjects in both treatment groups achieved HAI titers of ≥1:32 against each of the three influenza virus strains; the relationship between HAI titer levels and protection from influenza has not been established for LAIV (Fig. 3).
TABLE 3.
Summary of postvaccination strain-specific GMT ratios
| Dose group and strain | CAIV-T
|
LAIV
|
Adjusted GMT ratioa (CAIV-T/LAIV) | 95% CI for GMT ratio | ||
|---|---|---|---|---|---|---|
| No. of subjects | GMT | No. of subjects | GMT | |||
| Two-dose group (ages 5 to 8 yr) | ||||||
| H1N1 | 164 | 22.1 | 168 | 17.9 | 1.24 | 1.02, 1.49 |
| H3N2 | 164 | 143.5 | 168 | 140.7 | 1.02 | 0.88, 1.19 |
| B | 164 | 12.7 | 168 | 12.8 | 1.00 | 0.81, 1.24 |
| One-dose group (ages 9 to 49 yr) | ||||||
| H1N1 | 275 | 13.5 | 271 | 11.9 | 1.14 | 0.94, 1.36 |
| H3N2 | 275 | 68.3 | 271 | 61.3 | 1.12 | 0.95, 1.32 |
| B | 275 | 11.2 | 271 | 11.7 | 0.96 | 0.83, 1.10 |
Adjusted for baseline serostatus.
FIG. 2.
Seroconversion/seroresponse rates by a fourfold or greater rise in HAI titer. (A) Results for the 5- to 8-year-old cohort; (B) results for the 9- to 49-year-old cohort.
FIG. 3.
Proportion of subjects with postvaccination HAI titers of ≥1:32. (A) Results for the 5- to 8-year-old cohort; (B) results for the 9- to 49-year-old cohort.
Safety.
The overall incidence of REs occurring within 28 days after vaccination was slightly higher in the recipients of CAIV-T than in the recipients of LAIV (Table 4). The duration of REs was not significantly different between the treatment groups after any dose for either cohort. Most REs occurred during the first 10 days postvaccination and lasted for a median of 1 to 2 days. Among the subjects who received two doses of vaccine, the occurrence of any RE was higher after dose 1 than after dose 2 in both vaccination groups (CAIV-T, 69% and 57%, respectively; LAIV, 60% and 44%, respectively) and was significantly higher (P < 0.01) after dose 2 in CAIV-T recipients than in LAIV recipients. In both age cohorts, the most commonly reported REs (after any dose) were runny nose/nasal congestion, headache, cough, sore throat, and decreased activity level. In the 5- to 8-year-old cohort, a temperature >100°F was reported significantly more frequently by CAIV-T recipients than LAIV recipients after dose 2 (17% and 9%, respectively; P < 0.05) but not after dose 1 (12% and 14%, respectively). After dose 1, other REs were reported at similar rates in both vaccination groups (within 5 percentage points). After the second dose in the 5- to 8-year-old cohort, rate differences of ≥5 percentage points reported more frequently by CAIV-T recipients than LAIV recipients were also observed for runny nose/nasal congestion (41% and 29%, respectively; P < 0.05), sore throat (22% and 12%, respectively; P < 0.05), vomiting (12% and 6%, respectively; not significant), chills (7% and <1%, respectively; P < 0.01), and decreased activity level (15% and 10%, respectively; not significant). In the 9- to 49-year-old cohort, headaches were significantly more frequent in the CAIV-T group (44% versus 34% in the LAIV group; P < 0.05). Few subjects in either group experienced a temperature >103°F.
TABLE 4.
REs occurring within 0 to 28 days after doses 1 and 2
| RE | No. (%) of subjects
|
|||||
|---|---|---|---|---|---|---|
| Two-dose group (ages 5 to 8 yr)
|
One-dose group (ages 9 to 49 yr) after dose 1
|
|||||
| After dose 1
|
After dose 2
|
|||||
| CAIV-T (n = 185) | LAIV (n = 189) | CAIV-T (n = 168) | LAIV (n = 171) | CAIV-T (n = 283) | LAIV (n = 276) | |
| Any event | 128 (69.2) | 114 (60.3) | 96 (57.1) | 76 (43.7)b | 209 (73.9) | 184 (66.7) |
| Fevera | ||||||
| Temp, >100°F, oral or equivalent | 22 (11.9) | 26 (13.8) | 28 (16.7) | 15 (8.6)b | 17 (6.0) | 10 (3.6) |
| Temp, >101°F, oral or equivalent | 12 (6.5) | 15 (7.9) | 17 (10.1) | 10 (5.7) | 6 (2.1) | 4 (1.4) |
| Temp, >102°F, oral or equivalent | 5 (2.7) | 5 (2.6) | 9 (5.4) | 5 (2.9) | 2 (0.7) | 0 (0.0) |
| Temp, >103°F, oral or equivalent | 1 (0.5) | 0 (0.0) | 4 (2.4) | 2 (1.1) | 1 (0.4) | 0 (0.0) |
| Temp, >104°F, oral or equivalent | 0 (0.0) | 0 (0.0) | 2 (1.2) | 1 (0.6) | 1 (0.4) | 0 (0.0) |
| Runny nose/nasal congestion | 82 (44.3) | 79 (41.8) | 68 (40.5) | 51 (29.3)b | 142 (50.2) | 125 (45.3) |
| Sore throat | 35 (18.9) | 39 (20.6) | 37 (22.0) | 21 (12.1)b | 72 (25.4) | 69 (25.0) |
| Cough | 42 (22.7) | 42 (22.2) | 46 (27.4) | 41 (23.6) | 40 (14.1) | 52 (18.8) |
| Vomiting | 15 (8.1) | 17 (9.0) | 20 (11.9) | 10 (5.7) | 12 (4.2) | 10 (3.6) |
| Headache | 39 (21.1) | 39 (20.6) | 29 (17.3) | 25 (14.4) | 124 (43.8) | 94 (34.1)b |
| Muscle aches | 17 (9.2) | 12 (6.3) | 12 (7.1) | 4 (2.3)b | 36 (12.7) | 29 (10.5) |
| Chills | 11 (5.9) | 13 (6.9) | 11 (6.5) | 1 (0.6)c | 23 (8.1) | 17 (6.2) |
| Decreased activity | 39 (21.1) | 39 (20.6) | 25 (14.9) | 17 (9.8) | 66 (23.3) | 69 (25.0) |
| Irritability | 18 (9.7) | 23 (12.2) | 14 (8.3) | 8 (4.6) | 29 (10.2) | 24 (8.7) |
>100°F oral = >100.6°F rectal/tympanic or >99.6°F axillary; >101°F oral = >101.6°F rectal/tympanic or >100.6°F axillary; >102°F oral = >102.6°F rectal/tympanic or >101.6°F axillary; >103°F oral = >103.6°F rectal/tympanic or >102.6°F axillary; >104°F oral = >104.6°F rectal/tympanic or >103.6°F axillary.
P < 0.05 between CAIV-T and LAIV recipients by Fisher's exact test.
P < 0.01 between CAIV-T and LAIV recipients by Fisher's exact test.
AEs other than REs were infrequent (all <5%) and were similar between the treatment groups and the age groups (Table 5). No significant differences in the incidences of AEs were found between CAIV-T recipients and LAIV recipients. Two subjects reported SAEs during the study: gastroenteritis 28 days after dose 1 in a 24-year-old subject and lymphadenitis 30 days after dose 2 in a 7-year-old subject. Both events occurred in CAIV-T recipients and were judged by the blinded investigators to be unrelated to the study vaccine. In the 5- to 8-year-old cohort, four SNMCs were reported: three in CAIV-T recipients (mild asthma 165 days after dose 2, attention-deficit disorder 40 days after dose 1, and attention-deficit/hyperactivity disorder 126 days after dose 2) and one in a LAIV recipient (reactive airway disease 48 days after dose 1). In the 9- to 49-year-old cohort, five SNMCs (kidney stones, hypertension, sleep apnea, gallstones, and migraines) were reported in four CAIV-T recipients between 40 and 179 days after vaccination. Three subjects in the 5- to 8-year-old cohort who received LAIV withdrew from the study before receiving the second dose of vaccine for the following reasons: reactive airway disease, runny nose and cough, and a tooth abscess.
TABLE 5.
AEs reported in ≥1% of subjects 0 to 28 days after doses 1 and 2a
| System organ class preferred term | No. (%) of subjects
|
|||||
|---|---|---|---|---|---|---|
| Two-dose group (ages 5 to 8 yr)
|
One-dose group (ages 9 to 49 yr)
|
|||||
| Dose 1
|
Dose 2
|
|||||
| CAIV-T (n = 186) | LAIV (n = 189) | CAIV-T (n = 171) | LAIV (n = 176) | CAIV-T (n = 285) | LAIV (n = 280) | |
| All events | 36 | 33 | 28 | 25 | 53 | 45 |
| Subjects reporting one or more of the following events: | 27 (14.5) | 28 (14.8) | 23 (13.5) | 20 (11.4) | 43 (15.1) | 39 (13.9) |
| Diarrhea | 6 (3.2) | 3 (1.6) | 3 (1.8) | 5 (2.8) | 5 (1.8) | 3 (1.1) |
| Nausea | 4 (2.2) | 2 (1.1) | 1 (0.6) | 0 (0.0) | 1 (0.4) | 4 (1.4) |
| Upper abdominal pain | 1 (0.5) | 3 (1.6) | 4 (2.3) | 4 (2.3) | 3 (1.1) | 3 (1.1) |
| Stomach discomfort | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 3 (1.1) | 1 (0.4) |
| Streptococcal pharyngitis | 4 (2.2) | 1 (0.5) | 3 (1.8) | 1 (0.6) | 1 (0.4) | 0 (0.0) |
| Sneezing | 3 (1.6) | 2 (1.1) | 2 (1.2) | 0 (0.0) | 2 (0.7) | 1 (0.4) |
| Ear pain | 4 (2.2) | 1 (0.5) | 1 (0.6) | 1 (0.6) | 0 (0.0) | 2 (0.7) |
| Rash | 1 (0.5) | 3 (1.6) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Arthralgia | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 3 (1.1) | 0 (0.0) |
| Seasonal allergy | 2 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dizziness | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.1) |
No significant differences in the incidences of AEs were found between CAIV-T and LAIV recipients.
DISCUSSION
This randomized, double-blind, multicenter study showed equivalent immunogenicities and reactogenicities between the frozen formulation of LAIV and the refrigerated formulation of CAIV-T in healthy individuals 5 to 49 years of age. The seroconversion/seroresponse rates elicited by CAIV-T and LAIV were similar for both groups. The ratios of strain-specific GMTs for each vaccine in both age cohorts fell within a narrow range (0.96 to 1.24). As expected, the most robust immunogenicity was seen in children 5 to 8 years of age who were seronegative for the influenza vaccine strains before vaccination.
Because this study was conducted off season to limit potential confounding effects of possible exposure to circulating influenza viruses, it was not possible to compare the clinical efficacies of the two vaccines in preventing influenza illness. However, recent studies have shown that, compared with TIV, CAIV-T reduced the number of cases of culture-confirmed influenza by 35% to 55% in children 6 months to 17 years of age (1, 2, 9). In addition, live attenuated influenza vaccines have been shown to be effective against antigenically drifted influenza A virus strains. In children 6 to 59 months of age, the relative efficacy of CAIV-T was 79% compared with that of TIV against influenza A/H3N2 strains that were antigenically distinct from the vaccine strains (2). This finding is consistent with those of earlier studies with young children in which LAIV was up to 86% effective against antigenically drifted influenza virus strains that were not contained in the vaccine (3, 4, 19). In contrast, a recent study with adults concluded that LAIV was as effective as TIV against influenza A virus strains but less effective against influenza B virus strains during a season in which circulating A/H3N2 and B viruses were antigenically distinct from the vaccine strains (23). However, on the basis of relative efficacy estimates, the overall advantage of TIV over LAIV was not significant in adults in the study.
Multiple immune mechanisms influence protection from influenza virus, including serum HAI and neutralizing antibodies, mucosal antibodies, and cell-mediated immune responses (27). The ability of the intranasal LAIV and CAIV-T vaccines to elicit immune responses that recognize antigenically drifted influenza virus strains is probably a function of the diverse immunologic mechanisms stimulated with live vaccines. Whereas inactivated and attenuated influenza vaccines elicit comparable serum immunoglobulin G (IgG) responses in seronegative children, mucosal IgA and cytotoxic T-lymphocyte responses are greater after immunization with intranasal live vaccines than after immunization with injectable inactivated vaccines (6, 20).
In this study, the incidence of any RE was slightly higher among the recipients of CAIV-T than among the recipients of LAIV in both age cohorts. However, the rates for individual REs were similar (±5%) for the two vaccine formulations within each age cohort. In addition, the rates of REs were consistent or lower than those observed in previous studies with LAIV and CAIV-T in children and adults (5, 9, 15, 16, 18, 22, 26). Similarly, the number of subjects reporting AEs or SAEs was comparable between the two vaccine groups within each cohort.
This study has demonstrated the equivalent immunogenicity of CAIV-T, a recently licensed refrigerated formulation of LAIV, to that of the frozen formulation in healthy children and adults 5 to 49 years of age. Although the rates of REs and AEs were slightly higher with CAIV-T than with LAIV, the reduced volume of CAIV-T should be more appealing and more likely to be fully retained in the nose of the younger patient. Compared with LAIV, refrigerated CAIV-T has the advantages of more convenient storage requirements and greater ease of administration.
Acknowledgments
We thank the participating subjects and the parents of the children, the study nurses and coordinators, the clinical testing laboratory staff, and the clinical research associates and scientists at MedImmune. We also thank Gerard P. Johnson and Janet E. Stead, who provided medical writing and editorial assistance.
This study was supported by MedImmune.
The CAIV-T Study Group consists of Luis E. Angles, Heart America Research Institute, Shawnee Mission, KS; Nancy Wilson Ashbach, Radiant Research, Denver, CO; Gerald W. Bottenfield, R/D Clinical Research, Inc., Lake Jackson, TX; Tracy A. Bridges, Georgia Pollens Clinical Research Centers, Inc., Albany, GA; Robert S. Call, Commonwealth Clinical Research Specialists, Inc., Richmond, VA; John J. Champlin, Carmichael, CA; Shane Glade Christensen, J. Lewis Research, Inc., Foothill Family Clinic South, Salt Lake City, UT; Katherine Deiss, Advanced Clinical Research, Salt Lake City, UT; Frank Steven Eder, United Medical Associates, Binghamton, NY; Brandon Essink, Meridian Clinical Research, Omaha, NE; Thomas Fiel, Tempe Primary Care Associates, PC, Tempe, AZ; Mark B. Fischer, Plymouth Meeting Family Medicine, Plymouth Meeting, PA; Raul E. Gaona, Jr., Quality Assurance Research Center, San Antonio, TX; Fredric B. Garner, Burke, VA; Sandra M. Gawchik, Asthma and Allergy Research Associates, Upland, PA; Frank C. Hampel, Jr., Central Texas Health Research, New Braunfels, TX; Herschel Robert Lessin, Poughkeepsie, NY; Isaac Marcadis, Palm Beach Research Center, West Palm Beach, FL; Praful C. Mehta, Heartland Research Associates, LLC, Wichita, KS; Keith Pierce, Michigan Institute of Medicine, PC, Livonia, MI; Richard H. Schwartz, Advanced Pediatrics, Vienna, VA; Ronald J. Sell, Clinical Research Advantage, Inc., East Valley Family Physicians, PLC, Chandler, AZ; Gerald R. Shockey, Clinical Research Advantage, Inc., Desert Clinical Research, LLC, Mesa, AZ; and Paul P. Wisman Jr., Charlottesville Medical Research, Charlottesville, VA.
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Ashkenazi, S., A. Vertruyen, J. Arístegui, S. Esposito, D. McKeith, T. Klemola, J. Biolek, J. Kühr, T. Bujnowski, D. Desgrandchamps, K. Clarke, M. Saville, S.-M. Cheng, J. Skinner, A. Razmpour, W. Gruber, and B. Forrest for the CAIV-T Study Group. 2006. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr. Infect. Dis. J. 25:870-879. [DOI] [PubMed] [Google Scholar]
- 2.Belshe, R. B., K. M. Edwards, T. Vesikari, S. Black, R. E. Walker, M. Hultquist, G. Kemble, and E. M. Connor for the CAIV-T Comparative Efficacy Study Group. 2007. Comparative safety and efficacy of live attenuated and inactivated influenza vaccines in infants and young children. N. Engl. J. Med. 356:685-696. [DOI] [PubMed] [Google Scholar]
- 3.Belshe, R. B., and W. C. Gruber. 2000. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr. Infect. Dis. J. 19:S66-S71. [DOI] [PubMed] [Google Scholar]
- 4.Belshe, R. B., W. C. Gruber, P. M. Mendelman, I. Cho, K. Reisinger, S. L. Block, J. Wittes, D. Iacuzio, P. Piedra, J. Treanor, J. King, K. Kotloff, D. I. Bernstein, F. G. Hayden, K. Zangwill, L. Yan, and M. Wolff. 2000. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J. Pediatr. 136:168-175. [DOI] [PubMed] [Google Scholar]
- 5.Belshe, R. B., K. L. Nichol, S. B. Black, H. Shinefield, J. Cordova, R. Walker, C. Hessel, I. Cho, and P. M. Mendelman. 2004. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5-49 years. Clin. Infect. Dis. 39:920-927. [DOI] [PubMed] [Google Scholar]
- 6.Boyce, T. G., W. C. Gruber, S. D. Coleman-Dockery, E. C. Sannella, G. W. Reed, M. Wolff, and P. F. Wright. 1999. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine 18:82-88. [DOI] [PubMed] [Google Scholar]
- 7.Bridges, C. B., W. W. Thompson, M. I. Meltzer, G. R. Reeve, W. J. Talamonti, N. J. Cox, H. A. Lilac, H. Hall, A. Klimov, and K. Fukuda. 2000. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA 284:1655-1663. [DOI] [PubMed] [Google Scholar]
- 8.Efron, B., and R. J. Tibshirani. 1993. An introduction to the bootstrap, p. 168-177. Chapman & Hall, New York, NY.
- 9.Fleming, D., P. Crovari, U. Wahn, T. Klemola, Y. Schlesinger, A. Langussis, M. Luz Garcia, A. Krygier, H. Costa, U. Heininger, J. Pregaldien, S. Cheng, J. Skinner, A. Razmpour, M. Saville, W. Gruber, and B. Forrest. 2006. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr. Infect. Dis. J. 25:860-869. [DOI] [PubMed] [Google Scholar]
- 10.Hoberman, A., D. P. Greenberg, J. L. Paradise, H. E. Rockette, J. R. Lave, D. H. Kearney, D. K. Colborn, M. Kurs-Lasky, M. A. Haralam, C. J. Byers, L. M. Zoffel, I. A. Fabian, B. S. Bernard, and J. D. Kerr. 2003. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 290:1608-1616. [DOI] [PubMed] [Google Scholar]
- 11.Izurieta, H. S., W. W. Thompson, P. Kramarz, D. K. Shay, R. L. Davis, F. DeStefano, S. Black, H. Shinefield, and K. Fukuda. 2000. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N. Engl. J. Med. 342:232-239. [DOI] [PubMed] [Google Scholar]
- 12.Jefferson, T., S. Smith, V. Demicheli, A. Harnden, A. Rivetti, and C. Di Pietrantonj. 2005. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet 365:773-780. [DOI] [PubMed] [Google Scholar]
- 13.Kamada, M., T. Nagai, T. Kumagai, M. Igarashi, T. Ihara, T. Okafuji, H. Ochiai, H. Sakiyama, K. Shimomura, E. Suzuki, S. Torigoe, C. Miyazaki, A. Miyata, K. Yuri, Y. Ito, T. Nakayama, T. Kase, and Y. Okuno. 2006. Efficacy of inactivated trivalent influenza vaccine in alleviating the febrile illness of culture-confirmed influenza in children in the 2000-2001 influenza season. Vaccine 24:3618-3623. [DOI] [PubMed] [Google Scholar]
- 14.Kendal, A. P., and T. R. Cate. 1983. Increased sensitivity and reduced specificity of hemagglutination inhibition tests with ether-treated influenza B/Singapore/222/79. J. Clin. Microbiol. 18:930-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King, J. C., Jr., P. E. Fast, K. M. Zangwill, G. A. Weinberg, M. Wolff, L. Yan, F. Newman, R. B. Belshe, A. Kovacs, J. G. Deville, and M. Jelonek. 2001. Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr. Infect. Dis. J. 20:1124-1131. [DOI] [PubMed] [Google Scholar]
- 16.King, J. C., Jr., J. Treanor, P. E. Fast, M. Wolff, L. Yan, D. Iacuzio, B. Readmond, D. O'Brien, K. Mallon, W. E. Highsmith, J. S. Lambert, and R. B. Belshe. 2000. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J. Infect. Dis. 181:725-728. [DOI] [PubMed] [Google Scholar]
- 17.Lynd, L. D., R. Goeree, and B. J. O'Brien. 2005. Antiviral agents for influenza: a comparison of cost-effectiveness data. Pharmacoeconomics 23: 1083-1106. [DOI] [PubMed] [Google Scholar]
- 18.Mendelman, P. M., J. Cordova, and I. Cho. 2001. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold-adapted (CAIV-T) in healthy children and healthy adults. Vaccine 19:2221-2226. [DOI] [PubMed] [Google Scholar]
- 19.Mendelman, P. M., R. Rappaport, I. Cho, S. Block, W. Gruber, M. August, D. Dawson, J. Cordova, G. Kemble, K. Mahmood, G. Palladino, M. S. Lee, A. Razmpour, J. Stoddard, and B. D. Forrest. 2004. Live attenuated influenza vaccine induces cross-reactive antibody responses in children against an A/Fujian/411/2002-like H3N2 antigenic variant strain. Pediatr. Infect. Dis. J. 23:1053-1055. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, B. R., and M. L. Clements. 1989. The systemic and mucosal immune response of humans to influenza A virus. Curr. Top. Microbiol. Immunol. 146:107-116. [DOI] [PubMed] [Google Scholar]
- 21.National Institutes of Health. 1973. Specific immunity in influenza—summary of Influenza Workshop III. J. Infect. Dis. 127:220-236. [Google Scholar]
- 22.Nichol, K. L., P. M. Mendelman, K. P. Mallon, L. A. Jackson, G. J. Gorse, R. B. Belshe, W. P. Glezen, and J. Wittes. 1999. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 282:137-144. [DOI] [PubMed] [Google Scholar]
- 23.Ohmit, S. E., J. C. Victor, J. R. Rotthoff, E. R. Teich, R. K. Truscon, L. L. Baum, B. Rangarajan, D. W. Newton, M. L. Boulton, and A. S. Monto. 2006. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N. Engl. J. Med. 355:2513-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer, D. F., M. T. Coleman, W. R. Dowdle, and G. C. Schild. 1975. Advanced laboratory techniques for influenza diagnosis. Immunology series no. 6. U.S. Department of Health, Education, and Welfare, Washington, DC.
- 25.Quach, C., L. Piche-Walker, R. Platt, and D. Moore. 2003. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics 112:e197-e201. [DOI] [PubMed] [Google Scholar]
- 26.Redding, G., R. E. Walker, C. Hessel, F. S. Virant, G. H. Ayars, G. Bensch, J. Cordova, S. J. Holmes, and P. M. Mendelman. 2002. Safety and tolerability of cold-adapted influenza virus vaccine in children and adolescents with asthma. Pediatr. Infect. Dis. J. 21:44-48. [DOI] [PubMed] [Google Scholar]
- 27.Tamura, S., T. Tanimoto, and T. Kurata. 2005. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn. J. Infect. Dis. 58:195-207. [PubMed] [Google Scholar]
- 28.Weycker, D., J. Edelsberg, M. Elizabeth Halloran, I. M. Longini, Jr., A. Nizam, V. Ciuryla, and G. Oster. 2005. Population-wide benefits of routine vaccination of children against influenza. Vaccine 23:1284-1293. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. 2003. Addendum to the recommended composition of influenza virus vaccines for use in the 2003-2004 influenza season. Wkly. Epidemiol. Rec. 78:77.12674025 [Google Scholar]
- 30.Zangwill, K. M., and R. B. Belshe. 2004. Safety and efficacy of trivalent inactivated influenza vaccine in young children: a summary for the new era of routine vaccination. Pediatr. Infect. Dis. J. 23:189-197. [DOI] [PubMed] [Google Scholar]