Abstract
A vancomycin-resistant, anaerobic, gram-positive coccus containing the vanD and vanG-like genes (strain CCRI-16110) was isolated from a human fecal specimen during a hospital surveillance program to detect carriers of vancomycin-resistant enterococci. Comparison of the 16S rRNA gene sequence of strain CCRI-16110 with databases revealed a potentially novel Ruminococcus species that was most similar (<94% identity) to Clostridium and Ruminococcus species. Strain CCRI-16110 was highly resistant to vancomycin and teicoplanin (MICs of >256 μg/ml). The complete DNA sequence of the vanD cluster was most similar (98.2% identity) to that of Enterococcus faecium BM4339, containing the vanD1 allele. An intD gene with 99% identity with that of this E. faecium strain was found to be associated with the vanD gene cluster of this novel anaerobic bacterium. Strain CCRI-16110 also harbors genes encoding putative VanSG, VanG, and VanTG proteins displaying 56, 73.6, and 55% amino acid sequence identity, respectively, compared to the corresponding proteins encoded by the vanG1 and vanG2 operons of Enterococcus faecalis BM4518 and N03-0233. This study reports for the first time an anaerobic bacterium containing the vanD gene cluster. This strain also harbors a partial vanG-like gene cluster. The presence of vanD- and vanG-containing anaerobic bacteria in the human bowel flora suggests that these bacteria may serve as a reservoir for the vanD and vanG vancomycin resistance genes.
The glycopeptide antibiotics vancomycin and teicoplanin interfere with cell wall synthesis in gram-positive bacteria by binding to the terminal dipeptide d-alanyl-d-alanine regions of the pentapeptide precursors of peptidoglycan side chains. Resistance to glycopeptide antibiotics was first described for enterococci (23, 35) and has now spread worldwide. Six different genes (vanA, vanB, vanC, vanD, vanE, and vanG) have been shown to confer glycopeptide resistance in enterococci (7).
The VanD-type resistance is constitutively expressed in enterococci (8, 11) and is characterized by moderate MICs of vancomycin (64 to 128 μg/ml) and teicoplanin (4 to 64 μg/ml). This VanD-type resistance results from the acquisition of the vanRD, vanSD, vanYD, vanHD, vanD, and vanXD cluster of genes, which directs synthesis of peptidoglycan precursors terminating in d-alanyl-d-lactate (11). The vanD gene cluster is located on the chromosome and is not transferable to other enterococci by conjugation in vitro (11). The VanD-type resistance in enterococci is also characterized by the presence of an impaired d-alanyl-d-alanine (Ddl) ligase due to mutations in the chromosomal ligase-encoding gene ddl (10). Although the Ddl ligase is inactive, the VanD-type enterococci are able to grow even in the absence of glycopeptide because the vanD cluster is expressed constitutively as a result of mutations in the VanSD sensor or in the VanRD regulator (10).
The VanG-type resistance in enterococci is characterized by low-level resistance to vancomycin (MIC, 16 μg/ml) and susceptibility to teicoplanin (9). This VanG-type resistance results from the acquisition of the vanUG, vanRG, vanSG, vanYG, vanWG, vanG, vanXYG, and vanTG cluster of genes, which directs synthesis of peptidoglycan precursors terminating in d-alanyl-d-serine (d-Ala-d-Ser) (9).
To date, the vanD and vanG gene clusters have been described only for Enterococcus. However, the complete genome sequence of the Clostridium difficile strain 630 recently revealed the presence of a cluster of genes with high similarity to the vanG cluster in enterococci (32). Moreover, our group has recently described a high prevalence of vanB (4.8%), vanD (37.9%), and vanG (9.3%) genes in fecal specimens which are not associated with vancomycin-resistant enterococci, during a vancomycin-resistant enterococcus surveillance program implemented in two North American hospitals (13).
In this study, we describe for the first time an anaerobic bacterium of the human bowel which is resistant to both vancomycin and teicoplanin and which contains vanD and vanG-like gene clusters.
(This study was presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 27 to 30 September 2006.)
MATERIALS AND METHODS
Isolation procedure and identification.
A vanD-positive rectal specimen, ERV-110, collected from a patient at the Montreal General Hospital (Québec, Canada) in 2001 (13) was subcultured several times in brain heart infusion broth (Difco, Detroit, MI) supplemented with vitamin K (0.001 mg/ml), hemin (0.005 mg/ml), l-cystine (0.5 mg/ml), sodium lactate (10 mM), sodium pyruvate (10 mM), vancomycin (10 μg/ml), and aztreonam (100 μg/ml) under anaerobic conditions at 35 to 37°C. A subculture was then plated on agar medium containing the same supplement and incubated under the same anaerobic conditions for 7 days. Colonies were then screened for the presence of the vanD gene by PCR. Identification of the vanD-positive colony (strain CCRI-16110) was performed by methods previously described (15) and with the Rapid ID 32A system (bioMérieux, Marcy l'Étoile, France). Partial sequencing of a 1,466-bp region of the gene encoding 16S rRNA was performed as previously described (12).
Resistance studies.
MICs of vancomycin and metronidazole were determined by the agar dilution method for anaerobes according to the CLSI (formerly NCCLS) (24), whereas the MIC of teicoplanin was determined by the Etest method (AB Biodisk, Sweden). The medium used for antimicrobial susceptibility testing was brucella agar (Difco, Detroit, MI) supplemented with laked sheep blood, vitamin K (0.001 mg/ml), and hemin (0.005 mg/ml).
Bacterial strains and plasmids.
Enterococcus faecium BM4339 (resistant to vancomycin and teicoplanin and containing the vanD gene cluster) (5) and Enterococcus faecalis JH2-2 (resistant to fusidic acid and rifampin) (18) were obtained from P. Courvalin (Unité des Agents Antibactériens, Institut Pasteur, Paris, France) and from N. Woodford (Health Protection Agency, Collindale, London, United Kingdom), respectively. E. faecium BM4339 was used as a control strain, and E. faecalis JH2-2 was used as recipient in the conjugation experiments. Escherichia coli TOP10 (Invitrogen, Burlington, Ontario, Canada) was used as a host for recombinant plasmids. PCR products were cloned in the vector pCR2.1 using the TOPO TA cloning kit (Invitrogen, Burlington, Ontario, Canada).
Sequencing of the vanD gene cluster.
The primers used to characterize the vanD cluster are described in Table 1. PCRs were performed using standard conditions with purified genomic DNA and the Taq DNA polymerase (Promega, Mississauga, Ontario, Canada) as previously described (20). Purification of the amplification products and sequencing reactions were performed as previously described (20). Comparison with known sequences was carried out using the programs from the GCG package (Wisconsin package version 10.3; Accelrys Inc., San Diego, CA).
TABLE 1.
PCR primers used in this study to amplify and sequence the vanD and vanG-like clusters from Ruminococcus sp. strain CCRI-16110
| Cluster | Primer name | Oligonucleotide sequence (5′→3′) | Nucleotide positiona (ORF[s]) | Source or reference |
|---|---|---|---|---|
| vanD | RD-TSP1 | AAAAAACCGTTCACCCCATACTG | 899 (vanRD) | This study |
| RD-TSP2 | ACGCTCGCACAGATACCAAAG | 519 (vanRD) | This study | |
| RD-TSP3 | GGCTGCTCTTGCTGATTGTC | 443 (vanRD) | This study | |
| RD1 | CCGTTTAACCCGCTGGAA | 324 (vanRD) | 25 | |
| SD1 | CTATCATGATCGGGATG | 885 (vanSD) | 25 | |
| SD2 | CGAATGGTGGTATTCTC | 1576 (vanSD) | 25 | |
| YD1 | GATTCGTCAACCGCATG | 2453 (vanYD) | 25 | |
| YD3F | GGCTGCATGTGTCACAC | 2059 (vanYD) | This study | |
| YD2 | CTCTGGAACTGAGGGTA | 2214 (vanYD) | 25 | |
| HD1 | CGTAAGCCATAAAGCGGA | 3224 (vanHD) | 25 | |
| HD3a | AACGAAACATAGTCCGC | 3615 (vanHD) | This study | |
| D1 | TAAGGCGCTTGCATATACCG | 4425 (vanD) | 26 | |
| D2 | TGCAGCCAAGTATCCGGTAA | 4866 (vanD) | 26 | |
| XD2 | TATGTATCCGGGTATGG | 5637 (vanXD) | 25 | |
| ID1 | GTAAAGGCCCAGACAGT | 5995 (intD) | 5 | |
| ID-TSP1 | TCAACGCTTTTCTGGAGTTC | 6032 (intD) | This study | |
| ID-TSP2 | GCGGGAACTGTCTGAAGCGG | 6117 (intD) | This study | |
| ID-TSP3 | GGAAAAAACCGCACTGTTCTTCT | 6292 (intD) | This study | |
| ID2 | ATTCAAGATCCGCTCGTG | 6613 (intD) | 5 | |
| vanG like | V1 | GGIGARGAYGGIWSIHTICARGG | 1511 (vanG like) | 14 |
| V2ab | GTRAAICCIGGIADIGTRTT | 2120 (vanG like) | This study | |
| GG1-TSP1 | CCTTCCCCGTATATCTTTGC | 1760 (vanG like) | This study | |
| GG1-TSP2 | CCACAGCCTTCGGGACAGAT | 1639 (vanG like) | This study | |
| GG1-TSP3 | GAGGACGCCGCAGCCGACAA | 1560 (vanG like) | This study | |
| GP20 reverse | GGCAAGTTCAAGTAATCCCT | 1530 (vanG like) | This study | |
| GD1-TSP1 | TCCTCACTGTGGGGAGAGTC | 1887 (vanG like) | This study | |
| GD1-TSP2 | CTGACGGGTGGATTCTTTGA | 1919 (vanG like) | This study | |
| GD1-TSP3 | ACAGGAGACGGCAAAGAACA | 2017 (vanG like) | This study | |
| GP788 | GGCTGTCAGGCATGATATTA | 788 (vanZF like) | This study | |
| GN2720 | AGCGGTATCTTTTCAGTC | 2720 (vanTG like) | This study | |
| GP1421 | CCTGCGGTGCATGGAATACT | 1421 (vanG like) | This study | |
| GN2038 | AACATCCCAGCACTTGATAA | 2038 (vanG like) | This study | |
| GG2-TSP1 | GCATCGTCCTGAAAAATCTC | 941 (vanZF like) | This study | |
| GG2-TSP2 | CATTAGCGGCAACCTGTGTA | 866 (vanZF like) | This study | |
| GD2-TSP1 | AATGCGGAAAAGCTGGTGAG | 2421 (vanTG like) | This study | |
| GD2-TSP2 | GTTCGGGGACAGTTATTATG | 2696 (vanTG like) | This study | |
| GP107 | CCGATAAAATGCAGCGTGTAT | 107 (vanSG like) | This study | |
| GN3916 | ATTATGGGGCACTCAAAACAC | 3916 (vanTG like) | This study | |
| GN3098 | TGCCGGACTGACTGGTTTACA | 3098 (vanTG like) | This study |
Nucleotide positions refer to sequences of the vanD cluster (accession number EF508033) or vanG-like cluster (accession number EF508032) from Ruminococcus sp. strain CCRI-16110.
V2a represents the universal primer derived from reference 14 in which nucleotides GT have replaced the TG at the 5′ end.
Sequencing of the vanG-like gene cluster.
PCR amplification using universal primers V1 and V2a (Table 1), which were designed to amplify an internal region of the genes encoding Ddl ligases and related vancomycin-resistant proteins (14), was performed using genomic DNA purified from strain CCRI-16110. PCR products of the expected size (630 bp) were purified and cloned in the vector pCR2.1. The inserts from these recombinant plasmids were sequenced using the universal M13 forward and M13 reverse primers (Invitrogen). Nucleotide and deduced amino acid sequences from the cloned 630-bp PCR product obtained were analyzed by using the BLASTN, TBLASTN, and BLASTP softwares. Subsequently, the DNA sequence flanking the 630-bp fragment containing the vanG-like gene was obtained by using a combination of PCR amplifications with the DNA Walking SpeedUp kit (Bio/Can Scientific, Mississauga, Ontario, Canada) and cloning techniques as previously described (12). Sequences obtained by these methods were confirmed by sequencing PCR products generated after amplification of total genomic DNA from strain CCRI-16110, using specific primers overlapping the cloned fragments (Table 1). The Artemis software (27) was used to analyze and collate data from the complete sequence.
Phylogenetic analysis of the vanG-like gene from strain CCRI-16110.
To ascertain the phylogenetic position of the deduced protein sequence of the vanG-like ligase gene from strain CCRI-16110, phylogenetic analysis was performed using the deduced amino acid sequences from the Ddl ligases of gram-positive and gram-negative organisms as well as the d-Ala-d-Lac and d-Ala-d-Ser ligases of vancomycin-resistant organisms. Amino acid sequence alignment was performed with CLUSTALW (34). Phylogenetic analysis was carried out by the neighbor-joining method (28) using MEGA version 3.1 (21). The evolutionary distance was generated according to the Jones-Taylor-Thornton matrix for amino acid sequences (19). The tree topology was compared to that obtained by the maximum-parsimony method. Bootstrap values were calculated from 1,000 resamplings to test the robustness of the data and were displayed as percentages.
Filter mating.
To study the transfer by conjugation of the glycopeptide resistance phenotype of strain CCRI-16110, mating on filters was performed as described previously (12). E. faecalis JH2-2 was used as the recipient, while strain CCRI-16110 was used as a donor.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the vanD gene cluster and partial vanG-like gene cluster sequences of strain CCRI-16110 are EF508033 and EF508032, respectively.
RESULTS
Isolation and identification of Ruminococcus sp. strain CCRI-16110 containing vanD.
We isolated a strictly anaerobic, gram-positive coccus (strain CCRI-16110) from fecal specimen ERV-110. Biochemical tests performed in triplicate using the Rapid ID 32A system remained negative for all substrates tested with this system. Analysis of the partial sequence of its 16S rRNA gene revealed that strain CCRI-16110 was most similar to Clostridium and Ruminococcus species (<94% identity) belonging to the Clostridium coccoides cluster of organisms (rRNA cluster XIVa) (6). Based on these phenotypic and genotypic analyses, strain CCRI-16110 is a potentially novel Ruminococcus species. This strain was resistant to vancomycin and teicoplanin (MICs, >256 μg/ml) and susceptible to metronidazole (MIC, <0.125 μg/ml).
Characterization of the vanD gene cluster from Ruminococcus sp. strain CCRI-16110.
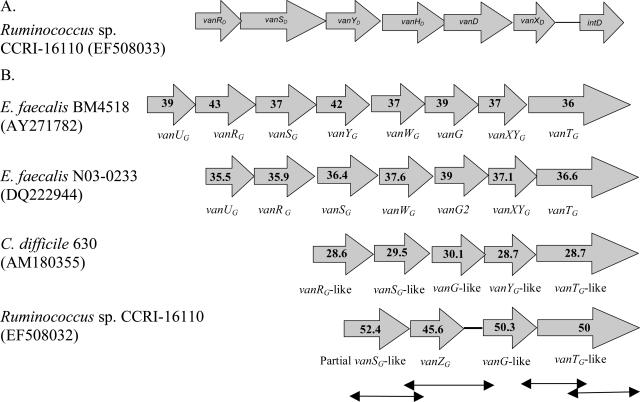
The vanD gene cluster from Ruminococcus sp. strain CCRI-16110 was characterized to determine its genetic organization. Nucleotide sequence analysis of the vanD gene cluster of this strain showed that the gene order of this cluster was similar to that of known vanD clusters (Fig. 1A). The complete DNA sequence of the CCRI-16110 vanD operon (from vanRD to vanXD, 5,655 bp) exhibited 98% identity with the vanD1 operon (accession number AF130997), 97% identity with the vanD3 operon (accession number AF175293), 95% identity with the vanD2 operon (accession number AF153050), 93% identity with the vanD5 operon (accession number AY489045), and 89% identity with the vanD4 operon (accession number AY082011). The amino acid sequences of VanRD, VanSD, VanHD, and VanXD of the vanD operon from this Ruminococcus strain were most similar to those of the vanD1 operon from E. faecium BM4339, while the amino acid sequences of VanYD and VanD were most similar to those of the vanD3 operon from E. faecium N97-330 (Table 2). The H, N, G1, F, and G2 conserved motifs found in histidine kinase proteins were present in the VanSD sensor of strain CCRI-16110 (10). Comparison of the sequence of the VanSD sensor of strain CCRI-16110 with that of BM4339 revealed point mutations in critical regions of these conserved motifs: (i) a V165A mutation within the H block beside the putative autophosphorylation site and (ii) a Q308K mutation beside the G1 ATP binding block. An intD gene, encoding a putative integrase-like protein showing 99.2% amino acid sequence identity with that of E. faecium BM4339, was found to be associated with the vanD gene cluster (Fig. 1A).
FIG. 1.
Organization of the vanD and vanG-like operons from Ruminococcus sp. strain CCRI-16110. (A) Arrows represent the ORFs of the vanD operon. (B) Schematic organization of the vanG operon from E. faecalis BM4518 (accession number AY271782), the vanG2 operon from E. faecalis N03-0233 (DQ222944), and the vanG-like operons from C. difficile 630 (AM180355) and Ruminococcus sp. strain CCRI-16110 (EF508032). Arrows represent the ORFs. The regions characterized by using the DNA walking method are indicated by two-headed arrows. The percent G+C is indicated within each arrow.
TABLE 2.
Comparison of nucleotide and amino acid sequences of the vanD gene cluster and intD gene from Ruminococcus sp. strain CCRI-16110 with those of the vanD1, vanD2, vanD3, vanD4, and vanD5 gene clusters
| Gene | Nucleotide/amino acid identity (%)a to:
|
||||
|---|---|---|---|---|---|
| vanD1 | vanD2 | vanD3 | vanD4 | vanD5 | |
| vanRD | 99.4/100 | NA | 97.3/98.7 | 9.3/98.7 | 97.0/100 |
| vanSD | 98.4/98.2 | NA | 97.0/96.0 | 90.0/94.0 | 92.0/96.0 |
| vanYD | 98.0/97.5 | 88.0/71.4 | 99.0/98.3 | 82.0/59.7 | 77.4/39.2 |
| vanHD | 98.0/100 | 96.5/98.1 | 98.5/100 | 80.0/88.0 | 94.0/96.6 |
| vanD | 98.1/98.3 | 97.3/97.1 | 98.3/99.1 | 82.6/89.5 | 85.8/91.0 |
| vanXD | 99.0/100 | 98.0/100 | 97.4/99.0 | 86.0/90.0 | 99.0/97.0 |
| intD | 99.3/99.6 | NA | NA | NA | NA |
Characterization of the vanG-like operon from Ruminococcus sp. strain CCRI-16110.
Since the ddl gene, encoding the Ddl ligase, is mutated in enterococci harboring the vanD gene (4, 5, 10), we searched for a ddl gene in strain CCRI-16110 by using the universal primers V1 and V2a (Table 1) (14). A similarity search using TBLASTN revealed that the sequence of the 630-bp PCR amplification product generated using these primers encoded a putative protein that was most similar (66% amino acid sequence identity) to the known VanG of enterococci, and hence this gene encoding the VanG variant was designated vanG like. From this partial vanG-like gene sequence, the successive combination of PCR amplification, cloning, and sequencing allowed us to characterize a 3,950-bp genomic DNA fragment. Sequence analysis of this genomic DNA fragment revealed the presence of three complete open reading frames (ORFs) and one partial ORF encompassing the vanG-like gene of strain CCRI-16110. Two complete ORFs displayed the highest amino acid sequence identities with the VanG d-Ala-d-Ser ligases (63.6% to 65.3%) and the VanTG serine racemases (58.6 to 59.6%) of the vanG operons of enterococci (Table 3). The partial ORF (which resulted from the shortened PCR fragment used for sequencing) was most similar to the VanSG histidine kinase sensors (63.4 to 64.8% identities). The fourth complete ORF displayed significant amino acid sequence identity to the VanZ protein encoded in the vanA operon of E. faecium BM4147 (27.1%) and the vanF operon of Paenibacillus popilliae (27.5%) (Table 3), as well as in the skin (sigK intervening sequence) element of C. difficile 630 (28.2%). Therefore, the gene encoding the putative VanZ protein in the vanG-like cluster of strain CCRI-16110 was named vanZG.
TABLE 3.
Percent identity of the amino acid sequences from the deduced proteins of the vanG-like gene cluster to those from other van gene clusters
| VanG-like cluster from CCRI-16110 | % Identity with Van typea:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VanA | VanB | VanD | VanC | VanE | VanF | VanG1 | VanG2 | VanG like (C. difficile) | Ddl (C. innocuum) | |
| VanSGb | 44.4 | 24.8 | 56.6 | 41.7 | 34.5 | 38.6 | 64.8 | 63.4 | 60 | NA |
| VanZG | 27.1 | NA | NA | NA | NA | 27.5 | NA | NA | NA | NA |
| VanG | 44.7 | 45.1 | 43.5 | 45.1 | 41.5 | 44.5 | 65 | 63.6 | 65.3 | 46.1 |
| VanTG | NA | NA | NA | 37.5 | 40.4 | NA | 58.6 | 59.6 | 57.8 | 36.1 |
Sequence accession numbers for the Van-type proteins are as follows: VanA, E. faecium BM4147 (M97297); VanB, E. faecalis V583 (EFU35369); VanD, E. faecium BM4339 (AF130997); VanC, E. gallinarum BM4174 (AF162694); VanE, E. faecalis N00-410 (AF430807); VanF, Paenibacillus popilliae ATCC 14706 (AF155139); VanG1, E. faecalis BM4518 (AY272782); VanG2, E. faecalis N03-0233 (DQ222944); VanG like, C. difficile 630 (AM180355); Ddl, C. innocuum NCIB 10674 (AY479979). The numbers in boldface indicate the highest percent identity with VanG-type proteins. NA, not applicable.
The partial deduced sequence of VanSG (190-amino-acid sequence) of CCRI-16110 was used for comparison.
Comparison of the organization of the partial vanG-like operon from strain CCRI-16110 (the region upstream of the vanSG-like gene has not been characterized) with those of the vanG operons from E. faecalis BM4518 (vanG1), E. faecalis N03-0233 (vanG2), and C. difficile 630 (vanG like) showed that the genes vanYG (encoding the carboxypeptidase), vanXYG (encoding the bifunctional dipeptidase and carboxypeptidase enzyme), and vanWG (with unknown function) were absent in strain CCRI-16110 (Fig. 1B). The vanZG gene, with unknown function, was present only in the vanG-like operon from CCRI-16110 (Fig. 1B). The percent G+C content of the genes forming the vanG-like operon of strain CCRI-16110 was higher than that of the vanG operons from enterococci and C. difficile (Fig. 1B).
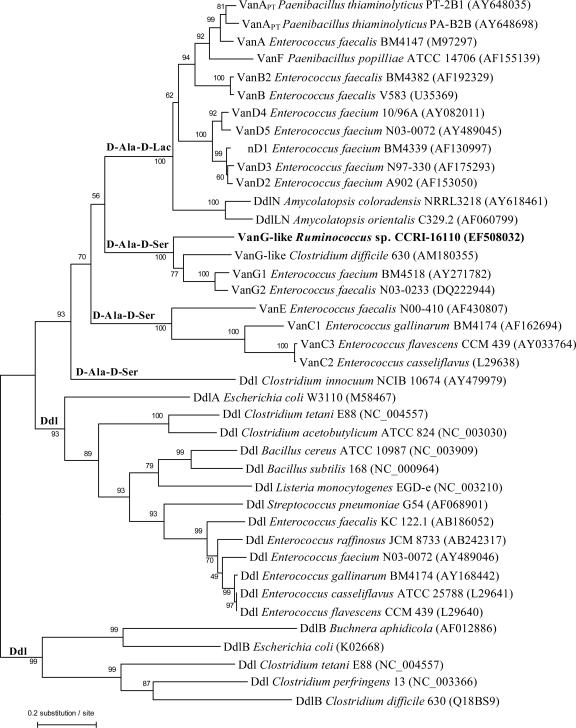
The branching pattern of the phylogenetic tree constructed using sequences of Ddl, d-Ala-d-Ser, and d-Ala-d-Lac ligases showed that the family of d-Ala-d-Ser ligases is organized in three distinct phylogenetic clades that were supported by significant bootstrap resampling values (Fig. 2). Clearly, the VanG-like protein of strain CCRI-16110 was most closely related to the VanG-type d-Ala-d-Ser family of ligases. The two other clades were formed by (i) the VanE and VanC d-Ala-d-Ser ligases and (ii) the d-Ala-d-Ser ligases of Clostridium innocuum.
FIG. 2.
Tree showing the phylogenetic relationships of the deduced amino acid sequences of the vanG-like genes from strain CCRI-16110 encoding the Ddl, d-Ala-d-Lac, and d-Ala-d-Ser ligases. The tree was constructed using the neighbor-joining method based on a comparison of 175 amino acid positions. Bootstrap values, expressed as a percentage of 1,000 replications, are given at each branching point. GenBank accession numbers are given in parentheses for each amino acid sequence.
Transfer of glycopeptide resistance from Ruminococcus sp. strain CCRI-16110 by conjugation.
We performed mating experiments between Ruminococcus sp. strain CCRI-16110 and E. faecalis JH2-2 to determine whether the glycopeptide resistance of Ruminococcus sp. strain CCRI-16110 was transferable by conjugation. No transfer was observed in several mating experiments.
DISCUSSION
We isolated for the first time a potentially novel species of Ruminococcus (strain CCRI-16110) displaying a high level of resistance to vancomycin and teicoplanin. This strain was isolated from a human fecal specimen and harbors the vanD gene. Work is in progress to characterize and assign a taxonomic name to this novel species of Ruminococcus.
Genetic analysis revealed that Ruminococcus sp. strain CCRI-16110 carries a vanD gene cluster closely related to the vanD1 gene cluster in enterococci. So far, the vanD gene cluster has been described only for enterococci (4, 10, 33), and the present report is the first descriptive study of the vanD gene cluster in a nonenterococcal species.
While searching for a ddl gene in Ruminococcus sp. strain CCRI-16110 and potential alterations in this gene, we identified a cluster of four ORFs, three of which displayed high identities to the vanSG, vanG, and vanTG genes of E. faecalis BM4518 and N03-0233, whereas one ORF was more closely related to the vanZ gene. The vanZ gene has been described in various genetic elements, including the enterococcal vanA operon, the vanF operon of Paenibacillus popilliae, and the skin element of C. difficile 630 (7, 32). The skin element of C. difficile 630 is a 14.66-kb prophage-like element inserted in the gene sigK, encoding a sporulation-specific sigma factor (7, 17, 32). The function of the vanZ gene is unknown, but it was shown to be involved in low-level resistance to teicoplanin in E. faecium BM4147 containing the vanA operon (1, 2).
Phylogenetic analysis showed that the VanG-like ligase of strain CCRI-16110 is closely related to the family of VanG-type d-Ala-d-Ser ligases, suggesting that these proteins share a common ancestor. However, the organization of the vanG operon of this strain differs from that of the vanG operons of enterococci and C. difficile. In CCRI-16110, the vanYG and vanWG genes are absent, while a vanZ-like gene is described for the first time in a vanG-like operon. The presence of the vanUG and vanRG genes as well as the entire sequence of the vanSG gene remains to be characterized in strain CCRI-16110. The percent G+C of the genes forming the vanG-like operon of this strain is higher than those of the vanG operons of enterococci and C. difficile, suggesting different origins for these genes. Therefore, the origin of the vanG and vanG-like operons could be due to a step-by-step mechanism of gene acquisition from various van operons as well as a one-step transfer of the vanG cluster of genes due to mobile genetic elements. Such a one-step acquisition of a vanG cluster was described during the transfer of the vanG operon from E. faecalis BM4518 to E. faecalis JH2-2 and was associated with the movement, from chromosome to chromosome, of genetic elements of ca. 240 kb (9). The transfer of vanG genes could explain the presence of a vanG-like operon in C. difficile 630, considering that this strain harbors several conjugative transposons, including CTn2, CTn4, and CTn5, which have a conjugation module related to that of Tn1549 (32), a conjugative transposon responsible for VanB-type vancomycin resistance (16). It has been shown that the vanG-like operon was not present in all C. difficile strains (32). This observation further supports the horizontal transfer of this operon in C. difficile. Despite the presence of a vanG-like operon in C. difficile 630, this strain is susceptible to glycopeptides (32). Based on these data, we can suggest that the presence of the vanG cluster of genes in some anaerobic bacteria is an evolutionary process towards the expression of glycopeptide resistance.
Ruminococcus sp. strain CCRI-16110 displays a high level of vancomycin and teicoplanin resistance (MICs of both antibiotics, >256 μg/ml), which usually corresponds to the VanA phenotype, although no vanA gene was detected in this strain (data not shown). Therefore, the presence of both vanD and vanG-like operons in Ruminococcus sp. strain CCRI-16110 could be responsible for the high level of resistance to vancomycin and teicoplanin. Moreover, mutations in the VanSD sensor could be responsible for constitutive expression of vancomycin resistance in CCRI-16110, as described for E. faecium BM4339 (10). However, the function of each operon as well as the role of the mutations in vanSD in the vancomycin and teicoplanin resistance phenotype of this strain remains to be described. In addition, the presence of a ddl gene encoding a Ddl ligase in this strain remains to be demonstrated.
The anaerobic bacteria from the intestinal flora seem to represent a reservoir of vanB, vanD, and vanG gene clusters (13) and could be involved in the dissemination of vancomycin resistance genes in other important anaerobic or aerobic gram-positive pathogens. However, in vitro conjugative transfer of the glycopeptide resistance phenotype from Ruminococcus sp. strain CCRI-16110 to E. faecalis JH2-2 could not be demonstrated. While the transfer of the vanG gene cluster has already been described (9), no other vanD gene cluster has been successfully transferred by conjugation in vitro to date (3, 10). The absence of in vitro transfer of the vanD and vanG-like gene clusters in the present study does not rule out the possibility of an in vivo horizontal transfer mechanism that could occur in the intestinal environment. Indeed, the transfer of the vanB gene cluster from Clostridium symbiosum to enterococcal strains has been demonstrated in the gut of gnotobiotic mice (22). Infact, the human gut represents a natural ecosystem where nutrients, biofilm bacteria, antibiotic resistance genes, and genetic material exchange are abundant (29-31).
Acknowledgments
This study was supported by grant PA-15586 from the Canadian Institutes of Health Research (CIHR) and by grant 2201-181 from the Valorisation Recherche Québec (VRQ). M.-C. Domingo is a research fellow from Bayer Healthcare (Bayer Healthcare/CIHR/AMMI Canada/FCMI).
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Arthur, M., F. Depardieu, C. Molinas, P. Reynolds, and P. Courvalin. 1995. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 154:87-92. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, M., F. Depardieu, P. Reynolds, and P. Courvalin. 1996. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol. Microbiol. 21:33-44. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, D. A., P. Kibsey, D. Roscoe, and M. R. Mulvey. 2004. Enterococcus faecium N03-0072 carries a new VanD-type vancomycin resistance determinant: characterization of the VanD5 operon. J. Antimicrob. Chemother. 54:680-683. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, D. A., M. A. Miller, and M. R. Mulvey. 2006. Enterococcus gallinarum N04-0414 harbors a VanD-type vancomycin resistance operon and does not contain a d-alanine:d-alanine 2 (ddl2) gene. Antimicrob. Agents Chemother. 50:1067-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadewall, B., P. E. Reynolds, and P. Courvalin. 2001. Regulation of expression of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339. J. Bacteriol. 183:3436-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 7.Courvalin, P. 2006. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 42(Suppl. 1):S25-S34. [DOI] [PubMed] [Google Scholar]
- 8.Dalla Costa, L. M., P. E. Reynolds, H. A. Souza, D. C. Souza, M. F. Palepou, and N. Woodford. 2000. Characterization of a divergent vanD-type resistance element from the first glycopeptide-resistant strain of Enterococcus faecium isolated in Brazil. Antimicrob. Agents Chemother. 44:3444-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depardieu, F., M. G. Bonora, P. E. Reynolds, and P. Courvalin. 2003. The vanG glycopeptide resistance operon from Enterococcus faecalis revisited. Mol. Microbiol. 50:931-948. [DOI] [PubMed] [Google Scholar]
- 10.Depardieu, F., M. Kolbert, H. Pruul, J. Bell, and P. Courvalin. 2004. VanD-type vancomycin-resistant Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 48:3892-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depardieu, F., P. E. Reynolds, and P. Courvalin. 2003. VanD-type vancomycin-resistant Enterococcus faecium 10/96A. Antimicrob. Agents Chemother. 47:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo, M. C., A. Huletsky, A. Bernal, R. Giroux, D. K. Boudreau, F. J. Picard, and M. G. Bergeron. 2005. Characterization of a Tn5382-like transposon containing the vanB2 gene cluster in a Clostridium strain isolated from human faeces. J. Antimicrob. Chemother. 55:466-474. [DOI] [PubMed] [Google Scholar]
- 13.Domingo, M. C., A. Huletsky, R. Giroux, K. Boissinot, F. J. Picard, P. Lebel, M. J. Ferraro, and M. G. Bergeron. 2005. High prevalence of glycopeptide resistance genes vanB, vanD, and vanG not associated with enterococci in human fecal flora. Antimicrob. Agents Chemother. 49:4784-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutka-Malen, S., C. Molinas, M. Arthur, and P. Courvalin. 1992. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a d-alanine:d-alanine ligase-related protein necessary for vancomycin resistance. Gene 112:53-58. [DOI] [PubMed] [Google Scholar]
- 15.Engelkirk, P. G., J. Duben-Engelkirk, and J. V. R. Dowell. 1992. Laboratory procedures, p. 331-357. In S. Hoffman (ed.), Principles and practice of clinical anaerobic bacteriology. Star Publishing Company, Belmont, CA.
- 16.Garnier, F., S. Taourit, P. Glaser, P. Courvalin, and M. Galimand. 2000. Characterization of transposon Tn1549, conferring VanB-type resistance in Enterococcus spp. Microbiology 146:1481-1489. [DOI] [PubMed] [Google Scholar]
- 17.Haraldsen, J. D., and A. L. Sonenshein. 2003. Efficient sporulation in Clostridium difficile requires disruption of the σK gene. Mol. Microbiol. 48:811-821. [DOI] [PubMed] [Google Scholar]
- 18.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, D. T., W. R. Taylor, and J. M. Thornton. 1992. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8:275-282. [DOI] [PubMed] [Google Scholar]
- 20.Ke, D., F. J. Picard, F. Martineau, C. Ménard, P. H. Roy, M. Ouellette, and M. G. Bergeron. 1999. Development of a PCR assay for rapid detection of enterococci. J. Clin. Microbiol. 37:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 22.Launay, A., S. A. Ballard, P. D. Johnson, M. L. Grayson, and T. Lambert. 2006. Transfer of vancomycin resistance transposon Tn1549 from Clostridium symbiosum to Enterococcus spp. in the gut of gnotobiotic mice. Antimicrob. Agents Chemother. 50:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leclercq, R., E. Derlot, J. Duval, and P. Courvalin. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157-161. [DOI] [PubMed] [Google Scholar]
- 24.NCCLS. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard, 6th ed. M11-A6. NCCLS, Wayne, PA.
- 25.Périchon, B., B. Casadewall, P. Reynolds, and P. Courvalin. 2000. Glycopeptide-resistant Enterococcus faecium BM4416 is a VanD-type strain with an impaired d-alanine:d-alanine ligase. Antimicrob. Agents Chemother. 44:1346-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Périchon, B., P. Reynolds, and P. Courvalin. 1997. VanD-type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob. Agents Chemother. 41:2016-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 28.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 29.Salyers, A. A., A. Gupta, and Y. Wang. 2004. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 12:412-416. [DOI] [PubMed] [Google Scholar]
- 30.Salyers, A. A., K. Moon, and M. S. Schlessinger. 2007. The human intestinal tract—a hotbed of resistance gene transfer? Part I. Clin. Microbiol. Newsl. 29:17-21. [Google Scholar]
- 31.Salyers, A. A., K. Moon, and M. S. Schlessinger. 2007. The human intestinal tract—a hotbed of resistance gene transfer? Part II. Clin. Microbiol. Newsl. 29:25-30. [Google Scholar]
- 32.Sebaihia, M., B. W. Wren, P. Mullany, N. F. Fairweather, N. Minton, R. Stabler, N. R. Thomson, A. P. Roberts, A. M. Cerdeno-Tarraga, H. Wang, M. T. Holden, A. Wright, C. Churcher, M. A. Quail, S. Baker, N. Bason, K. Brooks, T. Chillingworth, A. Cronin, P. Davis, L. Dowd, A. Fraser, T. Feltwell, Z. Hance, S. Holroyd, K. Jagels, S. Moule, K. Mungall, C. Price, E. Rabbinowitsch, S. Sharp, M. Simmonds, K. Stevens, L. Unwin, S. Whithead, B. Dupuy, G. Dougan, B. Barrell, and J. Parkhill. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38:779-786. [DOI] [PubMed] [Google Scholar]
- 33.Tanimoto, K., T. Nomura, H. Maruyama, H. Tomita, N. Shibata, Y. Arakawa, and Y. Ike. 2006. First VanD-type vancomycin-resistant Enterococcus raffinosus isolate. Antimicrob. Agents Chemother. 50:3966-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uttley, A. H., C. H. Collins, J. Naidoo, and R. C. George. 1988. Vancomycin-resistant enterococci. Lancet i:57-58. [DOI] [PubMed] [Google Scholar]