Abstract
The therapeutic benefits of current antiretroviral therapy are limited by the evolution of drug-resistant virus and long-term toxicity. Novel antiretroviral compounds with activity against drug-resistant viruses are needed. 2′,3′-Didehydro-3′-deoxy-4′-ethynylthymidine (4′-Ed4T), a novel thymidine analog, has potent anti-human immunodeficiency virus (HIV) activity, maintains considerable activity against multidrug-resistant HIV strains, and is less inhibitory to mitochondrial DNA synthesis in cell culture than its progenitor stavudine (D4T). We investigated the intracellular metabolism and anti-HIV activity of 4′-Ed4T. The profile of 4′-Ed4T metabolites was qualitatively similar to that for zidovudine (AZT), with the monophosphate metabolite as the major metabolite, in contrast to that for D4T, with relatively poor formation of total metabolites. The first phosphorylation step for 4′-Ed4T in cells was more efficient than that for D4T but less than that for AZT. The amount of 4′-Ed4T triphosphate (4′-Ed4TTP) was higher than that of AZTTP at 24 h in culture. There was a dose-dependent accumulation of 4′-Ed4T diphosphate and 4′-Ed4TTP on up-regulation of thymidylate kinase and 3-phosphoglycerate kinase expression in Tet-On RKO cells, respectively. The anti-HIV activity of 4′-Ed4T in cells persisted even after 48 h of drug removal from culture in comparison with AZT, D4T, and nevirapine (NVP). The order of increasing persistence of anti-HIV activity of these compounds after drug removal was 4′-Ed4T > D4T > AZT > NVP. In conclusion, with the persistence of 4′-Ed4TTP and persistent anti-HIV activity in cells, we anticipate less frequent dosing and fewer patient compliance issues than for D4T. 4′-Ed4T is a promising antiviral candidate for HIV type 1 chemotherapy.
The introduction of highly active antiretroviral therapy has significantly reduced human immunodeficiency virus (HIV)-associated morbidity and mortality (52). However, the treatment of HIV infection is a lifelong undertaking, and therapeutic benefits can be limited by the evolution of drug-resistant virus and long-term toxicity (28, 38). It has been speculated that it may take about 70 years of continuous highly active antiretroviral therapy to eradicate HIV type 1 (HIV-1) in an infected individual (7, 45). Therefore, there is an urgent need to develop novel antiviral agents that can inhibit drug-resistant HIV-1 replication while displaying favorable pharmacological and toxicity profiles.
Nucleoside analog reverse transcriptase (RT) inhibitors (NRTIs) were the first therapeutic agents to demonstrate clinical efficacy for HIV-1 infection, and they continue to play a central role in the treatment of HIV (http://AIDSinfo.nih.gov). The effectiveness of antiretroviral therapy may be offset by the increased burden of long-term drug toxicity mediated specifically by NRTI therapy (22, 23, 33, 47). All NRTIs require a stepwise phosphorylation to their triphosphate metabolites, which are incorporated into HIV DNA and cause premature termination of the viral DNA chain elongation (23). The inhibition of host DNA polymerase activity is responsible for many of the adverse effects of NRTIs. Mitochondrial DNA (mtDNA) polymerase γ, unlike nuclear DNA polymerases, lacks the ability to effectively discriminate against NRTI triphosphate metabolites in favor of endogenous deoxynucleoside triphosphates (31). NRTI-induced inhibition of mtDNA synthesis is proposed to induce depletion of cellular mtDNA and is ultimately responsible for the delayed toxicity (5, 6).
The unnatural β-l(−) configuration of dideoxynucleosides, exemplified by β-l-2′,3′-dideoxy-3′-thiacytidine (lamivudine) and its 5-fluoro analog (emtricitabine), maintain good anti-HIV-1 activity and favorable mitochondrial toxicity (8, 11, 14, 27, 29, 30). However, the rapid emergence of resistant virus limits the clinical utility of lamivudine and emtricitabine(26, 39, 43). In the search for novel inhibitors with potent anti-HIV-1 activity, low toxicity, and unique resistance profiles, several 4′-substituted thymidine analogs have been synthesized and evaluated for their antiviral activity and cytotoxicity (including their impact on mtDNA) (17, 18, 24, 32, 44). Our laboratory recently discovered a novel derivative of stavudine (D4T), 2′,3′-didehydro-3′-deoxy-4′-ethynylthymidine (4′-Ed4T) (17). 4′-Ed4T is more potent against HIV-1 replication and much less inhibitory to mtDNA synthesis in cell culture than its progenitor D4T (10, 17, 36). Moreover, a unique pattern of RT resistance mutations (P119S, T165A, and M184V) in the virus was observed under the selection pressure of 4′-Ed4T in vitro, and it was found to be active against multidrug-resistant HIV-1 clinical isolates (36). 4′-Ed4T is phosphorylated by human thymidine kinase 1 (TK1) to the monophosphate with an efficiency fourfold higher than that of its progenitor, D4T (10). The enzymes involved in the phosphorylation of 4′-Ed4T monophosphate (4′-Ed4TMP) to 4′-Ed4T triphosphate (4′-Ed4TTP) were recently elucidated (19). 4′-Ed4TMP is phosphorylated by thymidylate kinase (TMPK) while several enzymes, including nucleoside diphosphate kinase (NDPK), pyruvate kinase, creatine kinase, and 3-phosphoglycerate kinase (PGK), could phosphorylate 4′-Ed4TMP to 4′-Ed4TTP. The phosphorylation of 4′-Ed4T requires enzymes similar to those used by other thymidine analogs; however, the efficiencies are different for the different analogs. The formation of 4′-Ed4TTP implies that 4′-Ed4T could be incorporated into viral DNA by HIV RT and serve as a DNA chain terminator (23). Steady-state enzymatic analyses have confirmed that 4′-Ed4TTP exerts anti-HIV-1 activity by RT inhibition. In comparison with D4TTP, it is a more potent competitive inhibitor of RT with little inhibitory effect on major host DNA polymerases (51).
Taken together, the anti-HIV activity, phosphorylation efficiency, favorable cytotoxicity, and unique resistance profile of 4′-Ed4T make it an attractive candidate to be developed as a clinically useful anti-HIV drug with a potential for better clinical efficacy and a better toxicity profile than other thymidine analogs in clinical usage. We report here the intracellular metabolism of 4′-Ed4T and its superior persistence of antiviral activity even after drug removal in comparison with other RT inhibitors, using a novel application of the TZM-bl indicator cell line for a drug persistence assay.
MATERIALS AND METHODS
Chemicals.
4′-Ed4T was synthesized in the laboratory of Hiromichi Tanaka, School of Pharmaceutical Sciences, Showa University, Tokyo, Japan (17). Thymidine, D4T, and zidovudine (AZT) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). The purity of these compounds was verified by high-pressure liquid chromatography (HPLC) analysis. Radiochemicals, including [5′-3H]4′-Ed4T, [methyl-3H]D4T, and [methyl-3H]AZT, were purchased from Moravek Biochemicals Inc. (Brea, CA). All other chemicals used were of analytical grade or higher.
Cell lines and virus.
The CEM cell line was received from the AIDS Research and Reference Reagent Program of the National Institutes of Health (NIH) and was contributed by Robert Gallo. The TZM-bl indicator cell line (50), obtained from J. Kappes through the AIDS Research and Reference Reagent Program, is a HeLa cell line derivative that expresses high levels of CD4 and CCR5 along with endogenously expressed CXCR4. TZM-bl cells contain HIV long terminal repeat-driven β-galactosidase and luciferase reporter cassettes that are activated by HIV Tat expression. The Tet-On RKO cell line, which was derived from a human colorectal carcinoma with a Tet-On regulator plasmid, was a gift from Edward Chu (Yale University School of Medicine). Cells were cultured at 37°C in the presence of a humidified 5% CO2 atmosphere. The HIV-1 IIIB strain was received from John Mellors (University of Pittsburgh).
Analysis of intracellular metabolites of 4′-Ed4T, D4T, and AZT.
To evaluate the intracellular thymidine analog metabolites, CEM cells (2 × 107/ml) were incubated with 2 μM (75 mCi/mmol) of the various radiolabeled thymidine analogs in medium comprised of RPMI 1640 with 10% dialyzed fetal bovine serum and 100 μg/ml kanamycin at 37°C in 5% CO2 for 24 h as previously described (15, 53). At different time intervals (2, 4, 8, 12, 16, and 24 h), 2 × 107 of the cells were harvested by centrifugation and washed twice in ice-cold phosphate-buffered saline containing 20 μM dipyridamole (Sigma). The cell pellets were extracted with 15% trichloroacetic acid for 15 min on ice. The supernatant containing the thymidine analog and its phosphorylated metabolites was neutralized by two half-volume extractions with a 45:55 ratio of trioctylamine and 1,1,2-trichlorotrifluroethane. A 50-μl portion of the aqueous phase was injected unto the HPLC column. The metabolites in the soluble fraction were analyzed by HPLC (Shimadzu, Braintree, MA) connected to radiometric detector (flow scintillation analyzer 150TR; Packard) using a Partisil SAX column (Whatman, Clifton, NJ). The nucleotides were eluted by a gradient of H2O to 300 mM potassium phosphate buffer at a flow rate of 1.5 ml/min. The nucleoside and nucleotide peaks were determined using a diode array detector with a channel set at 265 nm, the λmax for 4′-Ed4T. The effluent from the UV detector directly enters an in-line radio spectrophotometer, where it was mixed with 3 ml/min of Monoflow 5 scintillation cocktail. This system allowed for the detection of nucleoside metabolites that were below the level of UV detection, and the performance parameters have been published previously (9).
To evaluate the intracellular stability of the radioactive metabolites of the nucleoside analogs, CEM cells (2 × 107/ml) were treated with 2 μM of [3H]4′-Ed4T, [3H]D4T, or [3H]AZT for 24 h. The cells were then washed and resuspended in fresh medium without drugs at 0, 2, and 4 h after 24 h of incubation. The intracellular metabolites of each analog were determined by HPLC at 0, 2, 4, 8, 12, and 24 h as described above.
To investigate the dose-dependent formation of 4′-Ed4T metabolites, CEM cells were cultured with various concentrations of [3H]4′-Ed4T (0.2, 0.5, 1, and 2 μM) for 12 h with double the radiospecificity (150 mCi/mmol). The cells were harvested after 12 h of incubation, and the intracellular metabolites of each analog were determined by HPLC as described above.
Establishment of inducible TMPK and PGK cell lines.
The inducible Tet-On TMPK cell line system was established by using the Tet-On gene expression system (Clontech Laboratories, Inc., Palo Alto, CA) as described previously (20). For PGK overexpression in Tet-On RKO cells (20), the coding sequence of phosphoglycerate kinase (PGK) was cloned into the pcDNA5/TO vector (Invitrogen Corporation, Carlsbad, CA) with a Tet-responsive element between the 5′ XhoI and 3′ BamHI sites (Invitrogen Corporation, Carlsbad, CA). After 48 h of transfection, the cells were grown in the presence of 0.5 mg/ml blasticidin for selection. To isolate the cell lines that stably expressed PGK, the transfected cells were passaged at a 1:60 dilution into fresh medium. At all times, the cells were grown in the presence of 0.5 mg/ml blasticidin and 0.5 mg/ml hygromycin. To induce the expression of PGK, the cells were grown in tissue culture medium with various concentrations of doxycycline (0, 50, and 100 ng/ml) for 72 h. Standard TMPK and PGK assays and Western blotting assays were used for evaluation of the TMPK and PGK activities and protein expression levels.
Metabolism of 4′-Ed4T in inducible TMPK and PGK RKO cells.
The impact of intracellular TMPK and PGK activities on the metabolism of 4′-Ed4T was examined by inducing the level of expression of these enzymes in RKO cells as previously described (20). The TMPK or PGK RKO cells were seeded at 5 × 105 cells per culture dish in the presence of increasing concentrations of doxycycline (0, 50, and 3,000 ng/ml and 0, 50 and 100 ng/ml, respectively) for TMPK and PGK induction, respectively. After 48 or 72 h of induction, 2 μM [5′-3H]4′-Ed4T (478 mCi/mmol) was added to the cells and left for 16 h. Cells were harvested in cold phosphate-buffered saline containing 20 μM dipyridamole (Sigma) and extracted with 15% trichloroacetic acid for 15 min on ice. The supernatant containing the nucleoside and its phosphorylated forms was neutralized by two extractions with a 45:55 ratio of trioctylamine and 1,1,2-trichlorotrifluroethane and subjected to HPLC analysis as previously described (19).
Assay for persistence of anti-HIV activity after drug removal.
TZM-bl cells maintained in Dulbecco modified Eagle medium were plated at 0.5 × 104 cells per well in a 96-well microtiter plate in 100 μl of phenol red-free RPMI 1640 medium and allowed to adhere for 15 to 18 h at 37°C. The medium was then changed, and the cells were treated with various concentrations of 4′-Ed4T, D4T, AZT, or nevirapine (NVP) for 24 h. Quintuple wells were used for each drug concentration. A batch of the plates was infected with HIV-1 IIIB at a multiplicity of infection (MOI) of 0.1 at the time of drug treatment. The culture medium was changed to remove extracellular drug from the second batch of plates and then infected with HIV-1 IIIB at an MOI of 0.1 at 0, 24, and 48 h after drug removal. After 24 h of infection, the cells in each well were lysed, and the luciferase activity of each well was measured using luciferase assay reagent (Promega, Madison, WI) and a luminometer (FARCyte; Amersham Biosciences Co., Piscataway, NJ). Background luminescence was determined from uninfected cells and subtracted from all experimental wells. Persistence of anti-HIV drug activity was measured as a function of inhibition of viral infectivity (percentage of control infection without drug), calculated by dividing the mean number of luciferase units at each concentration of a drug by the mean number from cells containing no drug. Persistence of anti-HIV activity was determined by plotting the percent inhibition on the y axis against drug concentration on the x axis.
RESULTS
Intracellular metabolism of 4′-Ed4T in CEM cells.
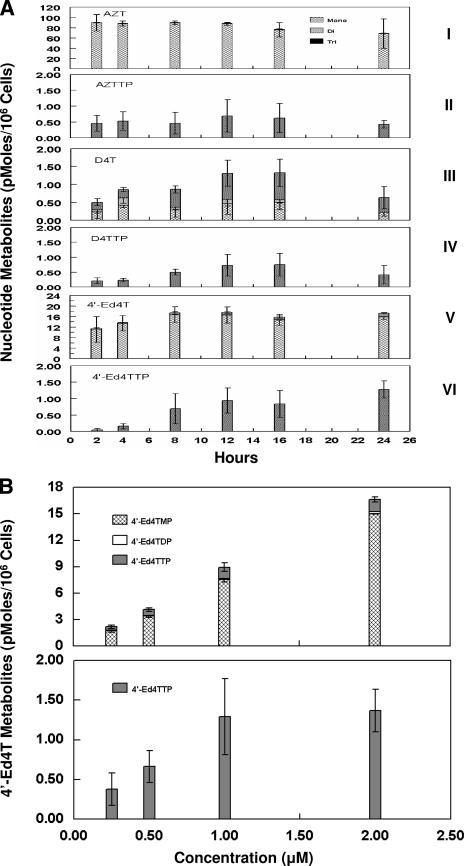
The profile of the intracellular metabolites of 4′-Ed4T was characterized in comparison with those of thymidine analogs in clinical use, D4T (the progenitor), and AZT. CEM cells were cultured in the presence of 2 μM (75 mCi/mmol) of [3H]4′-Ed4T, [3H]D4T, or [3H]AZT for 24 h. At different time intervals (2, 4, 8, 12, 16, and 24 h), 2 × 107 cells were harvested for acid extraction. The extracted samples containing radioactive metabolites of the nucleoside analogs were subjected to HPLC analysis. The identities of the peaks of the radiolabeled nucleotides of 4′-Ed4T, D4T, and AZT were determined by comparison with authentic elution time standards of unlabeled nucleotides of the respective analog (data not shown). The profile of 4′-Ed4T metabolites formed at 24 h was qualitatively similar to that for AZT, with the monophosphate metabolite as the major metabolite (Fig. 1A). This contrasted that of its progenitor D4T, with relatively poor formation of total metabolites and major metabolites being D4TMP and D4TTP. The AZTMP peak was higher than that of 4′-Ed4TMP, while 4′-Ed4T had the highest triphosphate accumulation. The radioactivity of each eluted sample was determined and plotted as a function of time. Figure 1A shows the time-dependent intracellular accumulation of the radioactive metabolites. The peak concentrations of the metabolites occurred at 2 h for AZT (90 pmol/106 cells) and at 12 h for both 4′-Ed4T (18 pmol/106 cells) and D4T (1.20 pmol/106 cells). 4′-Ed4T was phosphorylated to the triphosphate at a higher rate than D4T but less than AZT. However, the amount of triphosphate metabolites of 4′-Ed4T was higher than that of AZT at 24 h in culture. The triphosphate concentration of AZT reached a steady state at 2 h, while those of D4T and 4′-Ed4T continued to increase up to 24 h. We next investigated the dose-dependent formation of 4′-Ed4T metabolites. CEM cells were cultured with various concentrations of [3H]4′-Ed4T (0.2, 0.5, 1, and 2 μM) for 12 h with double the radiospecificity (150 mCi/mmol). The HPLC elution profile is shown in Fig. 1B. The total concentration of the metabolites exhibited a linear dose-response relationship without saturation at 12 h. However, the 4′-Ed4TTP reached a peak concentration at 1 μM, and at 2 μM, there was no appreciable increase in triphosphate formation. The major metabolite of 4′-Ed4T was the monophosphate, which contributed most to the linear increase over the 12-hour period. This is consistent with the behavior of 4′-Ed4T toward TMPK, the rate-limiting step, observed previously (19).
FIG. 1.
(A) Time-dependent intracellular accumulation of radioactive metabolites in CEM cells during incubation with 2 μM (75 mCi/mmol) of radiolabeled nucleotide. Panels: I and II, [3H]AZT (all metabolites and AZTTP only, respectively); III and IV, [3H]D4T (all metabolites and D4TTP only, respectively); V and VI, [3H]4′-Ed4T (all metabolites and 4′-Ed4TTP only, respectively). At different time intervals (2, 4, 8, 12, 16, and 24 h), 2 × 107 of the cells were harvested for acid extraction. The extracted samples containing radioactive metabolites of the nucleoside analogs were subjected to HPLC analyses. The detected radioactivity was calculated as picomoles of each type of metabolite for each nucleoside analog/106 cells. Each value is the mean ± standard deviation from three independent experiments. (B) Dose-dependent intracellular accumulation of radioactive metabolites of 4′-Ed4T in CEM cells. CEM cells were cultured with various concentrations of [3H]4′-Ed4T (0.25, 0.5, 1, and 2 μM) for 12 h, with doubling of the radiospecificity to 150 mCi/mmol. Cell extracts were prepared and subjected to HPLC analyses. The detected radioactivity was calculated as picomoles of each type of metabolite of 4′-Ed4T analog/106 cells. Each value is the mean ± standard deviation from three independent experiments.
Phosphorylation of mono- and diphosphate metabolites of 4′-Ed4T.
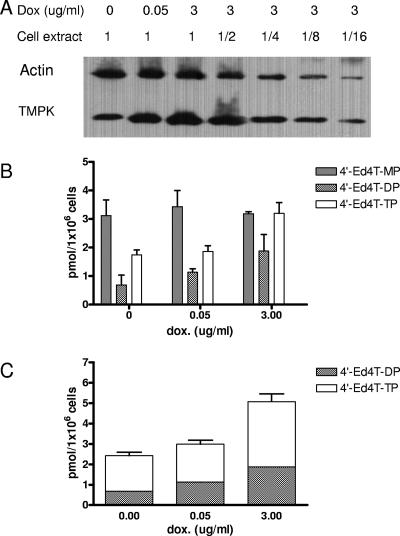
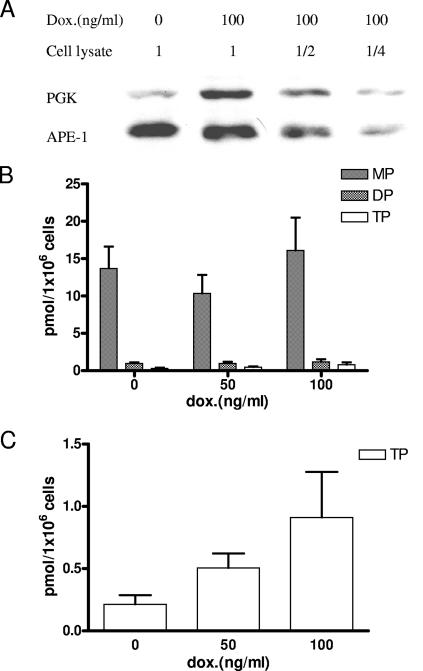
It has been reported that human TMPK can utilize 4′-Ed4TMP as a substrate (19). Furthermore, PGK appears to play a predominant role in the phosphorylation of 4′-Ed4TDP to the triphosphate metabolite instead of NDPK, which is preferentially used for the phosphorylation of naturally occurring TDP (19, 25, 40). We studied the relative contributions of TMPK and PGK to the metabolism of 4′-Ed4T in cells. The impact of intracellular TMPK activity on the metabolism of 4′-Ed4T was examined by regulating the TMPK response to various concentrations of doxycycline on stable Tet-On TMPK RKO cells. Tet-On TMPK RKO cells were cultured for 48 h in the presence of doxycycline at concentrations ranging from 0 to 3 μg/ml. Cellular extracts were prepared and analyzed by Western blotting with rabbit polyclonal TMPK antibody, using actin as an internal control. As shown in Fig. 2A, the TMPK activity increased in a doxycycline concentration-dependent manner up to 3 μg/ml. The maximum level of induction of TMPK expression achieved in the Tet-On TMPK RKO cells was approximately fivefold higher than that in control cells. The amounts of TK and PGK in the cells did not change (data not shown). There was also a dose-response relationship between doxycycline concentration and the amount of 4′-Ed4TDP and 4′-Ed4TTP formed (Fig. 2B and C). The graded increase in 4′-Ed4TDP and 4′-Ed4TTP to about threefold paralleled the fivefold increase in TMPK expression in the inducible cells over that in control cells. This suggests that the activity of TMPK in cells plays a critical role in increasing 4′-Ed4TMP metabolites. We next studied the effect of up-regulation of PGK expression on the phosphorylation of 4′-Ed4TDP to the triphosphate metabolite, using stable Tet-On PGK RKO cells. As shown in Fig. 3A, the level of PGK expression was threefold higher than that in the control RKO cells at a doxycycline concentration of 100 ng/ml. The HPLC elution profile of the phosphorylation of 4′-Ed4T to its triphosphate metabolite demonstrated a dose-dependent accumulation of 4′-Ed4TTP on up-regulation of PGK expression. There was a 2.3-fold increase in the accumulation of 4′-Ed4TTP in PGK-induced RKO cells compared to that in control cells. Moreover, the increase in the intracellular accumulation of the triphosphate metabolite (Fig. 3B and C) correlated with the level of PGK expression as seen in the Western blotting analysis (Fig. 3A). The data suggest that PGK activity is also critical in determining the formation of active anti-HIV metabolites of 4′-Ed4T in cells.
FIG. 2.
Intracellular TMPK activity in the metabolism of 4′-Ed4T in Tet-On TMPK RKO cells. (A) Expression of TMPK in Tet-On TMPK RKO cells, as detected by Western blotting. Actin was used as the internal control. (B) Doxycycline-induced TMPK RKO cells (5 × 105) were incubated with 2 μM [5′-3H]4′-Ed4T (478 mCi/mmol) for 16 h. Cell extracts were prepared and subjected to HPLC analyses. The detected radioactivity was calculated as picomoles of each type of metabolite of 4′-Ed4T analog/106 cells for each doxycycline concentration. (C) Total amounts of di- and triphosphate metabolites formed. Each value is the mean ± standard deviation from three independent experiments.
FIG. 3.
Intracellular PGK activity in the metabolism of 4′-Ed4T in Tet-On PGK RKO cells. (A) Expression of PGK in Tet-On PGK RKO cells, as detected by Western blotting. APE-1 was used as the internal control. (B) Doxycycline-induced PGK RKO cells (5 × 105) were incubated with 2 μM [5′-3H]4′-Ed4T (478 mCi/mmol) for 16 h. Cell extracts were prepared and subjected to HPLC analyses. The detected radioactivity was calculated as picomoles of each type of metabolite of 4′-Ed4T analog/106 cells for each doxycycline concentration. (C) Total amounts of di- and triphosphate metabolites formed. Each value is the mean ± standard deviation from three independent experiments.
Intracellular persistence of metabolites of 4′-Ed4T in CEM cells.
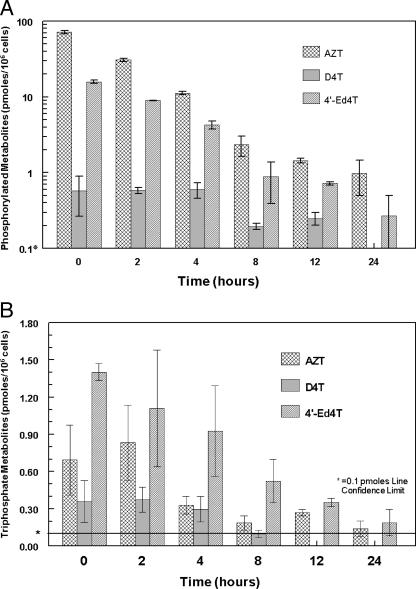
The intracellular stability of 4′-Ed4T metabolites in comparison with those of D4T and AZT was studied. CEM cells were treated with 2 μM of [3H]4′-Ed4T, [3H]D4T, or [3H]AZT for 24 h. The cells were then washed and resuspended in fresh medium without drugs. The intracellular metabolites of each analog were determined by HPLC at 0, 2, 4, 8, 12, and 24 h after removal of drug, as shown in Fig. 4A. As determined at 8 h after drug removal, the total metabolites of AZT, D4T, and 4′-Ed4T were 2.33 ± 0.52, 0.20 ± 0.01, and 1.07 ± 0.33 pmol/106 cells, respectively. Moreover, after 24 h of drug removal, the percentages of total metabolites remaining compared to the 0-h values were 1.5, 0, and 1.7, for AZT, D4T, and 4′-Ed4T, respectively. The retention of 4′-Ed4T metabolites was relatively longer than those for the other analogs. After 4 h of drug removal, the total amounts of triphosphate metabolites of AZT, D4T, and 4′-Ed4T were 47%, 83%, and 66% of the values at 0 h, respectively (Fig. 4B). After 24 h of drug removal, the amount of the triphosphate metabolites in cells was 0.14 (close to the limit of detection [0.1]), undetectable, and 0.2 pmol/106 cells for AZTTP, D4TTP, and 4′-Ed4TTP, respectively. The interconversion of the phosphorylated metabolites of the analogs makes it difficult to calculate the accurate half-life of each of the metabolites.
FIG. 4.
Intracellular persistence of radioactive metabolites in CEM cells during incubation of 106 cells with 2 μM (75 mCi/mmol) of [3H]AZT, [3H]D4T, or [3H]4′-Ed4T for 24 h. The cells were then washed and resuspended in fresh medium without nucleoside analogs at 0, 2, and 4 h after 24 h of incubation. The intracellular metabolites of each analog were determined by HPLC at 0, 2, 4, 8, 12, and 24 h. (A) The detected total radioactive metabolites were calculated as picomoles of each analog/106 cells. (B) Concentrations of triphosphates formed. *, confidence limit of detection. Each value is the mean ± standard deviation from three independent experiments.
Persistent anti-HIV activity of 4′-Ed4T after 4′-Ed4T removal from culture.
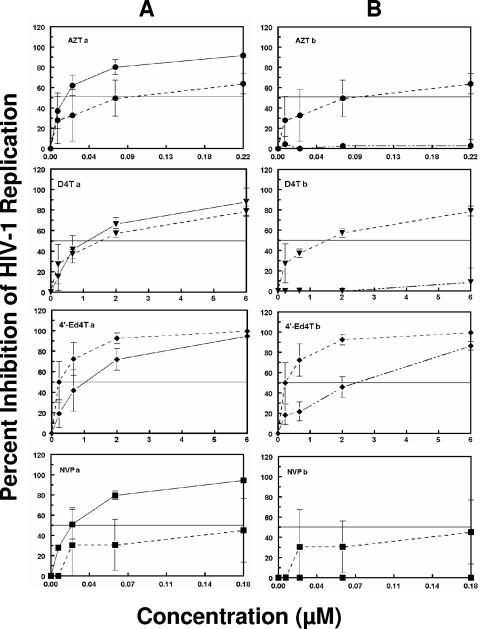
With the long retention of 4′-Ed4T metabolites and the persistence of its intracellular triphosphate metabolite as well as its potent activity against HIV RT (51), we next investigated whether cells treated with 4′-Ed4T will maintain anti-HIV-1 activity after its removal from culture, in comparison with D4T, AZT, and NVP. First, TZM-bl cells were initially cocultured with HIV-1 IIIB at an MOI of 0.1 in the presence of various concentrations of the analogs for 24 h (Fig. 5A), and the percentage of HIV-1 replication inhibition was determined by measuring the luciferase activity as previously described (49, 50). As shown in Fig. 5A, AZT and NVP achieved 50% inhibition of HIV-1 replication at the three higher concentrations tested. However, D4T and 4′-Ed4T, whose phosphorylated metabolites reached peak concentrations at 12 h, achieved 50% inhibition of viral replication only at drug concentrations of 2 or 6 μM. When the cells were cocultured with virus in the presence of drug for 24 h, all the drugs showed a dose-dependent inhibition of viral replication (Fig. 5A). Next, we tested the persistence of the anti-HIV activity of the analogs after drug removal from the culture. TZM-bl cells were cultured in the presence of various concentrations of 4′-Ed4T, D4T, AZT, or NVP for 24 h. The cells were then washed to remove extracellular drug, and at 0, 24, and 48 h after drug removal, the cells were infected with HIV-1 IIIB at an MOI of 0.1. The percentage inhibition of HIV-1 replication was determined after 24 h of infection by measuring the luciferase activity. As shown in Fig. 5A, when the viral infection of the cells occurred at 0 h after drug removal, the inhibition curves for all the analogs shifted to the right, except for 4′-Ed4T which achieved 50% inhibition at all the concentrations tested. Interestingly, NVP, with the longest plasma half-life (30 h) (35), was the least protective against cell infection; even at the 0.18 μM concentration (i.e., 1.8 times the 50% inhibitory concentration) (21), it did not achieve 50% inhibition. When the cells were pretreated and the drug was removed 24 h prior to the infection (i.e., the culture medium was changed twice prior to viral infection), only the inhibition curve of 4′-Ed4T crossed the 50% inhibitory concentration line; the rest of the analogs tested did not protect the cells from HIV infection (Fig. 5B). Moreover, 4′-Ed4T showed some inhibition when infection occurred after 48 h of drug removal (data not shown). On average the order of increasing persistence of anti-HIV activity of these compounds after drug removal was 4′-Ed4T > D4T > AZT > > NVP.
FIG. 5.
Persistence of anti-HIV-1 activity after removal of AZT, D4T, 4′-Ed4T, or NVP from culture medium. The persistence of nucleoside analog activity was determined using a novel TZM-bl indicator cell line-based assay described in Materials and Methods. TZM-bl cells were cultured in the presence of various concentrations of 4′-Ed4T, D4T, AZT, or NVP for 24 h. The cells were then washed to remove extracellular drug and infected with HIV-1 IIIB at an MOI of 0.1 at 0, 24, and 48 h of drug removal. The percent inhibition of HIV-1 replication was determined by measuring the luciferase activity. (A) Percent inhibition of HIV-1 replication when cells were infected at the time of drug treatment and incubated together for 24 h (solid lines) and percent inhibition when cells were infected at 0 h after drug removal (dotted lines) and incubated for 24 h. (B) Percent inhibition when cells were infected at 0 h after drug removal (dotted lines) and incubated for 24 h and percent inhibition when cells were infected at 24 h after drug removal (dashed lines) and incubated for 24 h (the infection was after two consecutive 24-h medium changes without drug replacement). The percent inhibition at 0 h after drug removal was plotted for ease of comparison. Error bars indicate standard deviations.
DISCUSSION
Novel treatment strategies are needed to overcome HIV-1 drug resistance, long-term toxicity, and treatment compliance issues. These may include (i) the discovery of novel inhibitors with potent anti-HIV-1 activity, low toxicity, and unique resistance profiles; (ii) the development of antiretroviral combinations that will compromise viral fitness and have improved side effect profiles; and (iii) less-frequent dosing to enhance treatment adherence (13, 46). The recently discovered 4′-Ed4T has potent anti-HIV activity (10, 17), maintains considerable activity against HIV mutant strains carrying the K65R or the Q151M complex (36), and is less inhibitory to mtDNA synthesis in cell culture than its progenitor D4T (51).
In the present study, we demonstrate that 4′-Ed4T is phosphorylated in CEM cells in a dose- and time-dependent manner (Fig. 1). The major metabolite of 4′-Ed4T, like for AZT, is the monophosphate. AZT could reach steady state in 2 h, in contrast to D4T and 4′-Ed4T, which reach steady state at 12 h. The intracellular concentrations of the phosphate metabolites in cells exposed to drugs for 12 h are in the order AZT > 4′-Ed4T > D4T. This is consistent with the in vitro studies of these compounds towards TK1, where AZT is better than 4′-Ed4T, which in turn is better than D4T (1, 10, 12, 48). TMPK is proposed to phosphorylate 4′-Ed4T with behavior similar to that of AZT but different from that of D4T (19). Cell culture studies have demonstrated the role of TMPK activity in the formation of AZT and D4T di- and triphosphate metabolites (20). In the present study, we demonstrate that the activity of TMPK in cells is also critical for the subsequent phosphorylation of 4′-Ed4T-MP. The up-regulation of TMPK expression in Tet-On TMPK RKO cells correlated with an increase in the formation of 4′-Ed4TDP (Fig. 2). The main enzyme involved in the phosphorylation of TDP to TTP is NDPK. However, the phosphorylation efficiencies of NDPK for AZTDP and D4TDP are at least 104-fold less and 103-fold less than those for naturally occurring TDP, respectively (40-42). Hsu et al. observed that 4′-Ed4TDP can also be phosphorylated by PGK and pyruvate kinase (19). The intracellular amount of 4′-Ed4TTP is also dependent on PGK activity; up-regulation of PGK expression in RKO cells corresponded to an increase in the formation of 4′-Ed4TTP (Fig. 3). PGK is critical for the formation of the active metabolite, 4′-Ed4TTP.
The use of thymidine analogs and especially D4T in HIV-1-infected individuals has been limited by delayed toxicity, notably peripheral neuropathy and myopathy caused by mitochondrial damage (3, 23, 34). Previous studies from our laboratory have demonstrated less mitochondrial toxicity of 4′-Ed4T in comparison with AZT and D4T (10). The lack of inhibitory activity of 4′-Ed4TTP against human DNA polymerase-γ could be part of the reason (51). Furthermore, the persistence of 4′-Ed4TTP in cells 24 h after removal of drug (Fig. 3C) may contribute to the superior anti-HIV-1 activity of 4′-Ed4T compared with D4T. Previous studies from our laboratory have demonstrated that 4′-Ed4TTP inhibits RNA-dependent DNA synthesis more efficiently than D4TTP, with Ki/Km (an index for evaluation of RT inhibition efficiency) values of 0.15 and 1.1 μM, respectively, using dTTP as the reference (51). With the observed intracellular persistence of 4′-Ed4TTP and its potency against HIV RT (51), we postulate that cells treated with 4′-Ed4T could inhibit HIV replication after drug removal. This will have implications for dosing schedule, with a better treatment compliance outcome. We tested whether the persistent intracellular 4′-Ed4TTP will translate to a more persistent anti-HIV-1 activity of 4′-Ed4T after removal of the drug from the culture medium. TZM-bl cells were incubated with drug (4′-Ed4T, D4T, AZT, or NVP) for 24 h, and then the cells were washed to remove the inhibitor from the culture medium and then infected with the HIV-1 IIIB strain at 0, 24, and 48 h after drug removal. After 24 h of infection, the cells were harvested and lysed, and the percentage of HIV-1 replication inhibition was determined by measuring the luciferase activity. As shown in Fig. 5A, when the cells were infected with virus in the presence of AZT for 24 h, 50% inhibition of viral replication was achieved at concentrations of 0.02 μM and above. The superiority of AZT may be due to its higher phosphorylation efficiency. The affinity of AZT for TK1 is comparable to that of the endogenous thymidine, with Km values of 3.0 μM and 2.9 μM, respectively (1, 12, 48). As shown in Fig. 1A, the peak concentrations of the phosphorylated metabolites of AZT were achieved within 2 h of incubation. NVP, which does not require phosphorylation for antiviral activity, provided significant inhibition of viral replication when infection occurred in the presence of NVP. NVP has a rapid onset of antiviral activity, and hence it is used during labor to prevent mother-to-child transmission of HIV by decreasing the maternal viral load (16). When the cells were treated with either D4T or 4′-Ed4T at the time of infection, the percent inhibition of viral replication achieved was less than that of AZT. This could be due to the fact that the peak concentrations of D4T and 4′-Ed4T phosphorylated metabolites lag behind that for AZT in cells. However, when the cells were preincubated with the analogs for 24 h and washed prior to HIV infection at 0, 24, and 48 h after drug removal, 4′-Ed4T was superior to all the analogs tested in resisting HIV replication even after 48 h of drug removal (Fig. 5A and B). Our data suggest that once formed, 4′-Ed4TTP may remain relatively stable and active in cells and that the pool of 4′-Ed4TMP may continue to replenish the critical concentration of 4′-Ed4TTP. The lack of persistence of anti-HIV activity of AZT may be due to the ability of AZT metabolites to permeate the cell membrane by nonfacilitated diffusion (54). Moreover, the uptake of AZT into cells is not sensitive to nucleoside transport inhibitors such as dipyridamol or nitrobenzylthioinosine and an excess of endogenous nucleosides (37). Therefore, the percolation of AZT metabolites from the cells may account for the lack of persistent anti-HIV activity after drug removal from the culture. Thus, the present data showing that 4′-Ed4TTP is retained in the cells longer and that its anti-HIV activity persists in cells longer than that of AZT, D4T, or NVP suggest that a less-frequent dosing of 4′-Ed4T in comparison with its progenitor, D4T, may be possible. In preclinical development of 4′-Ed4T, its use for HIV-1 postexposure chemoprophylaxis and as a component of HIV-1 microbicide formulations is worth investigating. Interestingly, in our persistence studies the 4′-Ed4T percent inhibition was superior to that of NVP. Single-dose NVP is used to prevent mother-to-child transmission of HIV in resource-limited settings because of its cost-effectiveness and favorable pharmacokinetics (half-life of 30 h) (2, 4, 16).
In conclusion, we have demonstrated that 4′-Ed4T satisfies the sine qua non of a nucleoside analog, i.e., recognition by host cellular kinases and conversion to a high concentration of the active triphosphate metabolite with a long retention time. The efficiency will depend on the intracellular activities of TK1, TMPK, and PGK. All three of these enzymes will be critical for its metabolism in cells. The variability of the activities of these enzymes in cells among different individuals could play a role in the response of HIV-1-infected individuals to 4′-Ed4T. The proposed clinical investigation may corroborate our in vitro data. Since 4′-Ed4T-treated cells could maintain anti-HIV activity longer than AZT- and D4T-treated cells upon drug removal, the possibility for less-frequent dosing should be investigated in preclinical studies. Taken together our data show that, 4′-Ed4T is a promising candidate for HIV-1 chemotherapy.
Acknowledgments
We thank Norman Chen for helpful suggestions.
This work was supported by Public Health Service grant AI-38204 from NIAID. Y.-C.C. is a fellow of the National Foundation for Cancer Research.
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Balzarini, J. 1994. Metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm. World Sci. 16:113-126. [DOI] [PubMed] [Google Scholar]
- 2.Bardsley-Elliot, A., and C. M. Perry. 2000. Nevirapine: a review of its use in the prevention and treatment of paediatric HIV infection. Paediatr. Drugs 2:373-407. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman, K., H. J. ter Hofstede, D. M. Burger, J. A. Smeitink, and P. P. Koopmans. 1998. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12:1735-1744. [DOI] [PubMed] [Google Scholar]
- 4.Cheeseman, S. H., S. E. Hattox, and M. M. McLaughlin. 1993. Pharmacokinetics of nevirapine: initial single-rising-dose study in humans. Antimicrob. Agents Chemother. 37:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, C. H., and Y. C. Cheng. 1989. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J. Biol. Chem. 264:11934-11937. [PubMed] [Google Scholar]
- 6.Chen, C. H., M. Vazquez-Padua, and Y. C. Cheng. 1991. Effect of anti-human immunodeficiency virus nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol. Pharmacol. 39:625-628. [PubMed] [Google Scholar]
- 7.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 8.Coates, J. A., N. Cammack, H. J. Jenkinson, A. J. Jowett, M. I. Jowett, B. A. Pearson, C. R. Penn, P. L. Rouse, K. C. Viner, and J. M. Cameron. 1992. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob. Agents Chemother. 36:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutschman, G. E., E. G. Bridges, S. H. Liu, E. Gullen, X. Guo, M. Kukhanova, and Y. C. Cheng. 1998. Metabolism of 2′,3′-dideoxy-2′,3′-didehydro-beta-l(−)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus beta-d(+) nucleoside analogs in vitro. Antimicrob. Agents Chemother. 42:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutschman, G. E., S. P. Grill, E. A. Gullen, K. Haraguchi, S. Takeda, H. Tanaka, M. Baba, and Y. C. Cheng. 2004. Novel 4′-substituted stavudine analog with improved anti-human immunodeficiency virus activity and decreased cytotoxicity. Antimicrob. Agents Chemother. 48:1640-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng, J. Y., E. Murakami, S. M. Zorca, A. A. Johnson, K. A. Johnson, R. F. Schinazi, P. A. Furman, and K. S. Anderson. 2004. Relationship between antiviral activity and host toxicity: comparison of the incorporation efficiencies of 2′,3′-dideoxy-5-fluoro-3′-thiacytidine-triphosphate analogs by human immunodeficiency virus type 1 reverse transcriptase and human mitochondrial DNA polymerase. Antimicrob. Agents Chemother. 48:1300-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furman, P. A., J. A. Fyfe, M. H. St Clair, K. Weinhold, J. L. Rideout, G. A. Freeman, S. N. Lehrman, D. P. Bolognesi, S. Broder, H. Mitsuya, et al. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 83:8333-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gathe, J. C., Jr., P. Ive, R. Wood, D. Schurmann, N. C. Bellos, E. DeJesus, A. Gladysz, C. Garris, and J. Yeo. 2004. SOLO: 48-week efficacy and safety comparison of once-daily fosamprenavir /ritonavir versus twice-daily nelfinavir in naive HIV-1-infected patients. AIDS 18:1529-1537. [DOI] [PubMed] [Google Scholar]
- 14.Gosselin, G., R. F. Schinazi, J. P. Sommadossi, C. Mathe, M. C. Bergogne, A. M. Aubertin, A. Kirn, and J. L. Imbach. 1994. Anti-human immunodeficiency virus activities of the beta-l enantiomer of 2′,3′-dideoxycytidine and its 5-fluoro derivative in vitro. Antimicrob. Agents Chemother. 38:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grove, K. L., and Y. C. Cheng. 1996. Uptake and metabolism of the new anticancer compound beta-l-(−)-dioxolane-cytidine in human prostate carcinoma DU-145 cells. Cancer Res. 56:4187-4191. [PubMed] [Google Scholar]
- 16.Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, C. Ducar, M. Deseyve, L. Emel, M. Mirochnick, M. G. Fowler, L. Mofenson, P. Miotti, K. Dransfield, D. Bray, F. Mmiro, and J. B. Jackson. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354:795-802. [DOI] [PubMed] [Google Scholar]
- 17.Haraguchi, K., S. Takeda, H. Tanaka, T. Nitanda, M. Baba, G. E. Dutschman, and Y. C. Cheng. 2003. Synthesis of a highly active new anti-HIV agent 2′,3′-didehydro-3′-deoxy-4′-ethynylthymidine. Bioorg. Med. Chem. Lett. 13:3775-3777. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa, H., S. Kohgo, K. Kitano, N. Ashida, E. Kodama, H. Mitsuya, and H. Ohrui. 2004. Potential of 4′-C-substituted nucleosides for the treatment of HIV-1. Antiviral Chem. Chemother. 15:169-187. [DOI] [PubMed] [Google Scholar]
- 19.Hsu, C. H., R. Hu, G. E. Dutschman, G. Yang, P. Krishnan, H. Tanaka, M. Baba, and Y. C. Cheng. 2007. Comparison of the phosphorylation of 4′-ethynyl 2′,3′-dihydro-3′-deoxythymidine with that of other anti-human immunodeficiency virus thymidine analogs. Antimicrob. Agents Chemother. 51:1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, R., L. Li, B. Degreve, G. E. Dutschman, W. Lam, and Y. C. Cheng. 2005. Behavior of thymidylate kinase toward monophosphate metabolites and its role in the metabolism of 1-(2′-deoxy-2′-fluoro-beta-l-arabinofuranosyl)-5-methyluracil (Clevudine) and 2′,3′-didehydro-2′,3′-dideoxythymidine in cells. Antimicrob. Agents Chemother. 49:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, J. B., S. Barnett, E. Piwowar-Manning, L. Apuzzo, C. Raines, C. Hendrix, F. Hamzeh, and J. Gallant. 2003. A phase I/II study of nevirapine for pre-exposure prophylaxis of HIV-1 transmission in uninfected subjects at high risk. AIDS 17:547-553. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, A. A., A. S. Ray, J. Hanes, Z. Suo, J. M. Colacino, K. S. Anderson, and K. A. Johnson. 2001. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J. Biol. Chem. 276:40847-40857. [DOI] [PubMed] [Google Scholar]
- 23.Kakuda, T. N. 2000. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22:685-708. [DOI] [PubMed] [Google Scholar]
- 24.Kitano, K., S. Kohgo, K. Yamada, S. Sakata, N. Ashida, H. Hayakawa, D. Nameki, E. Kodama, M. Matsuoka, H. Mitsuya, and H. Ohrui. 2004. Attempt to reduce cytotoxicity by synthesizing the l-enantiomer of 4′-C-ethynyl-2′-deoxypurine nucleosides as antiviral agents against HIV and HBV. Antiviriral Chem. Chemother. 15:161-167. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan, P., E. A. Gullen, W. Lam, G. E. Dutschman, S. P. Grill, and Y. C. Cheng. 2003. Novel role of 3-phosphoglycerate kinase, a glycolytic enzyme, in the activation of l-nucleoside analogs, a new class of anticancer and antiviral agents. J. Biol. Chem. 278:36726-36732. [DOI] [PubMed] [Google Scholar]
- 26.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 27.Lee, H., J. Hanes, and K. A. Johnson. 2003. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry 42:14711-14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenert, L. A., M. Feddersen, A. Sturley, and D. Lee. 2002. Adverse effects of medications and trade-offs between length of life and quality of life in human immunodeficiency virus infection. Am. J. Med. 113:229-232. [DOI] [PubMed] [Google Scholar]
- 29.Lin, T. S., M. Z. Luo, M. C. Liu, S. B. Pai, G. E. Dutschman, and Y. C. Cheng. 1994. Antiviral activity of 2′,3′-dideoxy-beta-l-5-fluorocytidine (beta-l-FddC) and 2′,3′-dideoxy-beta-l-cytidine (beta-l-ddC) against hepatitis B virus and human immunodeficiency virus type 1 in vitro. Biochem. Pharmacol. 47:171-174. [DOI] [PubMed] [Google Scholar]
- 30.Lin, T. S., M. Z. Luo, M. C. Liu, Y. L. Zhu, E. Gullen, G. E. Dutschman, and Y. C. Cheng. 1996. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-beta-l-cytidine (beta-l-d4C) and 2′,3′-dideoxy 2′,3′-didehydro-beta-l-5-fluorocytidine (beta-l-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J. Med. Chem. 39:1757-1759. [DOI] [PubMed] [Google Scholar]
- 31.Longley, M. J., P. A. Ropp, S. E. Lim, and W. C. Copeland. 1998. Characterization of the native and recombinant catalytic subunit of human DNA polymerase gamma: identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry 37:10529-10539. [DOI] [PubMed] [Google Scholar]
- 32.Maag, H., R. M. Rydzewski, M. J. McRoberts, D. Crawford-Ruth, J. P. Verheyden, and E. J. Prisbe. 1992. Synthesis and anti-HIV activity of 4′-azido- and 4′-methoxynucleosides. J. Med. Chem. 35:1440-1451. [DOI] [PubMed] [Google Scholar]
- 33.Martin, J. L., C. E. Brown, N. Matthews-Davis, and J. E. Reardon. 1994. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob. Agents Chemother. 38:2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina, D. J., C. H. Tsai, G. D. Hsiung, and Y. C. Cheng. 1994. Comparison of mitochondrial morphology, mitochondrial DNA content, and cell viability in cultured cells treated with three anti-human immunodeficiency virus dideoxynucleosides. Antimicrob. Agents Chemother. 38:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyer, T. P., Z. Temesgen, R. Enger, L. Estes, J. Charlson, L. Oliver, and A. Wright. 1999. Drug monitoring of antiretroviral therapy for HIV-1 infection: method validation and results of a pilot study. Clin. Chem. 45:1465-1476. [PubMed] [Google Scholar]
- 36.Nitanda, T., X. Wang, H. Kumamoto, K. Haraguchi, H. Tanaka, Y. C. Cheng, and M. Baba. 2005. Anti-human immunodeficiency virus type 1 activity and resistance profile of 2′,3′-didehydro-3′-deoxy-4′-ethynylthymidine in vitro. Antimicrob. Agents Chemother. 49:3355-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peter, K., and J. G. Gambertoglio. 1998. Intracellular phosphorylation of zidovudine (ZDV) and other nucleoside reverse transcriptase inhibitors (RTI) used for human immunodeficiency virus (HIV) infection. Pharm. Res. 15:819-825. [DOI] [PubMed] [Google Scholar]
- 38.Powderly, W. G. 2002. Long-term exposure to lifelong therapies. J. Acquir. Immune Defic. Syndr. 29(Suppl. 1):S28-S40. [DOI] [PubMed] [Google Scholar]
- 39.Richman, D. D. 1993. Resistance of clinical isolates of human immunodeficiency virus to antiretroviral agents. Antimicrob. Agents Chemother. 37:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider, B., R. Sarfati, D. Deville-Bonne, and M. Veron. 2000. Role of nucleoside diphosphate kinase in the activation of anti-HIV nucleoside analogs. J. Bioenerg Biomembr. 32:317-324. [DOI] [PubMed] [Google Scholar]
- 41.Schneider, B., Y. W. Xu, J. Janin, M. Veron, and D. Deville-Bonne. 1998. 3′-Phosphorylated nucleotides are tight binding inhibitors of nucleoside diphosphate kinase activity. J. Biol. Chem. 273:28773-28778. [DOI] [PubMed] [Google Scholar]
- 42.Schneider, B., Y. W. Xu, O. Sellam, R. Sarfati, J. Janin, M. Veron, and D. Deville-Bonne. 1998. Pre-steady state of reaction of nucleoside diphosphate kinase with anti-HIV nucleotides. J. Biol. Chem. 273:11491-11497. [DOI] [PubMed] [Google Scholar]
- 43.Shulman, N., and M. Winters. 2003. A review of HIV-1 resistance to the nucleoside and nucleotide inhibitors. Curr. Drug Targets Infect. Disord. 3:273-281. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqui, M. A., S. H. Hughes, P. L. Boyer, H. Mitsuya, Q. N. Van, C. George, S. G. Sarafinanos, and V. E. Marquez. 2004. A 4′-C-ethynyl-2′,3′-dideoxynucleoside analogue highlights the role of the 3′-OH in anti-HIV active 4′-C-ethynyl-2′-deoxy nucleosides. J. Med. Chem. 47:5041-5048. [DOI] [PubMed] [Google Scholar]
- 45.Siliciano, J. D., J. Kajdas, D. Finzi, T. C. Quinn, K. Chadwick, J. B. Margolick, C. Kovacs, S. J. Gange, and R. F. Siliciano. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9:727-728. [DOI] [PubMed] [Google Scholar]
- 46.Sosa, N., C. Hill-Zabala, E. Dejesus, G. Herrera, A. Florance, M. Watson, C. Vavro, and M. Shaefer. 2005. Abacavir and lamivudine fixed-dose combination tablet once daily compared with abacavir and lamivudine twice daily in HIV-infected patients over 48 weeks (ESS30008, SEAL). J. Acquir. Immune Defic. Syndr. 40:422-427. [DOI] [PubMed] [Google Scholar]
- 47.Squires, K. E. 2001. An introduction to nucleoside and nucleotide analogues. Antivir. Ther. 6(Suppl. 3):1-14. [PubMed] [Google Scholar]
- 48.Tornevik, Y., B. Jacobsson, S. Britton, and S. Eriksson. 1991. Intracellular metabolism of 3′-azidothymidine in isolated human peripheral blood mononuclear cells. AIDS Res. Hum. Retroviruses 7:751-759. [DOI] [PubMed] [Google Scholar]
- 49.Waheed, A. A., S. D. Ablan, M. K. Mankowski, J. E. Cummins, R. G. Ptak, C. P. Schaffner, and E. O. Freed. 2006. Inhibition of HIV-1 replication by amphotericin B methyl ester: selection for resistant variants. J. Biol. Chem. 281:28699-28711. [DOI] [PubMed] [Google Scholar]
- 50.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, G., G. E. Dutschman, C. J. Wang, H. Tanaka, M. Baba, K. S. Anderson, and Y. C. Cheng. 2007. Highly selective action of triphosphate metabolite of 4′-ethynyl D4T: a novel anti-HIV compound against HIV-1 RT. Antiviral Res. 73:185-191. [DOI] [PubMed] [Google Scholar]
- 52.Yeni, P. G., S. M. Hammer, M. S. Hirsch, M. S. Saag, M. Schechter, C. C. Carpenter, M. A. Fischl, J. M. Gatell, B. G. Gazzard, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 292:251-265. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, Y. L., D. E. Dutschman, S. H. Liu, E. G. Bridges, and Y. C. Cheng. 1998. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-beta-l(−)-5-fluorocytidine. Antimicrob. Agents Chemother. 42:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerman, T. P., W. B. Mahony, and K. L. Prus. 1987. 3′-Azido-3′-deoxythymidine. An unusual nucleoside analogue that permeates the membrane of human erythrocytes and lymphocytes by nonfacilitated diffusion. J. Biol. Chem. 262:5748-5754. [PubMed] [Google Scholar]