Abstract
Our studies on the role of cholesterol homeostasis in the pathogenesis of scrapie revealed abnormal accumulation of cholesterol esters in ex vivo peripheral blood mononuclear cells (PBMCs) and skin fibroblasts from healthy and scrapie-affected sheep carrying a scrapie-susceptible genotype compared to sheep with a resistant genotype. Similar alterations were observed in mouse neuroblastoma N2a cell lines persistently infected with mouse-adapted 22L and RML strains of scrapie that showed up to threefold-higher cholesterol ester levels than parental N2a cells. We now report that proteinase K-resistant prion protein (PrPres)-producing cell populations of subclones from scrapie-infected cell lines were characterized by higher cholesterol ester levels than clone populations not producing PrPres. Treatments with a number of drugs known to interfere with different steps of cholesterol metabolism strongly reduced the accumulation of cholesterol esters in ex vivo PBMCs and skin fibroblasts from scrapie-affected sheep but had significantly less or no effect in their respective scrapie-resistant or uninfected counterparts. In scrapie-infected N2a cells, inhibition of cholesterol esters was associated with selective antiprion activity. Effective antiprion concentrations of cholesterol modulators (50% effective concentration [EC50] range, 1.4 to 40 μM) were comparable to those of antiprion reference compounds (EC50 range, 0.6 to 10 μM). These data confirm our hypothesis that abnormal accumulation of cholesterol esters may represent a biological marker of susceptibility to prion infection/replication and a novel molecular target of potential clinical importance.
Prion diseases are fatal neurodegenerative pathologies of mammals and have genetic, sporadic, or infective origins. No proven treatments or conclusive means of diagnosis or prevention are currently available (2, 38). A number of therapeutic approaches are presently under investigation or in development, and they rely on (i) inhibitors of the structural β-transition (misfolding) of the cellular prion protein (PrPc) into its aberrant pathogenic isoform, termed scrapie PrP (PrPsc); (ii) compounds affecting fibril aggregation; and (iii) compounds able to reverse conformational changes (β-breakers) (5, 25). Although no treatment has been shown to halt or delay disease progression in animals or in humans (8, 39), the in vitro and in vivo results thus far obtained support the feasibility of pharmacological interventions and encourage research efforts aimed at increasing the potential chemical armamentarium against prions (14, 23). To this end, as well as the identification of new classes of inhibitors targeting the conventional prion conversion/aggregation steps and the improvement of currently available drugs in terms of selectivity, potency, and ability to cross the blood brain barrier, it is crucial to identify cellular processes that are directly or indirectly involved in prion generation/accumulation that could be exploited as novel selective targets. At present, given the lack of molecular markers allowing certain early diagnosis, any potentially effective therapy can be administered only after symptoms have developed, while the long incubation period characteristic of many prion disorders likely implies a considerable period of clinically silent disease progression (7). Besides the unquestionable significance of a genetic factor(s) (26), a particular metabolic state might be crucial in modulating cascades of pathogenic events, thus influencing the length of the incubation period.
Our recent investigations on the role of cholesterol in the pathogenesis of prion diseases indicated peculiar alterations in intracellular cholesterol homeostasis in prion-susceptible or prion-infected cells (28, 29). With respect to genetically scrapie-resistant (ARR genotype) sheep, abnormal accumulation of cholesterol esters (CE) was a constant and distinctive trait of ex vivo skin fibroblasts and peripheral blood mononuclear cells (PBMCs) from uninfected and scrapie-affected Sarda sheep carrying a susceptible ARQ genotype. Increased levels of CE were also observed in brain tissue homogenates from susceptible sheep compared to resistant sheep (28). Cell-prion in vitro systems, such as mouse neuroblastoma N2a cell lines persistently infected with the 22L and RML strains of scrapie, revealed similar alterations with up to threefold-higher CE levels than parental, uninfected N2a cells (29).
The results reported here further sustain the presence of a strong correlation between cell susceptibility to scrapie infection/replication and intracellular levels of CE. In scrapie-infected cell lines, proteinase K (PK)-resistant PrP (PrPres)-producing cell populations were characterized by higher levels of CE, and drugs that inhibited cholesterol esterification showed antiprion activity at comparable concentrations.
The drugs used in this study were the steroid hormone progesterone (PG) and the calcium-blocking verapamil, which target MDR1-PgP-mediated cholesterol transport from the plasma membrane to the endoplasmic reticulum (10, 27, 30, 31); Sandoz 58-035 (SaH), a known inhibitor of the enzyme ACAT-1, which catalyzes the esterification of the cholesterol moiety to free fatty acids in the endoplasmic reticulum (11); pioglitazone (PIO), a drug used in the treatment of non-insulin-dependent diabetes mellitus that appears to induce intracellular redistribution of free fatty acids (4, 15); and everolimus (EVE), an immunosuppressant agent that has been reported to inhibit cholesterol esterification in our and other laboratories (4, 16, 20).
MATERIALS AND METHODS
Chemicals.
Amphotericin B, Congo red (91%), dextran sulfate sodium salt (molecular weight, 500,000), verapamil hydrochloride (98%), cyclosporine A (≥98.5%), chlorpromazine hydrochloride, quinacrine dihydrochloride, PG, and dimethyl sulfoxide (DMSO) (99.5%), were purchased from Sigma-Aldrich (Italy). Tannic acid was purchased from MP Biomedicals. EVE and SaH were kindly provided by Novartis (Switzerland) and PIO by Takeda (Japan). Dextran sulfate (DX500) was solubilized in OptiMEM and stored at −20°C. EVE was solubilized in 100% ethanol and stored at 4°C. PIO and SaH were solubilized in 100% ethanol and stored at room temperature. Stock solutions of the other compounds were prepared in DMSO and stored at −20°C.
Sheep.
Sheep samples were a generous gift from Ciriaco Ligios and were collected at the Istituto Zooprofilattico Sperimentale of Sardinia, Sassari, Italy. The samples were collected from a total of 14 Sarda breed sheep, 4 of which carried the scrapie-resistant ARR/ARR genotype while 10 carried the scrapie-susceptible ARQ/ARQ genotype. Of the latter, two were mock infected, one had natural scrapie, and seven developed clinical disease following experimental inoculation of scrapie. The four scrapie-resistant sheep, which were scrapie infected in parallel with susceptible animals, and the two mock-infected susceptible animals did not develop any clinical signs and were alive and healthy at the time of this report. With the exception of the sheep affected by natural scrapie, all the animals used were raised under the same environmental conditions and were of the same age and sex in order to reduce physiological differences in experimental determinations. Samples from all sheep were collected at the time of the terminal clinical stage in the ill animals.
Isolation of sheep mononuclear leukocytes.
Blood was sampled from the anterior vena cava of each sheep, and PBMCs were separated by Ficoll-Hypaque density gradient. After extensive washings, the cells were suspended (1 × 106 cells/ml) in RPMI 1640 with 10% fetal calf serum (FCS) and incubated overnight. For determinations, 2 × 105 nonadherent cells/ml were incubated with phytohemoagglutinin (PHA) (10 μg/ml; Sigma-Aldrich) at 37°C in RPMI 1640 supplemented with 10% FCS. The numbers of viable cells were evaluated during time courses by counting trypan blue-excluding cells.
Isolation of sheep skin fibroblasts.
The isolation and culture procedures for skin fibroblasts from sheep dermal biopsies have been described (28). In brief, dermal biopsies were plated into six-well plates in Dulbecco's modified Eagle's medium (Gibco Laboratories) supplemented with 10% fetal bovine serum (Sigma), 100 U/ml penicillin/streptomycin (Sigma), and fungizone (Life Technologies, Inc.). After 4 weeks (37°C; 5% CO2), the fibroblasts were detached by repeated trypsinization (trypsin-EDTA, 0.05%/0.02%) and propagated to achieve a homogeneous population of spindle cells. Purified fibroblasts (1 × 106) were seeded in a 75-cm2 culture flask and grown to confluence. At that time, the cells were used for in vitro staining experiments or concentrated in cryopreservation medium (1 × 107 cells/ml) for long-term storage in liquid nitrogen and thawed according to need. Analytical assays were carried out on fibroblast cultures between the second and the fourth passage; cells were plated at a density of 5,000 cells/cm2 in six-well plates and brought to proliferative quiescence by serum depletion (0.2% FCS in minimal essential medium 199) for 48 h. Quiescent cells were stimulated to reenter the cell cycle by the addition of 10% FCS and processed as described below at the indicated times.
Cell lines.
The mouse neuroblastoma N2a cell line and the 22L-N2a and RML-N2a sublines, infected with the mouse-adapted 22L and RML strains of scrapie (Rocky Mountain Laboratories), respectively, were a generous gift of Byron Caughey, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, NIH. The cells were grown and maintained at 37°C and 5% CO2 in OptiMEM supplemented with 10% fetal bovine serum (Gibco-Invitrogen, Italy), 2 mM l-glutamine, 50 U/ml penicillin G sodium, and 50 μg/ml streptomycin sulfate (Gibco-Invitrogen, Italy) and passaged every 3 or 4 days at a 1:10 or 1:20 dilution, respectively. At times, the 22L-N2a and RML-N2a sublines were cloned by end point dilution (single-cell dilution) to isolate better PrPres producer populations. Cell lines and subclones were stored in liquid nitrogen, and working cultures were replaced at 2- to 3-month intervals in order to maintain the same intensity of PrPres signal throughout the experiment. All trials were carried out in cell cultures during exponential growth.
Lipid staining.
Intracellular neutral lipids (i.e., CE) were evaluated by the oil red O method (13, 24) at the indicated time points as previously described (28). In brief, cultures were washed three times with phosphate-buffered saline and fixed by soaking them in 10% formalin. The cells were then treated with isopropyl alcohol (60%), washed, stained with oil red O for intracellular neutral lipid droplets, and counterstained with Mayer's hematoxylin. Stained cells were examined by light microscopy, and digital images were recorded. The red color intensity in single cells, indicating neutral-lipid-bound oil red O, was measured with the NIH Image 1.63 Analysis Software program (Scion Image). Values are expressed as the mean color intensity per cell calculated for at least 30 single random cells in six different microscopic fields.
Detection of PrPres in cell cultures.
For the dot blot procedure, approximately 5,000 cells in 100 μl of growth medium were added to each well of a Microtest flat-bottom 96-well plate with a low-evaporation lid (Becton Dickinson). For drug testing, the cells were allowed to settle overnight before the addition of 10 μl (10× solutions) of different dilutions of test compounds. The DMSO concentration in the cell medium was never higher than 0.5% (vol/vol). Each drug concentration was tested in quadruplicate. After 4 days at 37°C in 5% CO2, the cells were processed for PrPres as described previously (22). In brief, the cells were lysed with 50 μl of cold lysis buffer (0.5% [wt/vol] Triton X-100, 0.5% [wt/vol] sodium deoxycholate, 5 mM Tris-HCl, pH 7.4, at 4°C, 5 mM EDTA, and 150 mM NaCl). After 15 min on ice, 25 μl of 0.1 mg/ml PK (Novagen) in Tris-buffered saline (TBS) (1.4 M NaCl, 1 M Tris-HCl, pH 7.6) was added to each well for 60 min at 37°C. A total of 225 μl of 1 mM Pefabloc (Roche-Novagen) was added to the wells of PK-treated and mock-treated cultures. The lysates were transferred to a 96-well dot blot apparatus (Schleicher & Schuell) over a 0.45-μm-pore-size polyvinylidene difluoride membrane (Immobilon-P; Millipore) and rinsed with TBS. The polyvinylidene difluoride membrane was removed, covered with a denaturing solution of 3 M Gdn SCN (Fluka) for 8 min at room temperature, blocked with 5% (wt/vol) nonfat dry milk (Bio-Rad) and 0.05% (vol/vol) Tween 20 (USB Corporation) in TBS for 60 min at room temperature, incubated with anti-PrP mouse monoclonal antibody 6H4 (Prionics, Zurich, Switzerland; 1:20.000) in TBS-T for 1 h, and then exposed to horseradish peroxidase-labeled anti-mouse immunoglobulin G antibody (GE Healthcare, United Kingdom; 1:50.000) in TBS-T for 1 h. After extensive washings, the membrane was soaked for 5 min in ECL-Plus reagent (GE Healthcare) and exposed to X-ray film (Hyperfilm ECL; GE Healthcare). The autoradiography images were captured in TIFF format, and the intensity of each dot was determined by using Scion Image software (Scion, Frederick, MD). The PrPres mean value at each drug concentration was expressed as a percentage of that of untreated controls, and the concentration resulting in 50% reduction (the 50% effective concentration [EC50]) was determined by linear regression analysis. For Western blot analysis, 4 × 106 cell samples were lysed in 500 μl of cold lysis buffer (see above) for 6 min. After centrifugation at 5,000 rpm for 5 min, the supernatants were collected and the total protein concentration was determined by the bicinchoninic acid protein assay (Sigma-Aldrich). Samples were digested with 20 μg/ml PK (Novagen) in TBS at 37°C for 30 min, and the digestion was stopped by incubation with 4 mM Pefabloc (Roche-Novagen) for 10 min on ice. PrPres was collected by precipitation with 4 volumes of methanol at −20°C (6). The resulting pellets were then solubilized by sonication in LDS sample buffer (Invitrogen), and 20-μg protein samples were loaded onto a 10% NuPage bis-Tris-polyacrylamide gel (Invitrogen) just after boiling. The protein bands were electroblotted onto an Immobilion-P membrane (Millipore), and PrPres was detected as described above.
Cytotoxicity assay.
Antiproliferative activity was evaluated in exponentially growing cell cultures. One hundred microliters of a cell suspension at a density of 5 × 104 cells/ml was added to each well of a flat-bottom 96-well plate 24 h before the addition of 100 μl of 2× dilutions of the test compounds. Each drug concentration was tested in quadruplicate. Cell viability was determined after 4 days by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (Sigma, Italy) method as previously described (12). Cell viability at each drug concentration was expressed as a percentage of that of untreated controls, and the concentration resulting in 50% viability (the 50% cytotoxic concentration [CC50]) was determined by linear regression analysis.
Statistical analysis.
All values are presented as the mean and standard deviation (in the table) or mean and standard error of the mean (in the figures). Statistical analysis was performed with the Student t test. For multiple comparisons, all significance values were corrected by the Bonferroni method for multiple tests. Significance was set at a P value of <0.05.
RESULTS
Comparative inhibition of CE in ex vivo skin fibroblasts and PBMCs from sheep genetically susceptible or resistant to scrapie.
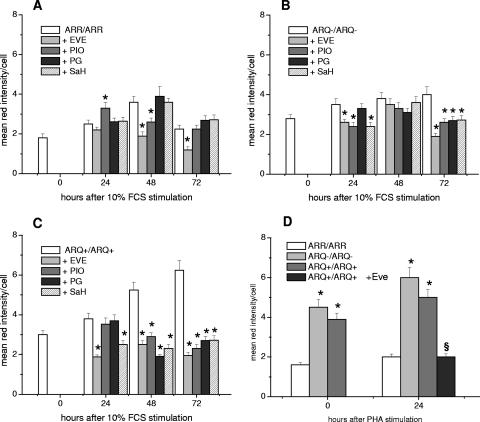
The ability shown by EVE and PIO to selectively reduce CE levels in prion-infected mouse neuroblastoma cell lines (29) prompted us to analyze the effects of a greater number of drugs acting as cholesterol modulators in skin fibroblast and PBMC cultures isolated from sheep genetically susceptible or resistant to scrapie (Fig. 1A to D). As expected, in untreated cultures, the basal content of CE was higher in skin fibroblasts and PBMCs from susceptible animals, and following growth stimulation, the levels of CE increased in all cultures irrespective of genotype (Fig. 1A to D, compare the controls). However, with respect to cells from scrapie-resistant animals, CE levels increased more in skin fibroblasts from all animals carrying a susceptible genotype: these reached twice the basal level observed in cultures from scrapie-affected sheep after 72 h (Fig. 1C). It is noteworthy that at that time point, CE in cells from scrapie-resistant sheep had returned to basal levels. In PBMCs from scrapie-susceptible animals, the levels of CE were already twofold higher than in cells from resistant sheep before mitogen stimulation, and they increased more in the susceptible cultures within the first 24 h of growth stimulus (Fig. 1D). Treatment of growth-stimulated skin fibroblasts with drugs affecting cholesterol esterification resulted in different patterns of CE inhibition depending on the sheep's genotype (Fig. 1A to C). In skin fibroblasts from resistant (ARR/ARR) sheep, EVE (50 nM) was able to reduce CE levels by about 50% at 48 h after growth stimulation and maintained similar inhibitory activity at 72 h (Fig. 1A). CE levels in PIO (40 μM)-treated cultures were slightly increased at 24 h, slightly reduced at 48 h, and not modified at 72 h. PG (20 μM) and SaH (40 μM) did not show any ability to significantly affect the CE content at any time point considered. In fibroblasts from the unaffected-susceptible (ARQ/ARQ−) sheep, all drugs exerted a significant inhibitory effect, ranging from 30% to 50%, at 72 h only (Fig. 1B). By contrast, incubation of skin fibroblasts from scrapie-affected (ARQ/ARQ+) sheep in the presence of the cholesterol modulators led to a reduction of intracellular CE levels from about 50% to about 70% at 48 h and 72 h after growth stimulation, respectively (Fig. 1C). EVE emerged as the most potent of all the inhibitors, both in terms of the effective concentration (50 nM versus 20 to 40 μM) and the rapidity of effect; it was the only drug able to reduce the CE levels by 50% within the first 24 h of growth stimulation. In PHA-stimulated PBMCs from the scrapie-affected sheep (Fig. 1D), 24 h of incubation with EVE (50 nM) resulted in strong CE inhibition (60%), whereas treatments with PIO, PG, and SaH showed lower (20% to 35%) inhibitory effects (not shown).
FIG. 1.
Neutral-lipid contents of growth-stimulated skin fibroblast and PBMC cultures from sheep with a scrapie-susceptible or scrapie-resistant genotype treated in vitro with modulators of cholesterol metabolism. Skin fibroblasts were serum stimulated in the absence or presence of EVE (50 nM), PIO (40 μM), PG (20 μM), and SaH (40 μM). PBMCs were PHA stimulated, and cells from scrapie-infected sheep were incubated in the absence and in the presence of EVE (50 nM). At the indicated times, the cells were stained by the oil red O method and processed for determination of neutral-lipid contents. Quantification of the intensity of lipid-bound red color was determined by densitometric analysis with Scion Image software (NIH). The values represent the mean plus standard error of the mean of red stain per cell in triplicate determinations from at least three independent experiments with each group of cultures. (A) Skin fibroblast cultures from four sheep with a scrapie-resistant (ARR/ARR) genotype. (B) Skin fibroblast cultures from two sheep with a scrapie-susceptible (ARQ/ARQ−) genotype. (C) Skin fibroblast cultures from eight scrapie-affected sheep with a scrapie-susceptible (ARQ/ARQ+) genotype. (D) PBMCs from four sheep with a scrapie-resistant (ARR/ARR) genotype, two sheep with a scrapie-susceptible (ARQ/ARQ) genotype, and eight scrapie-affected sheep with a scrapie-susceptible (ARQ/ARQ+) genotype. *, P < 0.05; considered statistically significant (P < 0.05/4 = 0.0125 after Bonferroni correction). §, P < 0.05 versus ARQ/ARQ+.
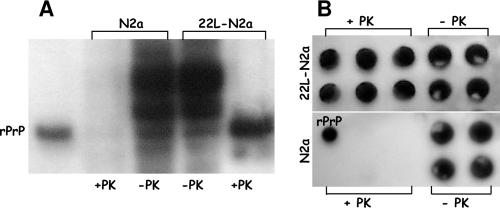
Correlation between the production of PrPres and CE levels in prion-infected mouse neuroblastoma cells.
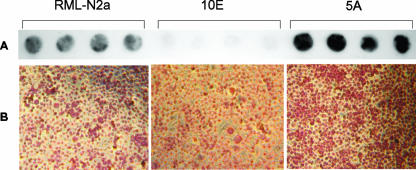
As expected, Western and dot blotting analyses of PrP in parental and prion-infected N2a cells revealed the presence of PrPc in both infected and uninfected cultures, while PrPres was present only in lysates from infected cells (Fig. 2A and B). In our hands, scrapie-infected cell lines underwent crises during which their production of PrPres was reduced to undetectable levels. On these occasions, we either thawed fresh cells or cloned the cell population to isolate sublines that were better producers of PrPres. On the basis of our previous observations showing that different CE levels differentiated uninfected and prion-infected N2a cell lines (29), we were interested in investigating PrPres production in relation to the CE contents in two subclones, 5A and 10E, recently isolated from the prion-infected RML-N2a cell line (Fig. 3A and B). With respect to the parental cells, the 5A clone was a better producer of PrPres, whereas the 10E clone scarcely produced any (Fig. 3A). Duplicate cultures evaluated for intracellular contents of CE showed a homogeneously more intense red stain in the 5A PrPres-producing population with respect to the parental RML-N2a cells, indicating higher levels of CE (Fig. 3B). On the contrary, absence of PrPres production in the 10E clone was accompanied by lower CE levels, as revealed by the diminished red stain in the entire cell population.
FIG. 2.
Detection of PrPc and PrPres in N2a and 22L-N2a mouse neuroblastoma cell lines. Lysates from uninfected and 22L scrapie-infected N2a cell lines were digested or not with PK (20 μg/ml) and analyzed with 6H4 antibody by Western (A) and dot (B) blotting procedures (see Materials and Methods). As a control, 0.05 μg of recombinant mouse PrP (rPrP) (Prionics) was used.
FIG. 3.
PrPres and neutral lipids in the RML-N2a cell line and subclones. (A) Lysates from RML-N2a cells and from 10E and 5A subclones selected from the RML-N2a cell population were digested with PK (20 μg/ml) and analyzed with 6H4 antibody by a dot blot procedure. (B) Duplicate cultures were stained for neutral lipids by the oil red O method and photographed (see Materials and Methods for details).
Antiprion activities of cholesterol modulators in mouse neuroblastoma 22L-N2a cells.
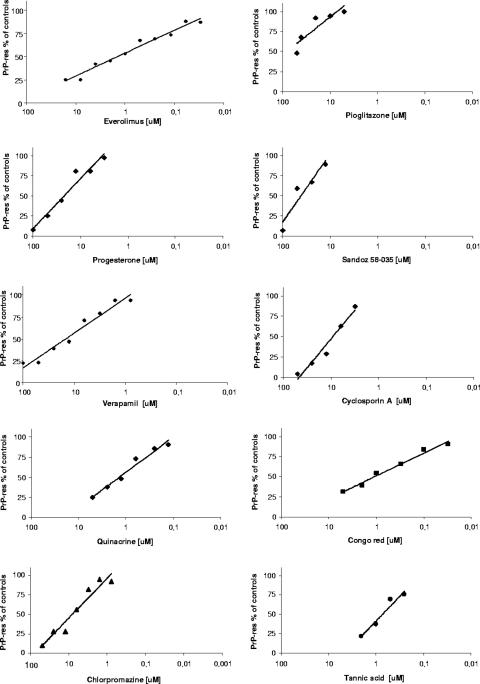
To investigate the influence of CE inhibition on PrP misfolding, we evaluated the effects of cholesterol modulators on the production of PrPres. Treatment of 22L-N2a cells with EVE, PIO, PG, verapamil, SaH, and cyclosporine A led to PrPres inhibition in a dose-dependent manner (Fig. 4). Although none of the drugs proved to be either very potent or very selective prion inhibitors, some of them showed antiprion activity at noncytotoxic concentrations, with EC50s ranging between 1.4 μM and 40 μM (Table 1). The most potent and selective was EVE (EC50 of 1.4 μM versus CC50 of >16 μM), followed by verapamil (EC50 of 15 μM versus CC50 of ≥100 μM), PG (EC50 of 35 μM versus CC50 of 95 μM), and PIO (EC50 of 40 μM versus CC50 of 60 μM), whereas SaH showed antiprion activity at concentrations close to the CC50. Dose-response curves of the antiprion activities of known prion inhibitors (Fig. 4), and their 50% inhibitory and cytotoxic concentrations (Table 1), were obtained under the same experimental conditions for comparative purposes and indicated antiprion activities in a similar dose range (0.6 to 10 μM).
FIG. 4.
Dose-response curves of antiprion activities of cholesterol modulators and known prion inhibitors. 22L-N2a cells seeded in growth medium at approximately 5,000 cells/well in 96-well plates were incubated in the absence and in the presence of serial dilutions of the different drugs. After 4 days, the cells were lysed and digested with PK (20 μg/ml). PrPres in the lysates was analyzed by a dot blot procedure with 6H4 antibody and quantized (see Materials and Methods). The mean value of PrPres at each drug concentration (four wells/concentration) was expressed as a percentage of that of untreated cultures, and the concentration resulting in 50% inhibition (the EC50) was determined by linear regression analysis. Each drug was tested at least three times.
TABLE 1.
Comparative cytotoxicities and anti-prion activities of cholesterol modulators and prion inhibitors in the 22L-N2a cell linea
| Cholesterol modulator | CC50 [μM] | PrPres EC50 [μM] | Prion inhibitor | CC50 [μM] | PrPres EC50 [μM] |
|---|---|---|---|---|---|
| EVE | >16 | 1.4 ± 0.8 | Quinacrine | 4.5 ± 1.3 | 1 ± 0.9 |
| PIO | 60.1 ± 11 | 40 ± 5.4 | Chlorpromazine | 83.4 ± 11.6 | 10 ± 3.9 |
| PG | 95.7 ± 18.6 | 35 ± 7.8 | Dextran sulfate 500 | >50 (μg/ml) | 0.2 (μg/ml) ± 0.1 |
| Verapamil | ≥100 | 15 ± 4.6 | Tannic acid | 6.6 ± 3.9 | 0.7 ± 0.3 |
| SaH | 68.4 ± 8.4 | 40 ± 6.4 | Amphotericin B | 28 ± 7 | 25 ± 5.7 |
| Cyclosporine A | 15.9 ± 4.6 | 10 ± 3.2 | Congo red | >10 | 0.6 ± 0.3 |
Values are the mean ± standard deviation of three or four independent experiments.
DISCUSSION
The data presented here confirm our previous evidence (28, 29) of a strong relationship between abnormal CE accumulation and cell susceptibility to scrapie infection/replication. In RML-N2a subclones, high CE levels were correlated with high PrPres production, whereas low levels were associated with barely detectable production of PrPres. Moreover, various drugs that inhibited cholesterol esterification by targeting different steps of cholesterol metabolism/trafficking had the following traits: (i) they were able to reduce CE levels in PBMCs and skin fibroblasts isolated from scrapie-affected sheep, (ii) they had significantly less or no effect on the scrapie-resistant counterparts, and (iii) they determined a parallel inhibition of PrPres in a prion-infected 22L-N2a cell line. Among all cholesterol modulators tested, EVE was determined to be the most active drug. It was the most potent inhibitor of cholesterol esterification, both in terms of the effective concentration (50 nM versus 20 to 40 μM) and the rapidity of effect, and it exerted the most potent antiprion activity in 22L-N2a cells with an EC50 (1.4 μM) similar to that obtained under the same experimental conditions with the prion inhibitors quinacrine (EC50, 1 μM), tannic acid (EC50, 0.7 μM), and Congo red (EC50, 0.6 μM). The cholesterol modulators PIO, PG, and verapamil resulted in a selective, although moderate, antiprion effect (EC50 range, 15 to 40 μM) comparable to that obtained with the antiprion chlorpromazine (EC50, 10 μM).
Drugs known to affect de novo cholesterol biosynthesis (i.e., statins) have been reported to inhibit PrPres accumulation (3), and more recently, evidence has been presented that quinacrine can exert its antiprion effect by influencing the intracellular redistribution of cholesterol (21). However, this is the first report that shows selective antiprion activities of drugs affecting the overall cholesterol esterification process.
In normal tissues, only a minor amount (1 to 10%) of total cholesterol is found as CE, the storage form of cholesterol in the cytoplasm, while more than 90% is in the form of free cholesterol and resides in the cholesterol-rich membrane domains (rafts). Because membrane cholesterol appears to be critical for the functions of raft-resident proteins (e.g., PrP, β- and γ-secretases of APP, and growth factor receptors), cells have developed a highly integrated set of homeostatic mechanisms that finely regulate free-cholesterol versus CE pools according to the cells' needs. In previous studies (reviewed in reference 31), we found abnormal CE accumulation to be a marker of pathological proliferation in contrast to a temporary CE increase in normal cells as a controlled physiologic response to growth stimuli (i.e., PHA-stimulated, interleukin-2-treated PBMCs from healthy humans). In this work, we showed that specific growth stimuli also led to a CE increase in sheep skin fibroblasts and PBMCs irrespective of the PrP genotype. However, cells from scrapie-susceptible and scrapie-affected animals showed basal CE levels higher than those of cells from scrapie-resistant sheep. Moreover, cells from scrapie-affected animals displayed a greater and more extended CE response than did cells from both uninfected and scrapie-resistant sheep; the CE increase was temporally regulated only in cells from scrapie-resistant animals, whose basal levels were restored after 72 h.
Several studies (1, 9, 17, 32, 35) have pointed out the essential role of cellular cholesterol for the proper folding and trafficking of PrPc, indicating that the conversion rate of PrPc into PrPsc may be modulated, at least in part, by cholesterol-homeostatic mechanisms. Conversely, PrPsc replication itself has been reported (34) to interfere with intracellular cholesterol metabolism and trafficking by displacing the cholesterol binding protein caveolin 1 from the membrane, thus suggesting that PrP perturbations may in turn exacerbate preexisting cholesterol alterations. In our study, sheep were infected in vivo, and the scrapie agent may have reached, in addition to the nervous and lymphatic systems, various host tissues, including the skin. As a matter of fact, Thomzig et al. showed PrPsc accumulation in the muscles (36) and, very recently, also in the skin (37) of experimentally scrapie-infected hamsters, as well as in naturally infected sheep.
We therefore suggest that abnormal cholesterol esterification could represent a phenotype predisposing a cell to the development of pathological processes involving abnormal activation, processing, and/or trafficking of membrane resident proteins and that cholesterol esterification inhibition may be a way to control disease progression.
In agreement with our findings, recent studies by Kovacs' group in models of Alzheimer's disease indicated a role for CE as modulators of the amyloidogenic processing of the amyloid precursor protein APP. Inhibition of CE by RNA interference-induced decrease of ACAT expression (18) or by a novel ACAT inhibitor, CP-113,818, prevented amyloid Aβ peptide generation (33) and led to more than 90% reduction of cerebral amyloid plaques in a mouse model of Alzheimer's disease (19).
Our previous (28, 29) and present findings have been the subject of U.S. patent applications (C. Anchisi, S. Dessì, P. La Colla, and A. Pani, U.S. patent application PCT/IT2007/000109; S. Dessì, P. La Colla, and A. Pani, U.S. patent application PCT/IT2007/000110), and further studies are already in progress to establish whether CE may truly be a target of clinical interest, as well as a biological marker of disease susceptibility applicable to prion diseases and to other protein-based neurodegenerative pathologies.
Acknowledgments
We are indebted to Ciriaco Ligios (Istituto Zooprofilattico Sperimentale, Sassari, Italy) and Byron Caughey (Rocky Mountain Laboratories, NIAID, NIH) for providing sheep samples and mouse neuroblastoma cell lines.
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Abid, K., and C. Soto. 2006. The intriguing prion disorders. Cell. Mol. Life Sci. 63:2342-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguzzi, A., M. Heikenwalder, and G. Miele. 2004. Progress and problems in the biology, diagnostics, and therapeutics of prion diseases. J. Clin. Investig. 114:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bate, C., M. Salmona, L. Diomede, and A. Williams. 2004. Squalestatin cures prion-infected neurons and protects against prion neurotoxicity. J. Biol. Chem. 279:14983-14990. [DOI] [PubMed] [Google Scholar]
- 4.Batetta, B., M. F. Mulas, F. Sanna, M. Putzolu, R. R. Bonatesta, A. Casperi-Campani, L. Roncuzzi, D. Baiocchi, and S. Dessì. 2003. Role of cholesterol ester pathway in the control of cell cycle in human aortic smooth muscle cells. FASEB J. 17:746-748. [DOI] [PubMed] [Google Scholar]
- 5.Cashman, N. R., and B. Caughey. 2005. Prion diseases—close to effective therapy? Nat. Rev. Drug Disc. 3:874-884. [DOI] [PubMed] [Google Scholar]
- 6.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collinge, J., J. Whitfield, E. McKintosh, J. Beck, S. Mead, D. J. Thomas, and M. P. Alpers. 2006. Kuru in the 21st century: an acquired human prion disease with very long incubation periods. Lancet 367:2068-2074. [DOI] [PubMed] [Google Scholar]
- 8.Collins, S. J., V. Lewis, M. Brazier, A. F. Hill, A. Fletcher, and C. L. Masters. 2002. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann. Neurol. 52:503-506. [DOI] [PubMed] [Google Scholar]
- 9.Critchley, P., J. Kazlauskaite, R. Eason, and T. J. Pinheiro. 2004. Binding of prion protein to lipid membranes. Biochem. Biophys. Res. Commun. 313:559-567. [DOI] [PubMed] [Google Scholar]
- 10.Debry, P., E. A. Nash, D. W. Neklason, and J. E. Metherall. 1997. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J. Biol. Chem. 272:1026-1031. [DOI] [PubMed] [Google Scholar]
- 11.De Medina, P., N. Boubekeur, P. Balaguer, G. Favre, S. Silvente-Poirot, and M. Poirot. 2006. The prototypical inhibitor of cholesterol esterification, Sah 58-035[3-[decyldimethylsilyl]-n-[2-(4-methylphenyl)-1-phenylethyl] propanamide], is an agonist of estrogen receptors. J. Pharmacol. Exp. Ther. 319:139-149. [DOI] [PubMed] [Google Scholar]
- 12.Denizot, F., and R. Lang. 1986. Rapid colorimetric assay for cell growth and survival. J. Immunol. Methods 89:271-277. [DOI] [PubMed] [Google Scholar]
- 13.Dichek, H. L., N. Agrawal, N. El Andaloussi, and K. Qian. 2006. Attenuated corticosterone response to chronic ACTH stimulation in hepatic lipase-deficient mice: evidence for a role for hepatic lipase in adrenal physiology. Am. J. Physiol. Endocrinol. Metab. 290:908-915. [DOI] [PubMed] [Google Scholar]
- 14.Doh-Ura, K., K. Ishikawa, I. Murakami-Kubo, K. Sasaki, S. Mohri, R. Race, and T. Iwaki. 2004. Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models. J. Virol. 78:4999-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman, D. A., and A. Romero. 2003. Effects of troglitazone on intracellular cholesterol distribution and cholesterol-dependent cell functions in MA-10 Leydig tumor cells. Biochem. Pharmacol. 66:307-313. [DOI] [PubMed] [Google Scholar]
- 16.Galantuomo, M., M. F. Mulas, P. Baire, C. Abete, E. Peiretti, S. Dessì, and M. Fossarello. 2005. Proliferative activity and cholesterol ester metabolism in primary culture of human pterygium fibroblasts. Investig. Ophthalmol. Vis. Sci. 46:3982-3985. [Google Scholar]
- 17.Gilch, S., C. Kehler, and H. M. Schatzl. 2006. The prion protein requires cholesterol for cell surface localization. Mol. Cell Neurosci. 31:346-353. [DOI] [PubMed] [Google Scholar]
- 18.Huttunen, H. J., C. Greco, and D. M. Kovacs. 2007. Knockdown of ACAT-1 reduces amyloidogenic processing of APP. FEBS Lett. 581:1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutter-Paier, B., H. J. Huttunen, L. Puglielli, C. B. Eckman, D. Y. Kim, A. Hofmeister, R. D. Moir, S. B. Domnitz, M. P. Frosch, M. Windisch, and D. M. Kovacs. 2004. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron 44:227-238. [DOI] [PubMed] [Google Scholar]
- 20.Kirchner, G. I., I. Meier-Wiedenbach, and M. P Manns. 2004. Clinical pharmacokinetics of everolimus. Clin. Pharmacokinet. 43:83-95. [DOI] [PubMed] [Google Scholar]
- 21.Klingenstein, R., S. Lober, P. Kujala, S. Godsave, S. R. Leliveld, P. Gmeiner, P. J. Peters, and C. Korth. 2006. Tricyclic antidepressants, quinacrine and a novel, synthetic chimera thereof clear prions by destabilizing detergent-resistant membrane compartments. J. Neurochem. 98:748-759. [DOI] [PubMed] [Google Scholar]
- 22.Kocisko, D. A., G. S. Baron, R. Rubenstein, J. Chen, S. Kuizon, and B. Caughey. 2003. New inhibitors of scrapie-associated prion protein formation in a library of 2,000 drugs and natural products. J. Virol. 77:10288-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korth, C., B. C. May, F. E. Cohen, and S. B. Prusiner. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 98:9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieber, J. G., and R. M. Evans. 1996. Disruption of the vimentin intermediate filament system during adipose conversion of 3T3-L1 cells inhibits lipid droplet accumulation. J. Cell Sci. 109:3047-3058. [DOI] [PubMed] [Google Scholar]
- 25.May, B. C. H., C. Govaerts, and F. E. Cohen. 2006. Developing therapeutics for the diseases of prion misfolding. Neurology 66(Suppl. 1):s118-s122. [DOI] [PubMed] [Google Scholar]
- 26.Mead, S., S. P. Mahal, J. Beck, T. Campbell, M. Farral, E. Fisher, and J. Collinge. 2001. Sporadic- but not variant-Creutzfeldt-Jakob disease is associated with polymorphisms upstream of PRNP exon 1. Am. J. Hum. Genet. 69:1225-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metherall, J. E., K. Waugh, and H. Li. 1996. Progesterone inhibits cholesterol biosynthesis in cultured cells. Accumulation of cholesterol precursors. J. Biol. Chem. 271:2627-2633. [DOI] [PubMed] [Google Scholar]
- 28.Pani, A., C. Abete, C. Norfo, C. Mulas, M. Putzolu, S. Laconi, M. D. Cannas, C. D. Orrù, P. La Colla, and S. Dessì. 2007. Cholesterol metabolism in brain and skin fibroblasts from Sarda breed sheep with scrapie resistant or susceptible genotype. Am. J. Infect. Dis. 3:143-150. [Google Scholar]
- 29.Pani, A., C. Abete, C. Norfo, C. Mulas, M. Putzolu, S. Laconi, C. D. Orrù, M. D. Cannas, S. Vascellari, P. La Colla, and S. Dessì. 2007. Accumulation of cholesterol esters in ex vivo lymphocytes from scrapie-susceptible sheep and in scrapie-infected mouse neuroblastoma cell lines. Am. J. Infect. Dis. 3:165-168. [Google Scholar]
- 30.Pani, A., B. Batetta, M. Putzolu, F. Sanna, O. Spano, S. Piras, M. F. Mulas, R. R. Bonatesta, C. Amat di San Filippo, L. Vargiu, T. Marceddu, L. Sanna, P. La Colla, and S. Dessì. 2000. MDR1, cholesterol esterification and cell growth: a comparative study in normal and multidrug-resistant KB cell lines. Cell. Mol. Life Sci. 57:1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pani, A., and S. Dessì (ed.). 2004. Cell growth and cholesterol esters. Kluwer Academic Press, New York, NY.
- 32.Prado, A. M., J. A. Silva, A. C. Magalhaes, V. F. Prado, R. Linden, V. R. Martins, and R. R. Brentani. 2004. PrPc on the road: trafficking of the cellular prion protein. J. Neurochem. 88:769-781. [DOI] [PubMed] [Google Scholar]
- 33.Puglielli, L., G. Konopka, E. Pack-Chung, L. A. Ingano, O. Berezovska, B. T. Hyman, T. Y. Chang, R. E. Tanzi, and D. M. Kovacs. 2001. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat. Cell. Biol. 3:905-912. [DOI] [PubMed] [Google Scholar]
- 34.Russelakis-Carneiro, M., C. Hetz, K. Maundrell, and C. Soto. 2004. Prion replication alters the distribution of synaptophysin and caveolin 1 in neuronal lipid rafts. Am. J. Pathol. 165:1839-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taraboulos, A., M. Scott, A. Semenov, D. Avrahami, L. Laszlo, and S. B. Prusiner. 1995. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 129:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomzig, A., C. Kratzel, G. Lenz, D. Kruger, and M. Beekes. 2003. Widespread PrPsc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 4:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomzig, A., W. Schulz-Schaeffer, A. Wrede, W. Wemheuer, B. Brenig, C. Kratzel, K. Lemmer, and M. Beekes. 2007. Accumulation of pathological prion protein PrPsc in the skin of animals with experimental and natural scrapie. PLoS Pathog. 3:659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trevitt, C. R., and J Collinge. 2006. A systemtic review of prion therapeutics in experimental models. Brain 129:2241-2265. [DOI] [PubMed] [Google Scholar]
- 39.Whittle, I. R., R. S. Knight, and R. G. Will. 2006. Unsuccessful intraventricular pentosan polysulphate treatment of variant Creutzfeldt-Jakob disease. Acta Neurochir. 48:677-679. [DOI] [PubMed] [Google Scholar]