Abstract
Pseudomonas aeruginosa causes vision-threatening keratitis and is difficult to treat due to emerging resistance. Human β-defensin 2 (hBD-2) is an antimicrobial peptide expressed by ocular surface epithelia with broad-spectrum activity against various pathogens, including P. aeruginosa. The activity of hBD-2 against P. aeruginosa in the presence of human tears or NaCl was studied. In some experiments, tears were heat-inactivated, filtered, and separated into cationic/anionic fractions or mucin MUC5AC was removed by immunoprecipitation before use. Immunoprecipitation was performed to study the interaction between hBD-2 and MUC5AC. hBD-2 activity was reduced by 40 to 90% in the presence of 17.5 to 70% (vol/vol) tears. NaCl reduced hBD-2 activity, but at most it could account for only 36% of the inhibitory effect of tears. Heat inactivation and filtration attenuated the ability of tears to inhibit hBD-2 activity by 65 and 68%, respectively. Anionic tear fractions significantly reduced (86%) the activity of hBD-2, whereas only a 22% reduction was observed with the cationic fractions. In the absence of MUC5AC, the activity of hBD-2 was restored by 64%. Immunoprecipitation studies suggested that the loss of hBD-2 activity in tears is due to a direct binding interaction with MUC5AC. Our data showed that the antimicrobial activity of hBD-2 is sensitive to the presence of human tears and that this is partly due to the salt content and also the presence of MUC5AC. These data cast doubt on the effectiveness of hBD-2 as an antimicrobial peptide, and additional studies are required to conclusively elucidate its role in innate immunity at the ocular surface in vivo.
Defensins constitute a major peptide family found in nature that have broad-spectrum antimicrobial properties, being active against many gram-positive and gram-negative bacteria and some fungi and viruses (18, 19, 58). Defensins are widely distributed throughout vertebrate species and are characterized by the presence of three intramolecular cysteine disulfide bonds and a β-sheet structure. Two classes of human defensins, referred to as classes α and β, have been identified according to the placement pattern and connectivity of the cysteine residues. α-Defensins were first found in neutrophils and are also produced by Paneth cells in the intestine, while β-defensins are expressed by epithelial tissues (17). This pattern of expression is in keeping with a role of these peptides in host defense. Defensins are believed to achieve their antimicrobial effect by creating pores or otherwise disrupting the cell membrane of target organisms, leading to the release of their cellular contents (28, 68). In addition to their antimicrobial effects, defensins have been shown to act as regulatory factors. For example, they enhance epithelial wound closure (1) and stimulate epithelial cell and fibroblast proliferation (41) and chemotaxis of T cells and dendritic cells (8, 70) and of monocytes (63). Defensins also modulate cytokine production in various cell types (7, 67) and simulate histamine release from mast cells (5, 46).
Human β-defensin-2 (hBD-2) has been identified at the ocular surface (34, 35, 43), and we have recently reported that it exerts activity against common ocular microbial pathogens in in vitro experiments conducted with 10 mM phosphate buffer (30). The expression of hBD-2 can be modulated; for example, it has been shown that hBD-2 expression by human corneal epithelial cells is upregulated by exposure to bacterial products such as lipopolysaccharide (37). Also, we have reported that the expression of hBD-2 mRNA and peptide is upregulated in the regeneration of the corneal epithelium during wound healing (34). We have also shown that the expression and secretion of hBD-2 by corneal and conjunctival epithelial cells are upregulated by inflammatory cytokines (35, 43). Furthermore, hBD-2 has been found to stimulate human corneal epithelial cell migration (52) and proliferation (A. M. McDermott, unpublished data). These in vitro findings suggest that in vivo hBD-2 may have an important role as an antimicrobial peptide at the ocular surface and may also be involved in the process of epithelial wound healing.
As multifunctional antimicrobial peptides, defensins and related molecules have been suggested to be potential candidates for novel pharmaceutical agents which may both protect against infection and accelerate epithelial wound healing (11, 38, 45). However, the protective function of antimicrobial peptides in vivo has been brought into question by observations that these peptides are relatively easily inactivated. It has been shown that the antimicrobial activities of most β-defensins are attenuated in the presence of physiological salt concentrations (4, 21, 24, 60). Evidence from one study has also shown that certain β-defensins are susceptible to degradation and inactivation by proteases in the airway surface fluids from cystic fibrosis patients (62). It has also been reported that certain proteins known to be present in the tear film may compromise the expected antimicrobial activities of α-defensins by complex formation (50, 57). Of note, a recent study reported that the in vitro antibacterial activity of a rabbit α-defensin was diminished in human tears and indicated that the loss of activity was due not only to salt but also to unidentified components of the tear film (36).
To gain insight into the effectiveness of hBD-2, whether it is endogenously expressed or exogenously applied as a novel pharmaceutical agent, the primary goal of this study was to investigate its activity against Pseudomonas aeruginosa (the leading cause of bacterial corneal infection, most commonly associated with extended contact lens wear) under more physiological conditions than those tested previously and to study the mechanisms by which the peptide may be inactivated in the presence of human tears in vitro.
MATERIALS AND METHODS
Tear sample collection from human subjects.
All procedures involving human subjects were performed with the approval of the University of Houston Institutional Review Board and in accordance with the tenets of the Declaration of Helsinki regarding research involving human subjects. All subjects had a complete optometric examination at the University Eye Institute (University of Houston) and were found to be free of any ocular surface disease. Fourteen subjects (eight males, six females; age range, 26 to 45 years) took part in this study; informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study. Unstimulated tears were collected from the inferior tear meniscus by using 5-μl microcapillary tubes (Drummond Scientific, Broomall, PA). Anesthetization of the ocular surface can lead to a reduction in tear production; therefore, tears were collected without the use of anesthetics (23). The osmolality of each sample was measured by using a vapor pressure osmometer (Vapro 5520; Wescor, Logan, UT). A total of 80 to 100 μl of tears was collected from each subject over a total of three visits spaced 2 to 3 days apart. Tear samples were stored at −80°C until analysis.
Preparation of Pseudomonas aeruginosa.
Pseudomonas aeruginosa (strains ATCC 27853 and ATCC 19660 and two clinical isolates from corneal scrapings of patients with bacterial keratitis) were tested in this study. ATCC 27853 is known to invade the cornea, while ATCC 19660 has been characterized as a cytotoxic strain; both strains are capable of inducing severe ocular infection in experimentally infected animal models of bacterial keratitis (12, 14, 27, 47, 51). The majority of the studies were carried out with ATCC 27853, and selected experiments were repeated with ATCC 19660 and the two clinically isolated P. aeruginosa strains. A single isolated P. aeruginosa colony was used to inoculate 5 ml of nutrient broth (NB) overnight at 37°C. Fifty microliters of this bacterial suspension was used to inoculate 50 ml of fresh NB, which was then incubated for 2.5 h with vigorous shaking at 37°C to achieve mid-log-phase growth. Twenty-five milliliters of the warm P. aeruginosa culture was centrifuged at 3,100 × g for 10 min, and the bacterial cell pellet was resuspended in phosphate buffer (PB; 8.2 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4). The optical density of the suspension was adjusted to 0.2 at 620 nm (approximately 107 CFU/ml) by adding an appropriate volume of PB.
Antimicrobial activity of hBD-2.
Synthetic hBD-2 peptide (Peninsula, San Carlos, CA) or recombinant hBD-2 peptide (Peprotech Inc., Rocky Hill, NJ) was dissolved in 0.01% acetic acid at a concentration of 1 mg/ml and stored at −20°C. The antimicrobial assay procedure was adapted from that described previously (22). Reaction mixtures (final volumes, 50 μl) containing 10 μl of 107 CFU/ml P. aeruginosa and 5 μl hBD-2 diluted in PB (final concentrations, 0.05, 0.1, 0.5, 1, 10, 25, 50, 75, 100, and 200 μg/ml) were incubated at 37°C for 2 h with vigorous shaking. In each experiment, reaction mixtures containing 5 μl of 0.01% acetic acid, the vehicle for the dilution of hBD-2, acted as a control. Additionally, in each experiment, the susceptibility of the organism to 5 μl of a 0.3% solution of ciprofloxacin HCl ophthalmic solution (Ciloxan; Alcon, Fort Worth, TX), a topical agent commonly prescribed for the treatment of ocular P. aeruginosa infections, was tested. At the end of the incubation, serial dilutions of each reaction mixture were used to inoculate NB agar plates. Samples (10 μl) were spread evenly over the surface of the plates by using sterile glass spreaders. After incubation at 37°C for 24 h, the agar plates were placed on a light board, and a digital image was captured with an Alpha Imager documentation system (Alpha Innotec, San Leandro, CA). The number of colonies was counted by using the colony count software of the Alpha Imager.
In vitro antimicrobial activity of hBD-2 in the presence of human tears and NaCl.
Antimicrobial assays were performed with 100 μg/ml hBD-2, which killed all of the organisms under the assay conditions used. The osmolalities of the reaction mixtures were measured with a vapor pressure osmometer (Vapro 5520; Wescor). Tears were diluted in PB to give final reaction mixtures containing 17.5% (vol/vol) tears (82 ± 10 mOsm/kg), 35% (vol/vol) tears (125 ± 20 mOsm/kg), and 70% (vol/vol) tears (228 ± 31 mOsm/kg). Due to the other constituents of the reaction mixture, 70% (vol/vol) was the maximum tear concentration obtainable in these experiments. NaCl solutions were diluted in PB to give final reaction mixtures with osmolalities of 89 mOsm/kg, 110 mOsm/kg, 212 mOsm/kg, and 303 mOsm/kg. These values approximately matched the osmolality conditions of the tear experiments, with the highest osmolality (303 mOsm/kg) matching that of undiluted tears (302 ± 11 mOsm/kg).
To further investigate the effects of tears on hBD-2 activity, a series of other experiments was performed. Reaction mixtures containing 35% (vol/vol) tears were incubated at 37°C for 15 min, 30 min, 45 min, 1 h, and 2 h. In some experiments, tears were heat inactivated by incubation at 65°C for 30 min before they were added to the reaction mixtures. In other experiments, tears were filtered through a Microcon-YM-50 (50-kDa cutoff) centrifugal filter device (Millipore, Billerica, MA) by centrifugation at 14,000 × g and 4°C for 12 min before they were added to the reaction mixtures. In separate experiments, tears were also separated into cationic or anionic fractions through Vivapure anionic or cationic ion-exchange spin columns (VivaScience, Carlsbad, CA), according to the manufacturer's instructions. Serial dilutions of each reaction mixture were used to inoculate NB agar plates, which were then incubated at 37°C for 24 h as described above. After the number of P. aeruginosa colonies on the NB agar plates was counted, data were expressed as the percentage of bacteria killed in the presence of tears or NaCl. The Wilcoxon signed-ranks test was used to compare data collected from the tears and NaCl experiments, with a P value of <0.05 considered significant.
IP.
Tears (50 to 100 μl) with or without added hBD-2 (100 μg/ml) were preincubated at 37°C for 2 h and were then incubated with 15 μl goat anti-human MUC5AC antiserum (donated by Marcia Jumblatt, University of Louisville), preimmune goat or rabbit serum, or 2.5 μg rabbit anti-human hBD-2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4°C. The antibodies were precipitated by addition of 10 μl of Protein A/G PLUS agarose beads (Santa Cruz Biotechnology) and end-over-end incubation overnight at 4°C. Immunoprecipitates were collected by centrifugation at 1,000 × g for 3 min at 4°C. The pellets were washed four times with phosphate-buffered saline (Invitrogen, Carlsbad, CA), with the centrifugation step repeated each time. After the final wash, the immunoprecipitated proteins were resuspended in electrophoresis sample buffer and analyzed, along with nontreated tear samples and residual tear fractions after immunoprecipitation (IP), by Western blotting.
Western blotting for hBD-2 and MUC5AC.
Untreated tears and antimicrobial assay reaction mixtures collected at the end of the 2-h incubation were analyzed for hBD-2. Five microliters of tears or reaction mixtures was mixed with 5 μl of sample buffer (Bio-Rad, Life Science, Hercules, CA) and separated on 16.5% Tris-Tricine polyacrylamide gels with a 4% stacking gel. The peptides were then transferred to Immobilon-P membranes (Millipore). The membranes were blocked with 5% BLOTTO and then incubated with a rabbit polyclonal hBD-2 antibody (1:5,000; donated by Tomas Ganz, UCLA) overnight at 4°C, washed, and subsequently incubated (1 h, room temperature) with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:10,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). hBD-2 was visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Untreated tears and immunoprecipitated samples were analyzed for MUC5AC, based on a protocol described by Jumblatt et al. (32). All samples and standards were boiled for 5 min before they were loaded onto 4 to 15% polyacrylamide gradient gels (Bio-Rad). The gels were run for 1 h at 100 mV and electroblotted onto nitrocellulose membranes (Sigma, St. Louis, MO). The blots were blocked overnight at 4°C with Tris-buffered saline containing 3% dried milk and 1% bovine serum albumin and were then incubated with the anti-MUC5AC antiserum (1:2,500) and subsequently incubated (1 h, room temperature) with horseradish peroxidase-conjugated rabbit anti-goat secondary antibody (1:20,000; DAKO, Carpinteria, CA). The immunoreactivity of MUC5AC was visualized by enhanced chemiluminescence.
RESULTS
Activity of hBD-2 against Pseudomonas aeruginosa in the presence of human tears and NaCl.
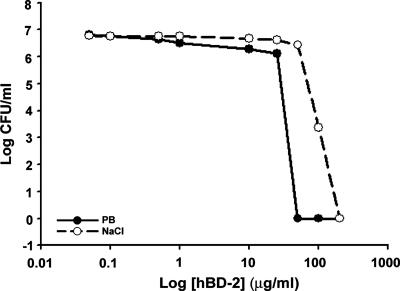
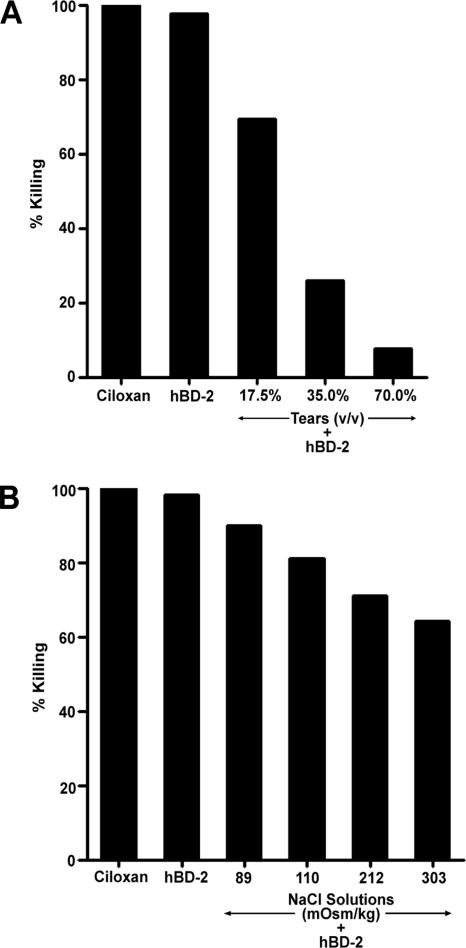
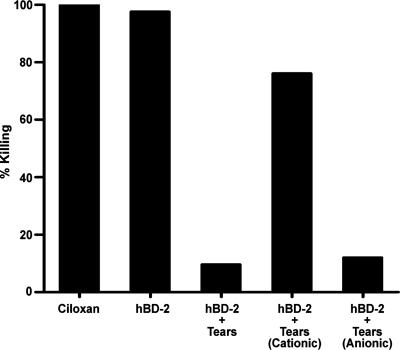
Antimicrobial activity assays were performed to compare the activity of hBD-2 against P. aeruginosa (ATCC 27853) under our previously described conditions (30) with that in the presence of tear fluid, a more physiologically relevant condition. As shown in Fig. 1, hBD-2 inhibited the growth of P. aeruginosa in a concentration-dependent manner; high concentrations of hBD-2 (50 to 200 μg/ml) completely killed P. aeruginosa and were as effective as the ciprofloxacin HCl ophthalmic solution. When the activity was tested in the presence of 150 mM NaCl (Fig. 1), the activity of hBD-2 was considerably impaired, with the 50% effective concentration (EC50) for killing P. aeruginosa being increased from 2.5 ± 1.0 (n = 3) to 32.5 ± 1.2 μg/ml (n = 3). NaCl alone did not inhibit the growth of P. aeruginosa (data not shown). When the activity was tested in the presence of tears, the antibacterial activity of 100 μg/ml hBD-2 was markedly reduced. Figure 2A summarizes the average data for tears from five different subjects. Tears alone were unable to inhibit the growth of all strains of P. aeruginosa tested (data not shown). With a reaction mixture containing P. aeruginosa (ATCC 27853) and 17.5% (vol/vol) tears (82 ± 10 mOsm/kg), 35% (vol/vol) tears (125 ± 20 mOsm/kg), or 70% (vol/vol) tears (228 ± 31 mOsm/kg), the effectiveness of hBD-2 was reduced by 39.5% (15 to 76%), 72.4% (51 to 93%), and 90.3% (76 to 99%), respectively, with the values in parentheses showing the range of the data from five different subjects. Similar results were obtained when the experiments were repeated with the three other P. aeruginosa strains and 70% (vol/vol) tears (n = 3). Incubations carried out for different lengths of time showed that the loss of hBD-2 activity against P. aeruginosa (ATCC 27853) occurred within 15 min, the shortest time tested (data not shown).
FIG. 1.
Bactericidal effect of hBD-2 against Pseudomonas aeruginosa. A total of 107 CFU/ml P. aeruginosa (ATCC 27853) was incubated with hBD-2 (0.05, 0.1, 1, 10, 25, 50, 75, 100, and 200 μg/ml) in the absence and presence of 150 mM NaCl for 2 h. The average data from three experiments are shown, and a concentration-dependent antimicrobial effect of hBD-2 is shown. The EC50 values were 2.5 ± 1.0 μg/ml and 32.5 ± 1.2 μg/ml for ATCC 27853 in the absence and presence of NaCl, respectively.
FIG. 2.
Antimicrobial activity of hBD-2 in the presence of human tears or NaCl solutions. Ciloxan, ciprofloxacin HCl ophthalmic solution. (A) A total of 107 CFU/ml P. aeruginosa (ATCC 27853) was incubated with 0.01% acetic acid, hBD-2 (100 μg/ml), or hBD-2 (100 μg/ml) and tears (17.5%, 35%, or 70% [vol/vol]) at 37°C for 2 h. Mean data, expressed as the percentage of bacteria killed by hBD-2 in tears obtained from five subjects, are shown. (B) A total of 107 CFU/ml P. aeruginosa (ATCC 27853) was incubated with 0.01% acetic acid, hBD-2 (100 μg/ml), or hBD-2 (100 μg/ml) and NaCl solutions at 37°C for 2 h. Average data from three experiments, expressed as the percentage of bacteria killed by hBD-2 in the presence of NaCl, are shown.
Having shown that the presence of tears leads to the loss of hBD-2 antibacterial activity, we examined if the NaCl within the tears is the major factor limiting the activity of hBD-2. As the NaCl concentration in tears is highly correlated with tear osmolality (10), we tested the activity of hBD-2 in the presence of reaction mixtures containing NaCl with osmolalities corresponding to those used in the tear experiments. As shown in Fig. 2B, NaCl-containing reaction mixtures of 89, 110, and 212 mOsm/kg inhibited the effect of hBD-2 by 8.7% (6.3 to 11.4%), 19.5% (14.8 to 23.5%), and 28.1% (24.6 to 33.8%), respectively (n = 5). Reaction mixtures containing NaCl solutions at an osmolality of 303 mOsm/kg, approximately equivalent to that of tears (mean tear osmolality of undiluted tears from five subjects, 302 ± 11 mOsm/kg), caused a 36% (27.1 to 44.6%) reduction in the antibacterial activity of hBD-2. Comparable findings were obtained when the experiments were repeated with the three other P. aeruginosa strains and 303 mOsm/kg (n = 3 per P. aeruginosa strain). Therefore, the salt content of tears could account for only 30 to 40% of the inhibitory effect of tears on the activity of hBD-2 against P. aeruginosa. Direct comparison between the tear and NaCl experiments (matched pairs, based on the mean osmolality of the reaction mixtures) indicated that the inhibitory effect of NaCl on hBD-2-mediated bacterial killing was significantly smaller than that obtained with tears (P = 0.005; Wilcoxon signed-ranks test).
hBD-2 is not degraded in tears.
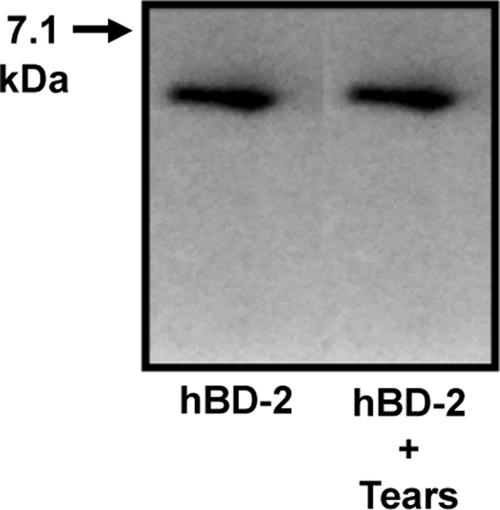
To determine if the loss of the antimicrobial activity of hBD-2 was due to degradation in the presence of tears, some reaction mixtures were analyzed by Western blotting at the end of the 2-h incubation. As seen in Fig. 3, there was no evidence of the degradation of hBD-2 in reaction mixtures containing tears (n = 3).
FIG. 3.
hBD-2 is not degraded in tears. Western blotting for hBD-2 was performed by using synthetic peptide (hBD-2) and reaction mixtures containing hBD-2 and tears (hBD-2 + Tears). Representative data from one of three subjects are shown.
Tear mucin MUC5AC interferes with the activity of hBD-2 against Pseudomonas aeruginosa.
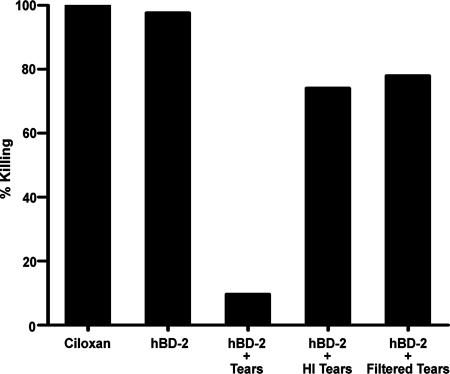
As the loss of antimicrobial activity of hBD-2 was not due to degradation in the presence of tears, we investigated other contributing factors by heat inactivating and filtering the tears to separate tear components into fractions according to size or charge. As seen in Fig. 4, there was markedly less of a reduction in the antibacterial activity of hBD-2 in the presence of 70% (vol/vol) heat-inactivated tears (24.0% reduction) compared to the activity of non-heat-inactivated tears (88.0% reduction) (n = 5). When filtered tears (with all molecules greater than 50 kDa eliminated) were used, the antibacterial activity of hBD-2 was reduced by only 20% (n = 5). As shown in Fig. 5, in reaction mixtures containing 70% (vol/vol) anionic tear fraction, the effectiveness of hBD-2 was reduced by 86.0%; however, when the cationic tear fraction was used, only a 22% reduction in the antibacterial activity of hBD-2 was observed (n = 3). These observations suggest that tears contain an anionic, heat-sensitive constituent greater than 50 kDa which is also (i.e., in addition to the salt content) responsible for hBD-2 inactivation.
FIG. 4.
Effect of heat-inactivated or filtered human tears on antimicrobial activity of hBD-2. A total of 107 CFU/ml P. aeruginosa (ATCC 27853) was incubated with 0.3% ciprofloxacin HCl ophthalmic solution (Ciloxan), hBD-2 (100 μg/ml), hBD-2 with 70% vol/vol tears (hBD-2 + Tears), hBD-2 with 70% (vol/vol) heat-inactivated tears (hBD-2 + HI Tears), or hBD-2 with 70% (vol/vol) filtered tears (hBD-2 + Filtered Tears) at 37°C for 2 h. Mean data from five experiments, expressed as the percentage of bacteria killed by hBD-2, are shown.
FIG. 5.
Antimicrobial activity of hBD-2 in the presence of cationic and anionic tear fractions. A total of 107 CFU/ml P. aeruginosa (ATCC 27853) was incubated with 0.3% ciprofloxacin HCl ophthalmic solution (Ciloxan), hBD-2 (100 μg/ml), hBD-2 with 70% (vol/vol) tears (hBD-2 + Tears), hBD-2 with 70% (vol/vol) cationic tear fraction [hBD-2 + Tears (Cationic)], or hBD-2 with 70% (vol/vol) anionic tear fraction [hBD-2 + Tears (Anionic)] at 37°C for 2 h. Mean data obtained from three experiments, expressed as the percentage of bacteria killed by hBD-2, are shown.
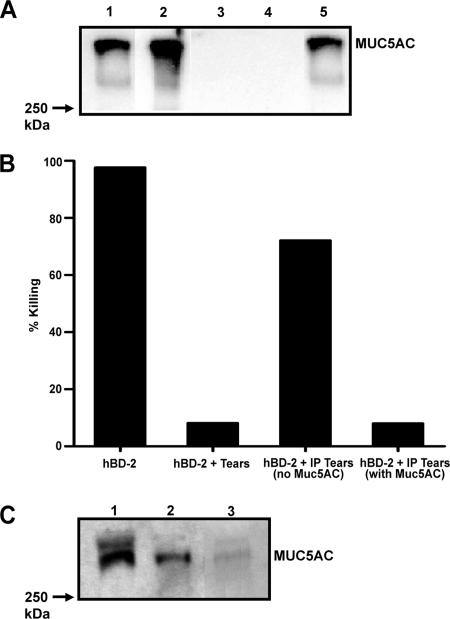
A previous study from our laboratory (29) indicated that an anionic mucin-like polymer, carboxymethyl cellulose, attenuated the antibacterial activity of hBD-2. Human tear fluid contains a number of secretory mucins, with MUC5AC being the major soluble form present (32). To determine if the MUC5AC present in tears was responsible for the loss of activity of hBD-2, MUC5AC was removed from tears by IP. As shown in Fig. 6A, MUC5AC was detected in both nontreated tears (lane 1) and the anti-MUC5AC-immunoprecipitated pellet (lane 2), whereas no MUC5AC immunoreactivity was present in the control IP with preimmune goat serum (lane 4). Antimicrobial assays were then performed with tears with or without MUC5AC. As shown in Fig. 6B, the activity of hBD-2 against P. aeruginosa (ATCC 27853) was restored by 64% in the absence of MUC5AC (n = 3), whereas MUC5AC-containing tears (either untreated tears or the residual tears from the IP with goat preimmune serum) almost completely (90 to 95%) impaired the activity of hBD-2 against P. aeruginosa (n = 3). Comparable findings were obtained with another laboratory strain (ATCC 19660) and two clinically isolated strains of P. aeruginosa (data not shown). IP was also performed to study the interaction between hBD-2 and MUC5AC in tears. Previously, it has been demonstrated that MUC5AC is capable of binding to cysteine-rich peptides, such as the trefoil proteins (54). To investigate if there is a direct interaction between hBD-2 and MUC5AC, exogenously added hBD-2 was immunoprecipitated from tears, followed by Western blotting with antiserum against MUC5AC. Figure 6C demonstrates the detection of MUC5AC immunoreactivity in the hBD-2 immunoprecipitate (lane 2), which suggests that a direct binding interaction between hBD-2 and MUC5AC occurs. In the absence of hBD-2, the immunoreactivity of MUC5AC was not detected in the immunoprecipitate (data not shown).
FIG. 6.
(A) IP of mucin MUC5AC. Tears (100 μl) were immunoprecipitated with anti-MUC5AC antiserum or goat preimmune serum and electrophoresed on a 4 to 15% gradient gel for MUC5AC immunodetection. Lane 1, nontreated tears; lane 2, anti-MUC5AC antiserum immunoprecipitate; lane 3 residual tears after anti-MUC5AC IP; lane 4, preimmune goat serum immunoprecipitated pellet; lane 5, residual tears after preimmune goat serum IP. Representative data from one of three experiments are shown. (B) Antimicrobial activity of hBD-2 in tears in the presence and absence of MUC5AC. A total of 107 CFU/ml P. aeruginosa (ATCC 27853) was incubated with hBD-2 (100 μg/ml), hBD-2 with 70% (vol/vol) tears (hBD-2 + Tears), hBD-2 with 70% (vol/vol) anti-MUC5AC immunoprecipitated tears [hBD-2 + IP Tears (no MUC5AC)], or hBD-2 with 70% (vol/vol) goat preimmune serum immunoprecipitated tears [hBD-2 + IP tears (with MUC5AC)] at 37°C for 2 h. Mean data from five experiments, expressed as the percentage of bacteria killed by hBD-2, are shown. (C) Direct interaction of hBD-2 and MUC5AC in human tears. Tears (50 μl) preincubated with hBD-2 (100 μg/ml) were immunoprecipitated with anti-hBD-2 polycolonal antibody or rabbit serum and electrophoresed on a 4 to 15% gradient gel for MUC5AC immunodetection. Lane 1, nontreated tears; lane 2, anti-hBD-2 antibody immunoprecipitated pellet; lane 3, rabbit serum immunoprecipitated pellet.
DISCUSSION
β-Defensins are produced endogenously by ocular surface epithelial cells, and notably, hBD-2 is upregulated in response to infection and inflammation (35, 37). Although β-defensins are described as natural antibiotics (16, 71) against both gram-positive and -negative bacteria, the in vitro antimicrobial activities of some β-defensins, including that of hBD-2, are sensitive to a high-salt environment (4, 60). As the salt content of human tears may hinder the activity of hBD-2, we studied the activity of this antimicrobial peptide against a common ocular pathogen under physiological salt conditions (150 mM NaCl) comparable to those in tear fluid (66). The current study showed that hBD-2 alone was effective in killing P. aeruginosa (ATCC 27853), with an EC50 of 2.5 μg/ml. Previously, Singh et al. (60) reported the EC50 for recombinant hBD-2 against P. aeruginosa (PAO1) to be 100 ng/ml, and Harder et al. (25) found the 50% lethal dose of an hBD-2 peptide purified from psoriatic scale extract against Escherichia coli and P. aeruginosa (ATCC 27853) to be near 10 μg/ml. In keeping with the results of other studies (4, 59, 60), we have also observed a reduction in the antimicrobial activity of hBD-2 with increasing salt concentrations, with an EC50 of 32.5 μg/ml in the presence of physiological NaCl concentrations.
We next investigated the activity of hBD-2 against P. aeruginosa in the presence and absence of human tears. Previously, Fleiszig et al. reported that tears were unable to retard the growth of some P. aeruginosa strains, findings suggesting that the antimicrobial components of the tear film are generally not effective against P. aeruginosa (14). In keeping with this, tears did not retard the growth of P. aeruginosa ATCC 27853 or ATCC 19660 (comparable to the findings of Fleiszig et al. [14]) or the two clinical isolates tested in our study. hBD-2 was not detected in the tears from our subjects (data not shown). Similarly, Zhou et al. have also reported the absence of β-defensins in human tears (72). This is to be expected, as the peptide is significantly expressed only during infection and inflammation and after injury (34). It should be noted that the levels of β-defensins detected in various tissues under both basal and inflammatory conditions are typically less than 1 μg/ml (26, 60, 65). Such concentrations are significantly lower than those required for antibacterial activity in vitro; however, some studies have suggested that local concentrating effects may allow the peptides to reach levels in situ that are compatible with effective antimicrobial activity (3, 48). The exact concentration of hBD-2 that can be achieved at the ocular surface (from epithelial cells of the cornea, the conjunctiva, and the lacrimal gland as possible sources) in vivo has yet to be determined, and it is unknown if the aforementioned local concentrating effects may contribute to raising the defensin concentration. For our experiments, we used a high concentration of the peptide (100 μg/ml) for the purpose of minimizing the expected effects of NaCl and other ionic tear constituents (64, 66) in order to investigate the effects of additional contributing factors in the tear fluid. We observed that tears almost completely abolished the effect of hBD-2 against P. aeruginosa in vitro. When the activity was tested in the presence of NaCl solution with an osmolality of 303 mOsm/kg (equivalent to the average osmolality of the tear film) (10), the activity of hBD-2 (100 μg/ml) was reduced by only 36%, therefore implying that additional factors present in the tears are responsible for hBD-2 inhibition. Other evidence that defensins lose their effect in biological fluids comes from a study in which Cole et al. reported that nasal mucus containing highly elevated levels of α-defensins and hBD-2 (collected from human subjects who were nasal carriers of Staphylococcus aureus or patients with acute rhinitis) failed to kill S. aureus in vitro or clear the colonizing bacteria in vivo (9). Similarly, McDermott et al. have recently reported that the microbicidal activity of a rabbit α-defensin, NP-3a, against P. aeruginosa was completely inhibited, although that of another defensin, NP-1, was only attenuated rather than completely blocked by human tears (36).
Defensins have previously been shown to act as substrates for bacterial and human matrix metalloproteinases (6, 69). For analysis of the possible degradation of hBD-2 in tears, we examined our samples by Western blotting. However, no evidence of degradation (neither a decrease in the signal nor the appearance of multiple bands) was observed, suggesting that the loss of hBD-2 activity is related to inactivation rather than degradation of the peptide. Our observation that the heat inactivation or the filtration of tears (which either eliminated all molecules greater than 50 kDa or removed negatively charged components from tears) was able to partially restore the antimicrobial activity of hBD-2 suggested that tears contain a heat-sensitive, anionic component larger than 50 kDa which, in addition to salt, is also responsible for hBD-2 inactivation.
Human tears are reported to be composed of greater than 500 components (15). Cationic antimicrobial peptides are known to interact with negatively charged macromolecules, and previous studies have shown that defensins can be inactivated by complex formation with large molecules such as the serpins, α1-proteinase inhibitor, α1-antichymotrypsin, and α2-macroglobulin (49, 50), all of which are known to be present in human tear fluid (2, 57). However, the concentrations of these proteins in tears (∼<0.1 μg/ml) are considerably lower than that required (3 to 4 μM or ∼200 μg/ml) to bind and neutralize the high concentration of hBD-2 used in our in vitro experiments (49, 50). In addition to the serpins, human tears contain other anionic molecules, including low-molecular-mass (17- to 18-kDa) prealbumin lipocalins and high-molecular-mass (>400- to 600-kDa) glycoprotein mucins (20, 53). Lipocalins are too small in terms of their molecular masses to be considered candidates as causes of hBD-2 inactivation, based on the data from the current study. Of note, results from our prior study indicated that carboxymethyl cellulose, an anionic mucoadhesive polymer commonly found in artificial tear solutions, interferes with the activity of hBD-2 against P. aeruginosa (29). Felgentreff et al. recently demonstrated that mucin binds to the cationic antimicrobial peptide cathelicidin (LL-37) and that this electrostatic interaction inhibits the activity of the peptide against P. aeruginosa (13).
Together, these findings point to mucins and, in particular, MUC5AC, which is the major gel-forming mucin in the tear film (32), as molecules possibly capable of influencing the activity of hBD-2. We observed that the antimicrobial activity of hBD-2 in tears was partially restored upon the elimination of MUC5AC, confirming a role for this mucin. Furthermore, an antibody to hBD-2 was able to immunoprecipitate MUC5AC out of tears, indicating a direct interaction between the two molecules.
The mechanism by which β-defensins achieve their antimicrobial effect requires the close proximity of the peptide to the bacterial surface through an electrostatic interaction, a process that can be interrupted by salt. Presumably, reversible binding of positively charged hBD-2 to negatively charged MUC5AC in tears can also disrupt this process, preventing the peptide from permeating the bacterial membrane. That heat-inactivated tears had a reduced ability to impair hBD-2 activity is attributable to this treatment that causes the dissociation of the negatively charged sialic acid residues from MUC5AC and denaturation of this glycoprotein (M. Berry, personal communication).
Overall, the inhibitory effects of MUC5AC and salt together accounted for an approximately 100% loss of the activity of hBD-2 in tears, indicating that these are the major constituents in tears that impaired the activity of hBD-2; however, this does not preclude the involvement of other tear components, such as those mentioned above, should they be present at high enough concentrations. As the interaction between hBD-2 and MUC5AC leads to a reduction in the antibacterial activity of hBD-2 in vitro, we speculate that this sequestering process will hinder bacterial killing by hBD-2 when it is applied exogenously onto the ocular surface in vivo. Indeed, such processes may have been responsible for the lack of effectiveness of synthetic antimicrobial peptides in a previous study of experimental bacterial keratitis in rabbits (33).
The antimicrobial activities of defensins in various tissues in vivo have been described. Mice deficient in matrilysin, a matrix metalloproteinase required for activation of mouse intestinal α-defensins, showed increased susceptibility to Salmonella enterica serovar Typhimurium infection (69). In addition, knockout of the mouse β-defensin 1 gene led to the delayed clearance of Haemophilus influenzae from the lung (40) and an increase in Staphylococcus colonization of the bladder (39). Salzman et al. also reported that transgenic mice expressing a human intestinal α-defensin showed increased resistance to S. enterica serovar Typhimurium (56). Collectively, these animal studies provide evidence that defensins are active in these tissues vivo.
The findings of the current study, however, bring into question the effectiveness of hBD-2, whether it is endogenously expressed or exogenously applied, as an antimicrobial peptide per se at the ocular surface in vivo. It has been proposed that the synergistic effects between antimicrobial peptides and other host defense proteins compensate for the low concentrations of peptides, and it has been demonstrated that their combined antimicrobial activities overcome the sensitivity to salt (42, 61). The full complement of antimicrobial peptides in and around the ocular surface is unknown, but we have observed that human corneal and conjunctival epithelial cells express three β-defensins (hBD-1, -2, and -3) (35, 43) and also cathelicidin LL-37/hCAP18 (31); thus, the potential for synergistic interactions among these peptides is high. However, it is uncertain if such an interaction is adequate to negate the effects of mucins in tears. Although data from the current study suggest that some antimicrobial peptides such as hBD-2 likely lose their effectiveness against microbial pathogens at the superficial ocular surface in vivo, it is possible that these peptides may still remain functionally active within the confines of the extracellular microenvironment of the corneal and conjunctival epithelia, where they are not subjected to tear components and local concentrating effects may operate.
One obvious extrapolation from the current study is that the MUC5AC within tears may reduce the activities of other cationic antimicrobial peptides; however, we have previously reported that human tears do not inhibit the activity of LL-37 (31) or hBD-3 (30), which suggests that not all antimicrobial peptides are susceptible to the effects of tears in terms of their antimicrobial functions. This is also supported by evidence from a recent study showing the differential effects of tears on two rabbit α-defensins (36). Collectively, these findings suggest that tear components selectively interact with certain peptides and that such an interaction may modulate their activities and lead to functional consequences.
An alternative interpretation is that an interaction between hBD-2 and MUC5AC may actually be advantageous, as defensins, in addition to being antimicrobial, are known to be chemotactic for various immune cells (8, 63, 70). One potential benefit for the “inactivation” of defensins by MUC5AC at the ocular surface is that it may prevent a prolonged chemotactic effect of the defensins, which may otherwise induce an excessive accumulation of immune cells, leading to a severe inflammatory response. Thus, mucins may act as reservoirs for the hBD-2 produced by epithelial cells in response to inflammation. The ability of MUC5AC to scavenge hBD-2 may therefore serve as a potential anti-inflammatory mechanism. Previous studies have also indicated that high concentrations of defensins can become cytotoxic to epithelial cells (44, 55); therefore, sequestration by MUC5AC may serve as a protective mechanism shielding the ocular surface from peptide-induced cytotoxicity.
The findings from the current study suggest that hBD-2 is unlikely to have significant direct antimicrobial activity in vivo, thus casting doubt on the role of this defensin in ocular surface innate immunity. However, it would be premature to strike other defensins or other cationic antimicrobial peptides from the list of potential therapeutic agents for ocular surface infections. As synergy exists among defensins, cathelicidin, and various antimicrobial factors known to be present in tear fluid, it is possible that these host defense molecules can act in tandem in the effort to maintain their activities at the ocular surface in vivo. The true therapeutic potential of cationic antimicrobial peptides will be apparent only when a complete understanding of their expression, interactions, and regulation has been gained.
Acknowledgments
We are very grateful to our subjects for donating their tears. We would like to sincerely thank Tomas Ganz of UCLA for antibodies to hBD-2, Marcia Jumblatt of University of Louisville for the antiserum to MUC5A, Bradley Mitchell of the Baylor College of Medicine for clinical isolates of P. aeruginosa, and William L. Miller of the University of Houston College of Optometry for assistance with examining the human subjects.
This work was supported by NIH grant EY13175 (to A.M.M.) and core grant P30 EY07751 to the University of Houston College of Optometry. L.C.H. was partially supported by a William C. Ezell Fellowship from the American Optometric Foundation.
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Aarbiou, J., R. M. Verhoosel, S. Van Wetering, W. I. De Boer, J. H. Van Krieken, S. V. Litvinov, K. F. Rabe, and P. S. Hiemstra. 2004. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am. J. Respir. Cell Mol. Biol. 30:193-201. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, C. S., T. McMahon, J. Sugar, L. Zhou, and B. Y. Yue. 1999. Levels of alpha1-proteinase inhibitor and alpha2-macroglobulin in the tear film of patients with keratoconus. Cornea 18:194-198. [DOI] [PubMed] [Google Scholar]
- 3.Akinbi, H. T., V. Narendran, A. K. Pass, P. Markart, and S. B. Hoath. 2004. Host defense proteins in vernix caseosa and amniotic fluid. Am. J. Obstet. Gynecol. 191:2090-2096. [DOI] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Befus, A. D., C. Mowat, M. Gilchrist, J. Hu, S. Solomon, and A. Bateman. 1999. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J. Immunol. 163:947-953. [PubMed] [Google Scholar]
- 6.Belas, R., J. Manos, and R. Suvanasuthi. 2004. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect. Immun. 72:5159-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaly, Y. V., E. M. Paleolog, T. S. Kolesnikova, I. I. Tikhonov, E. V. Petratchenko, and N. N. Voitenok. 2000. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Netw. 11:257-266. [PubMed] [Google Scholar]
- 8.Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. [DOI] [PubMed] [Google Scholar]
- 9.Cole, A. M., S. Tahk, A. Oren, D. Yoshioka, Y. H. Kim, A. Park, and T. Ganz. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin. Diagn. Lab. Immunol. 8:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig, J. P., P. A. Simmons, S. Patel, and A. Tomlinson. 1995. Refractive index and osmolality of human tears. Optom. Vis. Sci. 72:718-724. [DOI] [PubMed] [Google Scholar]
- 11.de Leeuw, E., and W. Lu. 2007. Human defensins: turning defense into offense? Infect. Disord. Drug Targets 7:67-70. [DOI] [PubMed] [Google Scholar]
- 12.Estrellas, P. S., Jr., L. G. Alionte, and J. A. Hobden. 2000. A Pseudomonas aeruginosa strain isolated from a contact lens-induced acute red eye (CLARE) is protease-deficient. Curr. Eye Res. 20:157-165. [PubMed] [Google Scholar]
- 13.Felgentreff, K., C. Beisswenger, M. Griese, T. Gulder, G. Bringmann, and R. Bals. 2006. The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides 27:3100-3106. [DOI] [PubMed] [Google Scholar]
- 14.Fleiszig, S. M., M. S. Kwong, and D. J. Evans. 2003. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun. 71:3866-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung, K., C. Morris, and M. Duncan. 2002. Mass spectrometric techniques applied to the analysis of human tears: a focus on the peptide and protein constituents. Adv. Exp. Med. Biol. 506:601-605. [DOI] [PubMed] [Google Scholar]
- 16.Gallo, R. L., M. Murakami, T. Ohtake, and M. Zaiou. 2002. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 110:823-831. [DOI] [PubMed] [Google Scholar]
- 17.Ganz, T., and R. I. Lehrer. 1998. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol. 10:41-44. [DOI] [PubMed] [Google Scholar]
- 18.Ganz, T., and R. I. Lehrer. 1995. Defensins. Pharmacol. Ther. 66:191-205. [DOI] [PubMed] [Google Scholar]
- 19.Ganz, T., M. E. Selsted, D. Szklarek, S. S. Harwig, K. Daher, D. F. Bainton, and R. I. Lehrer. 1985. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 76:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gipson, I. K. 2004. Distribution of mucins at the ocular surface. Exp. Eye Res. 78:379-388. [DOI] [PubMed] [Google Scholar]
- 21.Goldman, M. J., G. M. Anderson, E. D. Stolzenberg, U. P. Kari, M. Zasloff, and J. M. Wilson. 1997. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553-560. [DOI] [PubMed] [Google Scholar]
- 22.Gordon, Y. J., L. C. Huang, E. G. Romanowski, K. A. Yates, R. J. Proske, and A. M. McDermott. 2005. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 30:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta, A., T. Heigle, and S. C. Pflugfelder. 1997. Nasolacrimal stimulation of aqueous tear production. Cornea 16:645-648. [PubMed] [Google Scholar]
- 24.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 25.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 26.Haynes, R. J., J. E. McElveen, H. S. Dua, P. J. Tighe, and J. Liversidge. 2000. Expression of human beta-defensins in intraocular tissues. Investig. Ophthalmol. Vis. Sci. 41:3026-3031. [PubMed] [Google Scholar]
- 27.Hobden, J. A., S. A. Masinick, R. P. Barrett, and L. D. Hazlett. 1995. Aged mice fail to upregulate ICAM-1 after Pseudomonas aeruginosa corneal infection. Investig. Ophthalmol. Vis. Sci. 36:1107-1114. [PubMed] [Google Scholar]
- 28.Hoover, D. M., K. R. Rajashankar, R. Blumenthal, A. Puri, J. J. Oppenheim, O. Chertov, and J. Lubkowski. 2000. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J. Biol. Chem. 275:32911-32918. [DOI] [PubMed] [Google Scholar]
- 29.Huang, L. C., D. Jean, and A. M. McDermott. 2005. Effect of preservative-free artificial tears on the antimicrobial activity of human beta-defensin-2 and cathelicidin LL-37 in vitro. Eye Contact Lens 31:34-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, L. C., D. Jean, R. J. Proske, R. Y. Reins, and A. M. McDermott. 2007. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr. Eye Res. 32:595-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, L. C., T. D. Petkova, R. Y. Reins, R. J. Proske, and A. M. McDermott. 2006. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Investig. Ophthalmol. Vis. Sci. 47:2369-2380. [DOI] [PubMed] [Google Scholar]
- 32.Jumblatt, M. M., R. W. McKenzie, and J. E. Jumblatt. 1999. MUC5AC mucin is a component of the human precorneal tear film. Investig. Ophthalmol. Vis. Sci. 40:43-49. [PubMed] [Google Scholar]
- 33.Mannis, M. J. 2002. The use of antimicrobial peptides in ophthalmology: an experimental study in corneal preservation and the management of bacterial keratitis. Trans. Am. Ophthalmol. Soc. 100:243-271. [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott, A. M., R. L. Redfern, and B. Zhang. 2001. Human β-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr. Eye Res. 22:64-67. [DOI] [PubMed] [Google Scholar]
- 35.McDermott, A. M., R. L. Redfern, B. Zhang, Y. Pei, L. Huang, and R. J. Proske. 2003. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 44:1859-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott, A. M., D. Rich, J. Cullor, M. J. Mannis, W. Smith, T. Reid, and C. J. Murphy. 2006. The in vitro activity of selected defensins against an isolate of Pseudomonas in the presence of human tears. Br. J. Ophthalmol. 90:609-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamara, N. A., R. Van, O. S. Tuchin, and S. M. Fleiszig. 1999. Ocular surface epithelia express mRNA for human beta defensin-2. Exp. Eye Res. 69:483-490. [DOI] [PubMed] [Google Scholar]
- 38.Mookherjee, N., and R. E. Hancock. 2007. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 64:922-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, G., F. Kilanowski, D. Davidson, and J. Dorin. 2002. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect. Immun. 70:3053-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser, C., D. J. Weiner, E. Lysenko, R. Bals, J. N. Weiser, and J. M. Wilson. 2002. β-Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 70:3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, C. J., B. A. Foster, M. J. Mannis, M. E. Selsted, and T. W. Reid. 1993. Defensins are mitogenic for epithelial cells and fibroblasts. J. Cell. Physiol. 155:408-413. [DOI] [PubMed] [Google Scholar]
- 42.Nagaoka, I., S. Hirota, S. Yomogida, A. Ohwada, and M. Hirata. 2000. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 49:73-79. [DOI] [PubMed] [Google Scholar]
- 43.Narayanan, S., W. L. Miller, and A. M. McDermott. 2003. Expression of human beta-defensins in conjunctival epithelium: relevance to dry eye disease. Investig. Ophthalmol. Vis. Sci. 44:3795-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura, M., Y. Abiko, Y. Kurashige, M. Takeshima, M. Yamazaki, K. Kusano, M. Saitoh, K. Nakashima, T. Inoue, and T. Kaku. 2004. Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J. Dermatol. Sci. 36:87-95. [DOI] [PubMed] [Google Scholar]
- 45.Niyonsaba, F., I. Nagaoka, and H. Ogawa. 2006. Human defensins and cathelicidins in the skin: beyond direct antimicrobial properties. Crit. Rev. Immunol. 26:545-576. [DOI] [PubMed] [Google Scholar]
- 46.Niyonsaba, F., A. Someya, M. Hirata, H. Ogawa, and I. Nagaoka. 2001. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur. J. Immunol. 31:1066-1075. [DOI] [PubMed] [Google Scholar]
- 47.O'Callaghan, R. J., L. S. Engel, J. A. Hobden, M. C. Callegan, L. C. Green, and J. M. Hill. 1996. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Investig. Ophthalmol. Vis. Sci. 37:534-543. [PubMed] [Google Scholar]
- 48.Oren, A., T. Ganz, L. Liu, and T. Meerloo. 2003. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp. Mol. Pathol. 74:180-182. [DOI] [PubMed] [Google Scholar]
- 49.Panyutich, A., and T. Ganz. 1991. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am. J. Respir. Cell Mol. Biol. 5:101-106. [DOI] [PubMed] [Google Scholar]
- 50.Panyutich, A. V., P. S. Hiemstra, S. van Wetering, and T. Ganz. 1995. Human neutrophil defensin and serpins form complexes and inactivate each other. Am. J. Respir. Cell Mol. Biol 12:351-357. [DOI] [PubMed] [Google Scholar]
- 51.Pillar, C. M., and J. A. Hobden. 2002. Pseudomonas aeruginosa exotoxin A and keratitis in mice. Investig. Ophthalmol. Vis. Sci. 43:1437-1444. [PubMed] [Google Scholar]
- 52.Redfern, R. L., R. J. Proske, and A. M. McDermott. 2004. Effect of defensins on corneal cell migration and cytokine production. Investig. Ophthalmol. Vis. Sci. 45:E-abstr. 4866. [Google Scholar]
- 53.Redl, B. 2000. Human tear lipocalin. Biochim. Biophys. Acta 1482:241-248. [DOI] [PubMed] [Google Scholar]
- 54.Ruchaud-Sparagano, M. H., B. R. Westley, and F. E. May. 2004. The trefoil protein TFF1 is bound to MUC5AC in human gastric mucosa. Cell. Mol. Life Sci. 61:1946-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakamoto, N., H. Mukae, T. Fujii, H. Ishii, S. Yoshioka, T. Kakugawa, K. Sugiyama, Y. Mizuta, J. Kadota, M. Nakazato, and S. Kohno. 2005. Differential effects of alpha- and beta-defensin on cytokine production by cultured human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L508-L513. [DOI] [PubMed] [Google Scholar]
- 56.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522-526. [DOI] [PubMed] [Google Scholar]
- 57.Sathe, S., M. Sakata, A. R. Beaton, and R. A. Sack. 1998. Identification, origins and the diurnal role of the principal serine protease inhibitors in human tear fluid. Curr. Eye Res. 17:348-362. [DOI] [PubMed] [Google Scholar]
- 58.Schroder, J. M. 1999. Epithelial peptide antibiotics. Biochem. Pharmacol. 57:121-134. [DOI] [PubMed] [Google Scholar]
- 59.Schroder, J. M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 60.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh, P. K., B. F. Tack, P. B. McCray, Jr., and M. J. Welsh. 2000. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L799-L805. [DOI] [PubMed] [Google Scholar]
- 62.Taggart, C. C., C. M. Greene, S. G. Smith, R. L. Levine, P. B. McCray, Jr., S. O'Neill, and N. G. McElvaney. 2003. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J. Immunol. 171:931-937. [DOI] [PubMed] [Google Scholar]
- 63.Territo, M. C., T. Ganz, M. E. Selsted, and R. Lehrer. 1989. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Investig. 84:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomita, T., S. Hitomi, T. Nagase, H. Matsui, T. Matsuse, S. Kimura, and Y. Ouchi. 2000. Effect of ions on antibacterial activity of human beta defensin 2. Microbiol. Immunol. 44:749-754. [DOI] [PubMed] [Google Scholar]
- 65.Valore, E. V., C. H. Park, A. J. Quayle, K. R. Wiles, P. B. McCray, Jr., and T. Ganz. 1998. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Investig. 101:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Haeringen, N. J. 1981. Clinical biochemistry of tears. Surv. Ophthalmol. 26:84-96. [DOI] [PubMed] [Google Scholar]
- 67.Van Wetering, S., S. P. Mannesse-Lazeroms, M. A. Van Sterkenburg, M. R. Daha, J. H. Dijkman, and P. S. Hiemstra. 1997. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 272:L888-L896. [DOI] [PubMed] [Google Scholar]
- 68.White, S. H., W. C. Wimley, and M. E. Selsted. 1995. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 5:521-527. [DOI] [PubMed] [Google Scholar]
- 69.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 70.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]
- 71.Zasloff, M. 1992. Antibiotic peptides as mediators of innate immunity. Curr. Opin. Immunol. 4:3-7. [DOI] [PubMed] [Google Scholar]
- 72.Zhou, L., L. Q. Huang, R. W. Beuerman, M. E. Grigg, S. F. Li, F. T. Chew, L. Ang, M. E. Stern, and D. Tan. 2004. Proteomic analysis of human tears: defensin expression after ocular surface surgery. J. Proteome Res. 3:410-416. [DOI] [PubMed] [Google Scholar]