Abstract
We evaluated the effects of dihydroxyacetone (DHA) on Trypanosoma brucei bloodstream forms. DHA is considered an energy source for many different cell types. T. brucei takes up DHA readily due to the presence of aquaglyceroporins. However, the parasite is unable to use it as a carbon source because of the absence of DHA kinase (DHAK). We could not find a homolog of the relevant gene in the genomic database of T. brucei and have been unable to detect DHAK activity in cell lysates of the parasite, and the parasite died quickly if DHA was the sole energy source in the medium. In addition, during trypanosome cultivation, DHA induced growth inhibition with a 50% inhibitory concentration of about 1 mM, a concentration that is completely innocuous to mammals. DHA caused cell cycle arrest in the G2/M phase of up to 70% at a concentration of 2 mM. Also, DHA-treated parasites showed profound ultrastructural alterations, including an increase of vesicular structures within the cytosol and the presence of multivesicular bodies, myelin-like structures, and autophagy-like vacuoles, as well as a marked disorder of the characteristic mitochondrion structure. Based on the toxicity of DHA for trypanosomes compared with mammals, we consider DHA a starting point for a rational design of new trypanocidal drugs.
Trypanosoma brucei is the causative agent of sleeping sickness, a fatal illness that is endemic in many countries in sub-Saharan Africa. Nearly half a million people are currently infected with the parasite, and 50,000 to 60,000 new cases appear every year (40). Available drugs are not effective against all stages of the disease. Melarsoprol, a trivalent organic arsenical that was discovered in 1949, is still the first line of defense against sleeping sickness. This compound (together with eflornithine, a drug effective only against Trypanosoma gambiense) is the most effective medication for the late stage of the disease (5); however, it is not an ideal drug. Although melarsoprol has a high cure rate (80 to 90%), it remains ineffective in a number of cases. In addition, there has been an increase of treatment failure of up to 30% due to the emergence of melarsoprol resistance (17). Most importantly, however, the drug causes grave side affects. About 10% of patients develop a severe posttreatment reactive encephalopathy, which is fatal in approximately 50% of cases (17, 18). This scenario clearly highlights the importance of a rational search for new trypanocidal compounds and/or biochemical targets to develop effective drugs that are safe, easy to use under field conditions, and inexpensive to produce.
Recently, the three aquaglyceroporins (TbAQP1 to -3) of T. brucei have been cloned and characterized (38). They show high permeability for water and glycerol compared with other aquaglyceroporins. Surprisingly, however, this study also revealed for the first time that aquaglyceroporins from protozoa are highly permeable for dihydroxyacetone (DHA), a three-carbon sugar. This substance permeates these channels in the same range as, or even more effectively than, glycerol (38). The compound DHA is considered an energy source for different cells from bacteria to mammals (7, 36). Erythrocytes, for example, efficiently convert DHA into l-lactate via glycolysis at a rate comparable to their capacity to convert glucose (35). Likewise, in mammals, this triose is an important gluconeogenic precursor (34). T. brucei, however, seems not to possess enzymes involved in DHA consumption, as has been demonstrated by extensive data searches (38). An example is DHA kinase (DHAK), an enzyme involved in DHA metabolism in many organisms (7, 24) and particularly in the detoxification of high concentrations of DHA in Saccharomyces cerevisiae (23). Based on these facts and the results presented in this study, DHA may be considered a starting point for drug development. Here, we evaluated cell cycle progression and cell death, as well as the ultrastructural alterations produced by DHA treatment in bloodstream forms of T. brucei.
MATERIALS AND METHODS
Chemicals and cultures.
DHA and all other chemicals used were purchased from Sigma Chemicals (Deisenhofen, Germany) unless otherwise stated. Tetramethylrhodamine (TMRE) and dichlorofluorescein diacetate were obtained from Invitrogen (Karlsruhe, Germany) and the annexin V-Fluos kit from Roche (Mannheim, Germany). Rapamycin (Biaffin, Kassel, Germany) was dissolved in ethanol (50 mM stock solution) and used at a final concentration of 5 μM. The experiments in this work were performed using bloodstream forms of T. brucei MITat 1.2 (VSG variant 221) of the monomorphic strain EATRO 427. The parasites were grown in axenic culture at 37°C and 5% CO2 as described previously (12, 13). Experiments to evaluate the ability of DHA to serve as an energy source were performed in the same medium lacking glucose and fetal calf serum.
Cytotoxicity assay.
The 50% inhibitory concentration (IC50) was determined by measuring the phosphatase activity according to the method of Bodley et al. (3). Briefly, exponentially growing parasites were diluted to 2 × 105 cells/ml, placed in a 96-well microtiter plate (199 μl), and grown with or without DHA (1 μl/well) at concentrations between 1 and 4 mM. After a 24-h incubation, cell growth was stopped by the addition of 20 μl lysis buffer containing p-nitrophenyl phosphate (20 mg/ml in 1 M sodium acetate, pH 5.5, containing 1% Triton X-100). Finally, following a 6-h incubation at 37°C, the phosphatase activity was measured at a λ of 405 nm using an enzyme-linked immunosorbent assay reader (MRX II; Dynex Technologies, Middlesex, England).
Measurement of DHAK activity.
Following lysis of 3 × 109 parasites, the DHAK activity assay was performed according to the method of Johnson et al. (16). Aliquots of 1.5 × 107 cell lysates were added to the reaction mixture containing 50 mM triethanolamine, 2 mM MgCl2, 10 mM 2,2-bipyridyle, 5 mM ATP, 0.4 mM NADH, and 8 U/ml glycerol-3-phosphate dehydrogenase. The reaction was started in the presence or absence of 10 mM glycerol by adding 60 mM DHA. For monitoring, the decrease in absorption at 340 nm, due to the oxidation of NADH, was measured.
Light and fluorescence microscopy.
DHA-treated or untreated trypanosomes were stained after a 24-h incubation with the fluorescent dye DAPI (4,6-diamidino-2-phenylindole) (purchased from Sigma). Aliquots of each culture were withdrawn and washed twice with phosphate-buffered saline (PBS). The cells were resuspended in 200 μl PBS, placed on a slide, and fixed with methanol. DAPI staining was performed for 5 min (0.1-μg/ml final concentration). The slides were then washed three times and examined using an Olympus BH2 fluorescence microscope.
Transmission electron microscopy (TEM).
Trypanosomes (108) were fixed in 2% (vol/vol) glutaraldehyde in 0.2 M sodium cacodylate buffer containing 0.12 M sucrose for 1 h at 4°C. After overnight incubation in the same sodium cacodylate buffer, the cells were postfixed in osmium tetroxide (1.5% [wt/vol]) and stained in 0.5% uranyl acetate (14). Dehydration in ethanol, clearing in propylene oxide, and embedding in Agar 100 (equivalent to Epon 812) were performed according to standard procedures (10). Sections were stained in 5% (wt/vol) uranyl acetate and 0.4% (wt/vol) lead citrate.
Scanning electron microscopy (SEM).
After fixation and staining identical to the TEM procedure, cells were sequentially dehydrated in 50%, 75%, 95%, and 100% ethanol. Critical-point drying and gold-palladium sputter staining were performed using standard protocols.
Cellular permeability.
Plasma membrane integrity was tested using propidium iodide (5 μg/ml) staining. DHA-treated and untreated trypanosomes (2 × 106 cells/ml) were incubated for 10 min with propidium iodide and analyzed by flow cytometry in a FACSCalibur apparatus (Becton Dickinson Co., New Jersey). Digitonin-treated cells (6 μM) were used as a positive control for necrosis.
DNA content.
To determine the nuclear-DNA content, a propidium iodide staining method was used (25). Briefly, at least 5 × 105 cells were washed once with PBS and hypotonically lysed for 30 min at room temperature using phosphate buffer (10 mM, pH 7.4, containing 6 μM digitonin). Nuclei were stained with a propidium iodide solution (10-μg/ml final concentration in 10 mM phosphate buffer) and analyzed by fluorescence-activated cell sorter (FACS).
Mitochondrial-membrane potential.
The mitochondrial-membrane potential was evaluated by TMRE staining. Trypanosomes (106 cells/ml) were incubated in a culture medium containing 25 nM of TMRE for 30 min at 37°C and immediately analyzed by flow cytometry. Valinomycin (100 nM) was used to cause depolarization of the inner mitochondrial membrane as a positive control.
Phosphatidylserine exposure.
To detect phosphatidylserine exposure, annexin V-Fluos was used following the manufacturer's instructions. Briefly, cells were washed in HEPES buffer (10 mM, pH 7.4, containing 140 mM NaCl and 5 mM CaCl2) and incubated for 15 min at 4°C with annexin V-Fluos. Thereafter, the cells were assayed by FACS.
Reactive oxygen species (ROS).
To measure intracellular oxidative stress, control and treated trypanosomes were incubated for 1 h at 37°C with 10 μM dichlorofluorescein diacetate, and the cellular fluorescence intensity was measured in the FACSCalibur apparatus.
RESULTS
Evaluation of DHA as an energy source for T. brucei.
DHA can be used as an energy source by many cell types (7, 36). For T. brucei bloodstream forms, DHA would be an energy substrate if it could be converted to DHA phosphate, a regular glycolytic metabolite, as these forms depend exclusively on glycolysis for ATP production (22). However, despite the fact that putative DHAK genes are present in the Trypanosoma cruzi and Leishmania major databases, no orthologue gene could be found in the T. brucei genome database that showed a convincing level of homology. Likewise, our attempts to measure DHAK activity in trypanosome lysates using a well-established enzyme assay with glycerolphosphate dehydrogenase and NADH consumption as indicator reaction gave negative results, confirming the absence of the enzyme (data not shown).
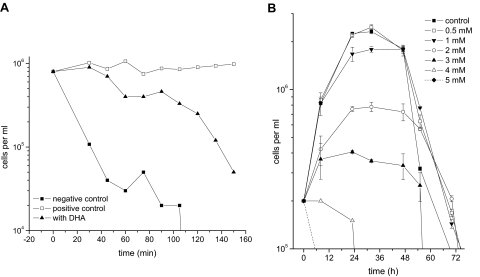
For further investigation of whether T. brucei bloodstream forms are able to use DHA as a carbon source, parasites were incubated in basic medium without glucose and serum in the presence or absence of DHA. Parasites cultivated without any energy source (the negative control) started to die immediately, showing a steep fall in cell density. In contrast, trypanosomes incubated in the presence of 1 mM glucose (the positive control) maintained their cell numbers and motility for hours (Fig. 1A). However, in the absence of glucose and the presence of 1 mM DHA, trypanosomes survived for a longer time than the negative control but showed a drastic diminution of their motility and cell density within minutes (Fig. 1A). In addition, DHA concentrations higher or lower than 1 mM did not improve the survival of the parasite (data not shown), and survival of parasites in the presence of glucose and DHA was virtually identical to that of the positive control. Indeed, cultivation of trypanosomes under the latter conditions showed only a slight decrease in cell growth (Fig. 1B).
FIG. 1.
Evaluation of DHA as either an energy source or a toxic compound for T. brucei bloodstream forms. (A) The use of DHA as a carbon source was evaluated by incubating cells in basic medium without supplementation with glucose or serum (negative control), in basic medium in the presence of 1 mM glucose (positive control), or in basic medium in the presence of 1 mM DHA. The cells died rapidly without any carbon source and survived in the presence of glucose. DHA as the sole carbon source sustained cell viability for only 40 min but led to cell death thereafter. (B) The effect of DHA on cellular proliferation was tested by cultivating parasites in complete medium (containing 33 mM glucose and 15% serum) in the presence of different concentrations of DHA. All experiments were performed in triplicate. The error bars represent standard deviations.
Taken together, these results show that DHA is a rather poor energy substrate for T. brucei, probably due to the absence of DHAK in the parasite. The marginally increased survival of the parasite in the presence of 1 mM DHA as the sole carbon source is readily explained by assuming a limited side reaction of the trypanosomal glycerol kinase, similar to glycerol kinases of other cells (15). Instead, DHA is toxic to trypanosomes, as judged by the induced growth inhibition at concentrations above 1 mM.
Cytotoxicity of DHA for T. brucei.
The obvious lack of DHAK in T. brucei changes DHA from an energy substrate to a toxic compound. In fact, the addition of different DHA concentrations to trypanosome cultures led to a dose-dependent decrease in cell density, with 4 and 5 mM DHA killing almost 100% of parasite cells within 24 h (Fig. 1B). The IC50 of DHA for T. brucei calculated according to the method of Bodley et al. (3) was 1.03 ± 0.14 mM. To our knowledge, this is the lowest IC50 for this substance reported to cause toxic effects, as the sugar is widely used as a carbon source (7, 23, 36).
Morphological changes in T. brucei after treatment with DHA.
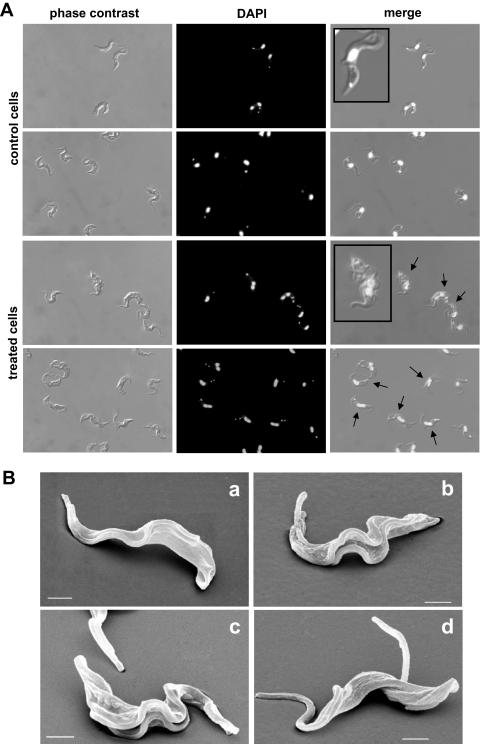
Parasites treated with 3 mM DHA for 24 h showed clear morphological alterations by light microscopy. Among these, the presence of two or more flagella, an increase in cell size, and atypical combinations of the numbers of nuclei and kinetoplasts (Fig. 2A) were the most obvious features, and the frequency of their appearance was clearly DHA concentration dependent.
FIG. 2.
Morphological changes in T. brucei bloodstream forms treated with DHA for 24 h. (A) Phase-contrast and DAPI-stained images of control cells and 3 mM DHA-treated cells; the arrows indicate cells containing more than one flagellum, kinetoplast, and/or nucleus. (B) SEM images of control cells (a) and cells treated with 5 mM DHA (b to d). Bars, 1 μm.
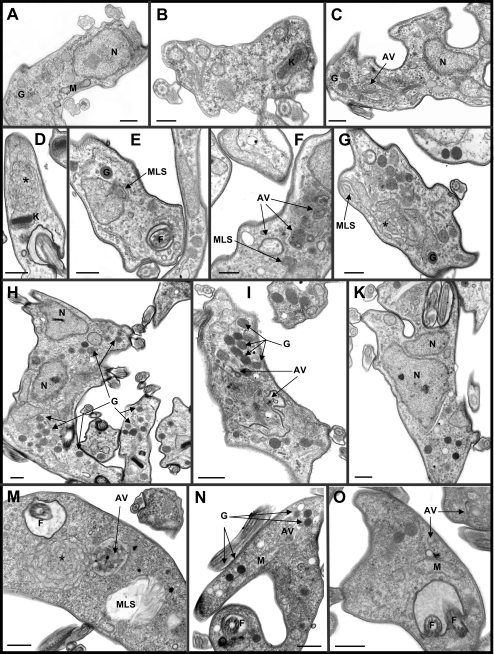
These results were confirmed using SEM and TEM. The vast majority of trypanosomes grown for 20 h in the presence of 5 mM DHA showed two or more flagella but no other alterations of cell morphology by SEM (Fig. 2B). However, significant ultrastructural changes in DHA-treated trypanosomes were detected using TEM, as summarized in Fig. 3. Cells treated for 24 h with 3 mM DHA showed very similar subcellular alteration patterns, and the variety, intensity, and frequency of such morphological changes correlated well with the DHA concentration used. In line with light microscopy findings, cells containing one or two nuclei and two flagella were observed (Fig. 3H and K). DHA-treated parasites showed a clear increase in cisternal and vesicular structures within the cytosol (Fig. 3E, F, G, and I), in parallel with a high frequency of autophagic vacuole structures (Fig. 3C, D, and F). Multivesicular bodies and related structures, such as myelin-like structures (i.e., concentric membranes), were also frequently observed (Fig. 3E to G). In contrast, cell nuclei seemed unaffected. Interestingly, the most affected organelle was the mitochondrion. It showed an increase in size, dilatation of membranes, disorder of its characteristic structure, and the appearance of multiple membrane folds (Fig. 3D and G).
FIG. 3.
Ultrastructural alterations in T. brucei bloodstream forms. After 24 h of treatment with 3 mM DHA, trypanosomes displayed several ultrastructural changes (C to K) compared with control parasites (A and B). The major alterations included the presence of one or two nuclei and two flagella per cell; an increase in vesicular structures; marked frequency of autophagy vacuoles, multivesicular bodies, and related structures, such as myelin-like structures; and dilatation of mitochondrial membranes and multiple membrane folds. (M to O) Trypanosomes treated with 5 μM rapamycin to induce autophagy as a positive control. AV, autophagy vacuoles; F, flagellum; G, glycosomes; K, kinetoplast; L, lysosomes; M, mitochondrion; MLS, myelin-like structures; N, nucleus; *, characteristic autophagic structure probably of mitochondrial origin. Bars, 0.5 μm.
Analysis of cell cycle progression.
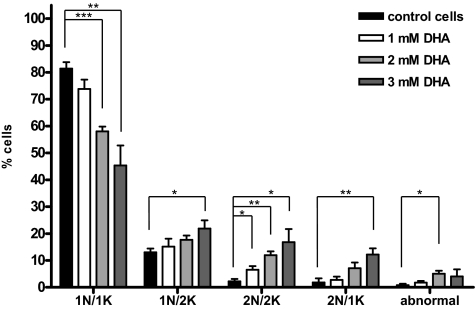
The increase in the number of cells containing more than one nucleus, kinetoplast, or flagellum suggested that the cell cycle was affected by DHA treatment. In order to investigate cell cycle arrest, the nuclei and kinetoplasts from DHA-treated trypanosomes were quantified at different DHA concentrations. About 80% of untreated cells contained one nucleus and one kinetoplast. This percentage gradually diminished with the addition of DHA, leading to an increase in cells with two nuclei and two kinetoplasts and with two nuclei and one kinetoplast (Fig. 4).
FIG. 4.
Representation of the DHA effect on the cell cycle of T. brucei bloodstream forms. Phase-contrast and DAPI-stained images from untreated and DHA-treated cells were used to determine the percentages of cells containing one nucleus and one kinetoplast (1N1K), one nucleus and two kinetoplasts (1N2K), two nuclei and two kinetoplasts (2N2K), or two nuclei and one kinetoplast (2N1K). All other cells were labeled abnormal. Statistical significance was evaluated by Student's t test (***, P < 0.001; **, P < 0.01; *, P < 0.05; n = 4). The error bars indicate standard deviations.
For cell cycle quantification, the DNA content per parasite was determined by FACS analysis as described in Materials and Methods. Following a 24-h cultivation, about 70% and 20% of untreated cells were in G1 and G2 phases, respectively. In contrast, these values were inverted for DHA-treated trypanosomes. The most effective concentration was 2 mM DHA, causing about 70% of cells to be arrested in G2 (Fig. 5, DNA content) after a 24-h incubation.
FIG. 5.
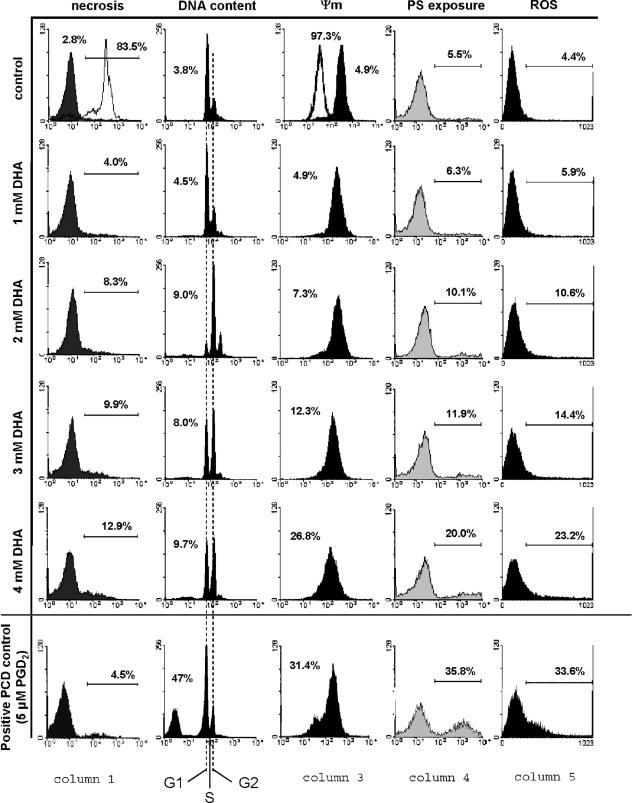
Evaluation of cell death markers and ROS production. After 24 h of treatment with different concentrations of DHA, cells were analyzed for necrosis, DNA content, mitochondrial-membrane potential (Ψm), phosphatidylserine exposure (PS exposure), and ROS production by FACS analysis. Column 1 shows the evaluation of necrosis as detected by propidium iodide staining; the white histogram included in the control panel shows necrosis induced by digitonin (83.5%). Column 2 shows the analysis of degraded DNA and, in addition, the distribution of intact DNA in G1, S, and G2 phases. The DHA-induced decrease in the mitochondrial-membrane potential, as represented by a shift to lower fluorescence intensities, is plotted in column 3; the white histogram in the control panel represents a positive control using valinomycin (97.3%). The increase of phosphatidylserine exposure is indicated in column 4 by annexin V-Fluos staining of the outer membrane. Likewise, DHA-induced increase of intracellular ROS is shown in column 5. Histograms showing a positive PCD control in trypanosomes (induced with prostaglandin D2 [PGD2]) are at the bottom of each column.
Evaluation of cell death markers.
The high frequency and variety of autophagy vacuoles and related structures observed in the parasites after treatment with different concentrations of DHA suggested that this process was the one mainly responsible for cell death. For further support of this hypothesis, other types of programmed cell death (PCD), as well as necrosis, were evaluated. Three classical markers of PCD (i.e., DNA degradation, loss of mitochondrial-membrane potential, and phosphatidylserine exposure) were evaluated by FACS analyses. In addition, the concentration of ROS, a known mediator of PCD in T. brucei bloodstream forms (9), was also investigated.
As shown in Fig. 5 (column 1), untreated control trypanosomes showed only 2.8% necrosis after 24 h of incubation. DHA-treated cells showed a minor increase in necrosis, which reached a maximum of about 13% at 4 mM DHA. Apoptosis-like PCD in trypanosomes is characterized by a significant increase in phosphatidylserine exposure in the outer leaflet of the plasma membrane, intracellular ROS production, and a loss of the mitochondrial-membrane potential (Fig. 5, Ψm) (8). In the case of DHA treatment, only minor (although concentration-dependent) changes (Fig. 5, columns 2, 3, 4, and 5) have been observed compared to prostaglandin D2-induced apoptosis (8).
Taken together, the observed changes are too small to support necrosis- or apoptosis-like PCD as the main cause of DHA-induced cell death. Instead, the electron microscopy data strongly suggest that treated cells die primarily by autophagy, as substantiated by rapamycin-induced autophagy as a positive control (Fig. 3M to O) (27, 28).
DISCUSSION
It has been clearly demonstrated that aquaglyceroporins are involved in drug uptake. Human aquaglyceroporins are permeable for, and are able to modulate the action of, arsenic trioxide (2, 20), a compound currently used against acute promyelocytic leukemia (31). Likewise, an aquaglyceroporin of Leishmania (LmAQP1) is able to take up trivalent antimony (11), the active compound of pentostam, the first line of treatment against leishmaniasis (11). In addition, down regulation of LmAQP1 mRNA in the antimony-resistant Leishmania species correlates well with the level of resistance to the drug (21). We cloned and characterized the aquaglyceroporins of T. brucei, showing that these channels are highly permeable for DHA, a triose sugar (38). DHA cannot be used as an energy source by T. brucei due to the absence of DHAK; homologous genes for this enzyme are absent from the genomic database of the parasite (38), and we did not find any DHAK activity in trypanosomal lysates. Most importantly, DHA as the sole carbon source in culture media could not sustain the survival of the parasite.
DHA is toxic, as demonstrated by its antiproliferative effect against trypanosomes, showing an IC50 of about 1 mM. Parasites treated with DHA were not able to progress through the cell cycle, with up to 70% of cells blocked in the G2/M phase, as observed by FACS analysis. This result is consistent with the morphological changes presented: the appearance of more than one nucleus, kinetoplast, or flagellum per cell, as evidenced by light microscopy, TEM, and SEM. Blockage of cell cycle progression by DHA has been reported previously; keratinocytes also arrest in G2/M phase when treated with DHA, although at a concentration between 25 and 100 mM (26). As the authors point out, this effect could be due to the nonenzymatic glycation called the Maillard reaction, which is known to be caused by glucose and other sugars, including DHA (37). This fact could explain some of the DHA effects; glycation of DNA may result in the modification of this structure, which in turn can induce the known cell cycle block in the G2/M phase to protect the genome by providing the time necessary to repair the damage caused (39).
Parasites treated with different concentrations of DHA showed profound ultrastructural changes, as evidenced by TEM (Fig. 3), which are comparable to those caused by other drugs considered promising against trypanosomiasis. The frequent appearance of multivesicular bodies, myelin-like structures, and autophagy vacuoles has also been reported for leishmania and T. cruzi treated with different drugs that affect the lipid metabolism of these parasites (29, 30). Interestingly, myelin-like structures are considered an indicator of both the high activity of organelle recycling and the degradation of damaged membranes by autophagy (6). Therefore, the common presence of these features (multivesicular bodies, myelin-like structures, and autophagy vacuoles) could indicate the reparative response of the cells to the damage caused by these drugs to membrane structure and function. In this sense, the deleterious action of DHA on the normal membranous structure (organelle, vesicular, and cisternal systems), probably mediated by the known Maillard reaction, leads to a marked increase in autophagy and, finally, by exacerbation of this mechanism, to parasite death by autophagy (or PCD type II). This type of cell death is strongly supported by the fact that none of the other types of cell death were detectable: caspase-dependent PCD does not occur in protozoa, as they do not possess these enzymes (19), and necrosis, as well as caspase-independent apoptosis (or apoptosis-like PCD), were eliminated by FACS analysis and TEM (Fig. 3 and 5). In fact, PCD and necrosis were significantly increased only at concentrations above 4 mM DHA. However, since these concentrations killed nearly the whole cell population within 24 h, the data are not really representative.
The toxic effect of DHA on trypanosomes is due to two principal factors. First is the absence of DHAK. It was shown that S. cerevisiae became sensitive to DHA upon disruption of the DHAK genes but was unaffected in the presence of 200 mM DHA after overexpression of these genes (23). The second factor is the high permeability of T. brucei aquaglyceroporins for DHA. Again, a yeast mutant without its glycerol channel (ΔFps1) grew well in the presence of 200 mM DHA, while this mutant was unable to proliferate when heterologously expressing TbAQP1, -2, or -3 (38).
These characteristics make DHA a possible candidate to be studied as a rational starting point for drug development. The most important disadvantage of DHA is the relatively high concentration that must be maintained in the blood in order to be effective. However, in principle, it is possible for a metabolizable compound to reach a relatively high blood concentration, since glycerol administrated simultaneously with salicylhydroxamic acid (SHAM) can rapidly clear trypanosomes from the blood of infected rodents (4). An alternative strategy would be to make DHA even more specific for TbAQPs, either to be exclusively taken up by the parasite or to block the channel for glycerol secretion. In the latter case, a synergistic effect with SHAM would occur, which should cure the parasitemia permanently rather than temporarily as with SHAM and glycerol. In this regard, relevant results of combination therapy approaches to the treatment of diseases caused by Leishmania and T. cruzi in experimental animal infections have been published (1, 30).
In conclusion, we have demonstrated that DHA is toxic for T. brucei, as it induces cell cycle arrest in the G2/M phase and autophagy, as judged by characteristic ultrastructural alterations. These effects are mainly due to the absence of DHAK and the presence of aquaglyceroporins in the parasite, which have an exceptionally high permeability for DHA. Therefore, we consider DHA, which is innocuous to humans (32, 33), an interesting candidate model for developing new antitrypanosomal drugs.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft. K.F. and N.L.U. are recipients of personal grants from DAAD (Germany) and CDCH (Venezuela), respectively.
Footnotes
Published ahead of print on 6 August 2007.
REFERENCES
- 1.Benaim, G., J. M. Sanders, Y. Garcia-Marchan, C. Colina, R. Lira, A. R. Caldera, G. Payares, C. Sanoja, J. M. Burgos, A. Leon-Rossell, J. L. Concepcion, A. G. Schijman, M. Levin, E. Oldfield, and J. A. Urbina. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J. Med. Chem. 49:892-899. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee, H., J. Carbrey, B. P. Rosen, and R. Mukhopadhyay. 2004. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem. Biophys. Res. Commun. 322:836-841. [DOI] [PubMed] [Google Scholar]
- 3.Bodley, A. L., M. W. McGarry, and T. A. Shapiro. 1995. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J. Infect. Dis. 172:1157-1159. [DOI] [PubMed] [Google Scholar]
- 4.Brohn, F. H., and A. B. J. Clarkson. 1978. Quantitative effects of salycylhydroxamic acid and glycerol on Trypanosoma brucei glycolysis in vitro and in vivo. Acta Trop. 35:23-33. [PubMed] [Google Scholar]
- 5.Burri, C., and R. Brun. 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 90:49-52. [DOI] [PubMed] [Google Scholar]
- 6.Dezfuli, B. S., E. Simoni, L. Giari, and M. Manera. 2006. Effects of experimental terbuthylazine exposure on the cells of Dicentrarchus labrax (L.). Chemosphere 64:1684-1694. [DOI] [PubMed] [Google Scholar]
- 7.Erni, B., C. Siebold, S. Christen, A. Srinivas, A. Oberholzer, and U. Baumann. 2006. Small substrate, big surprise: fold, function and phylogeny of dihydroxyacetone kinases. Cell. Mol. Life Sci. 63:890-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figarella, K., M. Rawer, N. L. Uzcategui, B. K. Kubata, K. Lauber, F. Madeo, S. Wesselborg, and M. Duszenko. 2005. Prostaglandin D(2) induces programmed cell death in Trypanosoma brucei bloodstream form. Cell Death Differ. 12:335-346. [DOI] [PubMed] [Google Scholar]
- 9.Figarella, K., N. L. Uzcategui, A. Beck, C. Schoenfeld, B. K. Kubata, F. Lang, and M. Duszenko. 2006. Prostaglandin-induced programmed cell death in Trypanosoma brucei involves oxidative stress. Cell Death Differ. 13:1802-1814. [DOI] [PubMed] [Google Scholar]
- 10.Glauert, A. M., A. E. Butterworth, R. F. Sturrock, and V. Houba. 1978. The mechansim of antibody-dependent, eosinophil-mediated damage to schistosomula of Schistosoma mansoni in vitro: a study by phase-contrast and electron microscopy. J. Cell Sci. 34:173-192. [DOI] [PubMed] [Google Scholar]
- 11.Gourbal, B., N. Sonuc, H. Bhattacharjee, D. Legare, S. Sundar, M. Ouellette, B. P. Rosen, and R. Mukhopadhyay. 2004. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J. Biol. Chem. 279:31010-31017. [DOI] [PubMed] [Google Scholar]
- 12.Hamm, B., A. Schindler, D. Mecke, and M. Duszenko. 1990. Differentiation of Trypanosoma brucei bloodstream trypomastigotes from long slender to short stumpy-like forms in axenic culture. Mol. Biochem. Parasitol. 40:13-22. [DOI] [PubMed] [Google Scholar]
- 13.Hesse, F., P. M. Selzer, K. Muhlstadt, and M. Duszenko. 1995. A novel cultivation technique for long-term maintenance of bloodstream form trypanosomes in vitro. Mol. Biochem. Parasitol. 70:157-166. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch, J. G., and M. E. Fedorko. 1968. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and “postfixation” in uranyl acetate. J. Cell Biol. 38:615-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin, R. Z., R. G. Forage, and E. C. Lin. 1982. Glycerol kinase as a substitute for dihydroxyacetone kinase in a mutant of Klebsiella pneumoniae. J. Bacteriol. 152:1303-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, E. A., S. K. Burke, R. G. Forage, and E. C. Lin. 1984. Purification and properties of dihydroxyacetone kinase from Klebsiella pneumoniae. J. Bacteriol. 160:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, P. 2004. Human African trypanosomiasis of the CNS: current issues and challenges. J. Clin. Investig. 113:496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy, P. G. 2006. Diagnostic and neuropathogenesis issues in human African trypanosomiasis. Int J. Parasitol. 36:505-512. [DOI] [PubMed] [Google Scholar]
- 19.Koonin, E. V., and L. Aravind. 2002. Origin and evolution of eukaryotic apoptosis: the bacterial connection. Cell Death Differ. 9:394-404. [DOI] [PubMed] [Google Scholar]
- 20.Liu, Z., J. Shen, J. M. Carbrey, R. Mukhopadhyay, P. Agre, and B. P. Rosen. 2002. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA 99:6053-6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquis, N., B. Gourbal, B. P. Rosen, R. Mukhopadhyay, and M. Ouellette. 2005. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol. Microbiol. 57:1690-1699. [DOI] [PubMed] [Google Scholar]
- 22.Michels, P. A., V. Hannaert, and F. Bringaud. 2000. Metabolic aspects of glycosomes in trypanosomatidae—new data and views. Parasitol. Today 16:482-489. [DOI] [PubMed] [Google Scholar]
- 23.Molin, M., J. Norbeck, and A. Blomberg. 2003. Dihydroxyacetone kinases in Saccharomyces cerevisiae are involved in detoxification of dihydroxyacetone. J. Biol. Chem. 278:1415-1423. [DOI] [PubMed] [Google Scholar]
- 24.Molin, M., and A. Blomberg. 2006. Dihydroxyacetone detoxification in Saccharomyces cerevisiae involves formaldehyde dissimilation. Mol. Microbiol. 60:925-938. [DOI] [PubMed] [Google Scholar]
- 25.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 26.Petersen, A. B., H. C. Wulf, R. Gniadecki, and B. Gajkowska. 2004. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat. Res. 560:173-186. [DOI] [PubMed] [Google Scholar]
- 27.Raught, B., A. C. Gingras, and N. Sonenberg. 2001. The target of rapamycin (TOR) proteins. Proc. Natl. Acad. Sci. USA 98:7037-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigden, D. J., M. Herman, S. Gillies, and P. A. M. Michels. 2005. Implications of a genomic search for autophagy-related genes in trypanosomatids. Biochem. Soc. Trans. 33:972-974. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues, J. C., J. A. Urbina, and W. de Souza. 2005. Antiproliferative and ultrastructural effects of BPQ-OH, a specific inhibitor of squalene synthase, on Leishmania amazonensis. Exp. Parasitol. 111:230-238. [DOI] [PubMed] [Google Scholar]
- 30.Santa-Rita, R. M., R. Lira, H. S. Barbosa, J. A. Urbina, and S. L. de Castro. 2005. Anti-proliferative synergy of lysophospholipid analogues and ketoconazole against Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae): cellular and ultrastructural analysis. J. Antimicrob. Chemother. 55:780-784. [DOI] [PubMed] [Google Scholar]
- 31.Soignet, S. L., P. Maslak, Z. G. Wang, S. Jhanwar, E. Calleja, L. J. Dardashti, D. Corso, A. DeBlasio, J. Gabrilove, D. A. Scheinberg, P. P. Pandolfi, and R. P. J. Warrell. 1998. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 339:1341-1348. [DOI] [PubMed] [Google Scholar]
- 32.Stanko, R. T., R. J. Robertson, R. J. Spina, J. J. Reilly, Jr., K. D. Greenawalt, and F. L. Goss. 1990. Enhancement of arm exercise endurance capacity with dihydroxyacetone and pyruvate. J. Appl. Physiol. 68:119-124. [DOI] [PubMed] [Google Scholar]
- 33.Stanko, R. T., R. J. Robertson, R. W. Galbreath, J. J. Reilly, Jr., K. D. Greenawalt, and F. L. Goss. 1990. Enhanced leg exercise endurance with a high-carbohydrate diet and dihydroxyacetone and pyruvate. J. Appl. Physiol. 69:1651-1656. [DOI] [PubMed] [Google Scholar]
- 34.Sumida, K. D., S. C. Crandall, P. L. Chadha, and T. Qureshi. 2002. Hepatic gluconeogenic capacity from various precursors in young versus old rats. Metabolism 51:876-880. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi, T., S. Murase, and I. Miwa. 2002. Glyceraldehyde metabolism in human erythrocytes in comparison with that of glucose and dihydroxyacetone. Cell Biochem. Function 20:223-226. [DOI] [PubMed] [Google Scholar]
- 36.Tang, J., E. Martin, and E. C. Lin. 1982. Derepression of an NAD-linked dehydrogenase that serves an Escherichia coli mutant for growth on glycerol. J. Bacteriol. 152:1001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tessier, F. J., V. M. Monnier, L. M. Sayre, and J. A. Kornfield. 2003. Triosidines: novel Maillard reaction products and cross-links from the reaction of triose sugars with lysine and arginine residues. Biochem. J. 369:705-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uzcategui, N. L., A. Szallies, S. Pavlovic-Djuranovic, M. Palmada, K. Figarella, C. Boehmer, F. Lang, E. Beitz, and M. Duszenko. 2004. Cloning, heterologous expression, and characterization of three aquaglyceroporins from Trypanosoma brucei. J. Biol. Chem. 279:42669-42676. [DOI] [PubMed] [Google Scholar]
- 39.Wang, J. Y., and S. K. Cho. 2004. Coordination of repair, checkpoint, and cell death responses to DNA damage. Adv. Protein Chem. 69:101-135. [DOI] [PubMed] [Google Scholar]
- 40.WHO. 2006. African trypanosomiasis or sleeping sickness. Fact sheet no. 259. WHO, Geneva, Switzerland.