Abstract
Empirical use of beta-lactam antibiotics, the preferred agents for treating uncomplicated skin and soft tissue infections, may no longer be appropriate for these infections because of the increasing prevalence of community strains of methicillin-resistant Staphylococcus aureus (MRSA). Retrospective studies, however, suggest that outcomes are good even when beta-lactams are used. We conducted a randomized, double-blind trial of 166 outpatient subjects comparing placebo to cephalexin at 500 mg orally four times for 7 days after incision and drainage of skin and soft tissue abscesses. The primary outcome was clinical cure or failure 7 days after incision and drainage. S. aureus was isolated from 70.4% of abscess cultures. Of the isolates tested 87.8% were MRSA, 93% of which were positive for Panton-Valentine leucocidin genes. Clinical cure rates were 90.5% (95% confidence interval, 0.82 to 0.96) in the 84 placebo recipients and 84.1% (95% confidence interval, 0.74 to 0.91) in the 82 cephalexin recipients (difference in the two proportions, 0.0006; 95% confidence interval, −0.0461 to 0.0472; P = 0.25). The 90.5% cure rate observed in the placebo arm and 84.1% cure rate observed in the cephalexin arm provide strong evidence that antibiotics may be unnecessary after surgical drainage of uncomplicated skin and soft tissue abscesses caused by community strains of MRSA.
There is evidence that antibiotics may not be necessary for treatment of uncomplicated skin and soft tissue infections (SSTIs) such as abscesses, including those caused by methicillin-resistant Staphylococcus aureus (MRSA) (10, 14). In recent studies, treatment outcomes for SSTIs were the same whether or not the antibiotic prescribed was actually active against the cultured organism (10, 11, 14, 20). When MRSA isolates were cultured from SSTI patients, infections resolved even when beta-lactam antibiotics, which are presumably inactive against MRSA, were used (11, 20, 30). It is standard practice to treat patients with SSTIs with an antibiotic (30). A 2005 survey found that 87% of health care providers continue to prescribe antibiotics after incision and drainage of abscesses, and a beta-lactam antibiotic is typically used (24). Overuse of antibiotics has adverse consequences, including untoward side effects and financial costs, and may contribute to the spread of antibiotic-resistant organisms (9, 15, 18, 25).
The alarming increase in the prevalence of community-associated MRSA may be a consequence of years of antibiotic misuse. The virulence of community MRSA strains, which typically carry genes for Panton-Valentine leucocidin (PVL), has been of particular concern (3, 5, 23). PVL has been associated with furunculosis; severe SSTIs, including necrotizing fasciitis (19); and necrotizing pneumonia (10, 16). These developments have necessitated a reassessment of the management and antimicrobial therapy of staphylococcal infections. The purposes of this study were (i) to compare a current “standard-of-care” antibiotic, cephalexin, to placebo after surgical incision and drainage of uncomplicated skin abscesses; (ii) to establish the prevalence of MRSA in the population under study; and (iii) to prospectively determine whether discordance between therapy and isolate susceptibility affected outcome.
MATERIALS AND METHODS
Study setting.
This study was conducted at the ISIS Clinic at the San Francisco General Hospital from November 2004 to March 2005. The patient population at this clinic has high rates of illicit drug injection use; homelessness; and infection with hepatitis C, hepatitis B, or human immunodeficiency virus (HIV) (30). The clinic provides uninterrupted, 7-days-per-week treatment from 9 a.m. to 5 p.m. on weekdays and 9 a.m. to 12 p.m. on weekends and holidays for all patients on a walk-in, outpatient basis (12).
Study population.
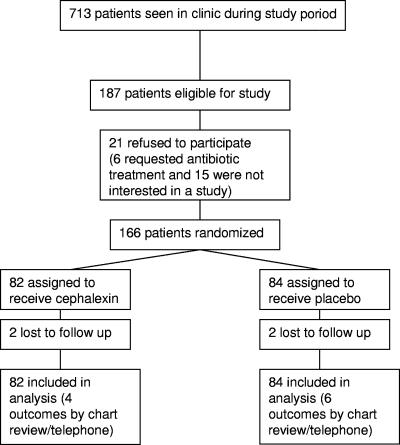
All patients presenting to the ISIS Clinic are assessed by a surgeon to determine if an abscess is present and if surgical drainage is necessary. All study participants were recruited from the group of patients considered to have a surgically drainable abscess (Fig. 1). We included patients with medical comorbidities such as intravenous drug use, hepatitis B and C, HIV infection, and diabetes. Subjects were eligible for enrollment if they were over the age of 18 years and had an abscess that the attending surgeon believed required surgical intervention and was severe enough that a duration of 5 or more days of antibiotic therapy was anticipated. Diagnostic criteria for an abscess were as follows: (i) acute onset within 7 days prior to enrollment; (ii) purulent drainage or purulent aspirate; (iii) erythema, induration (≥2 cm in diameter), or tenderness; and (iv) evidence of loculated fluid at time of enrollment. Our clinical standard is to prescribe antibiotics when two or more of these criteria are met. Subjects were ineligible for enrollment if they were unlikely to survive through the treatment period and evaluations or had toxic shock syndrome or toxic shock-like syndrome, shock or hypotension, oliguria (urine output of <20 ml/h) not responsive to fluid challenge, an incisional wound extending into visceral compartments, suspected or proven contiguous bone or joint involvement, ischemic ulcers or wounds associated with severe arterial insufficiency or gangrene, infection of prosthetic materials or venous catheters that could not be removed as part of the treatment of the current infection, infection of a full-thickness burn wound or burn wound that was >20% of total body area, allergy to penicillin or cephalexin, or renal compromise requiring adjusted dosing of cephalexin.
FIG. 1.
Enrollment and outcomes.
Febrile patients were included. The current standard of care at ISIS does not include routine blood drawings, and thus, white blood cell counts were not a component of the study criteria.
The Committee on Human Research at the University of California approved the study, and all patients gave written informed consent.
The drug trial is registered under identifier NCT00187759 at http://www.clinicaltrials.gov.
Study protocol.
At the initial visit, participants underwent a directed history and a physical examination, including complete examination of the skin. Abscesses were measured, and local anesthetic was injected along the line of the anticipated incision. Using a no. 11 blade, the attending surgeon used a sawing motion to create a wide opening and completely drain the pus. The cavity was probed for loculations and further drained if necessary. The cavity was packed from deep to superficial levels with plain gauze for healing by secondary intention. Participants were then randomized to treatment assignments by the pharmacist, using a block randomization scheme to generate a 1:1 ratio of subjects in each group. Assignments were placed in sequentially numbered, sealed envelopes, which were opened and recorded after all other data had been entered.
The antibiotic treatment regimen consisted of the current standard of care in the community, oral cephalexin at 500 mg four times daily for 7 consecutive days. Patients in the placebo group received oral placebo capsules identical in appearance to the cephalexin capsules for the same length of time. All subjects were seen daily in the clinic by a nurse who assessed wound healing and changed dressings until the wound showed the following signs of healing: absence of purulent wound drainage, erythema, fluctuance, localized warmth, pain/tenderness, and edema/induration. Patients were also asked to return 7 days after initial study enrollment for their follow-up visit. All patients, investigators, and clinic staff were blinded to study group assignment.
Microbiologic cultures and susceptibility testing.
Wound cultures were obtained from patients during the incision and drainage procedure. The intact skin over the abscess was cleansed with 10% povidone-iodine solution (Smith and Nephew, Largo, FL) prior to incision. A sterile Dacron swab (Becton, Dickinson & Co., Cockeysville, MD) was rotated within the cavity immediately after surgical incision. Wet swabs were sent to the clinical microbiology laboratory at San Francisco General Hospital for routine aerobic and anaerobic culture and antimicrobial susceptibility testing. Since staphylococci and streptococci are the two most prevalent organisms in SSTIs, the laboratory tested only for these. MICs were determined by Microscan (Microscan Walkaway instrument; Dade International, West Sacramento, CA). Guidelines from the Clinical and Laboratory Standards Institute (formerly NCCLS) were used throughout to assess susceptibility (22). Samples identified as MRSA were tested for the presence of PVL genes using the method of Lina et al. (16).
Clinical response.
The primary study outcome was either clinical cure or clinical failure, which was determined according to a predetermined set of criteria and based on the clinical judgment of trained nurse practitioners. There is currently no validated instrument available to assess cure or failure of an abscess; the goal of this study was not to create such an instrument but rather to reflect clinical practice. Determination of clinical cure was made by one of the study investigators (P.R.) and five trained study nurses at the 1-week follow-up visit if there was resolution of the following signs and symptoms—purulent wound drainage, erythema, fluctuance, localized warmth, pain/tenderness, and edema/induration—such that no further antibiotics or surgical procedure was needed. Treatment failure, defined as the presence of any of those symptoms, was confirmed by a nurse practitioner in the ISIS Clinic if the infection did not resolve as expected and thereby required antibiotics or a surgical procedure anytime after study entry. All those who assessed study outcome were trained by a study investigator. Patients who came to the clinic after the designated 1-week follow-up visit were also included. Treatment adherence was based on patient self-report.
Patients were instructed not to take any other antibiotics except the study drug during the study period. If they reported taking additional antibiotics for their abscess or for any unrelated condition, treatment was considered a failure and patients were followed in the clinic until their infection resolved.
Statistical analysis.
This was an intent-to-treat analysis of all patients who enrolled in the study. The primary clinical end point was the proportion of patients whose wounds were considered to be clinically cured. Early stopping rules were set in the event that the overall cure rate was high, irrespective of treatment group. Patients who missed their follow-up visit and whose treatment was not already considered a failure were contacted via telephone by one of the study investigators, and their medical records were reviewed. If sufficient information was available to determine clinical outcome, these patients were included in the analysis. Patients who did not return for follow-up, who could not be contacted by phone, and whose outcome could not be determined from chart review were deemed failures.
The sample size was designed to provide 80% power to detect a difference of 10% or more between the two groups (6). A one-tailed Fisher exact test with a 5% level of significance was used to compare the primary outcomes, as placebo was not expected to be better than cephalexin.
Statistical analyses were performed using Microsoft Access 2000 and SAS software, versions 8.2 and 9.1. Cure rates are presented as proportions with corresponding 95% confidence intervals. Dichotomous variables were analyzed using Fisher's exact test. Ordinal variables were analyzed using the Kruskal-Wallis test. Continuous variables were analyzed with Mann-Whitney tests. Two-sided P values of <0.05 were set to indicate statistical significance for all other comparisons.
RESULTS
Characteristics of the study subjects.
A total of 166 eligible subjects were enrolled and randomized to receive either cephalexin or placebo (Fig. 1). Subjects randomized to the two study groups were similar with respect to baseline characteristics (Table 1). A total of four subjects were lost to follow-up.
TABLE 1.
Baseline characteristics of the 166 study subjects included in the analyses, by study group
| Characteristic | Value for study group:
|
P value | |
|---|---|---|---|
| Cephalexin | Placebo | ||
| Age, median (range), yr | 43 (22-66) | 45 (22-88) | 0.3191 |
| Male sex (%) | 72.0 | 81.0 | 0.2313 |
| Race (%) | 0.2431 | ||
| American Indian | 6.2 | 0 | |
| Pacific Islander | 1.5 | 1.5 | |
| Asian | 4.6 | 1.5 | |
| African-American | 38.5 | 46.2 | |
| Caucasian | 49.2 | 50.8 | |
| Homeless (%) | 35.4 | 38.1 | 0.7493 |
| Diabetes (%) | 2.4 | 6.0 | 0.4432 |
| Hepatitis B or C (%) | 30.5 | 33.3 | 0.7408 |
| HIV infection (%) | 15.9 | 7.1 | 0.0915 |
| Abscess size of >5 cm in dimension (%) | |||
| Length | 17.7 | 18.1 | 1.0000 |
| Width | 19.2 | 24.1 | 0.5668 |
| Depth | 1.4 | 5.3 | 0.3678 |
| Surface area, mean (range), cm2 | 18.7 (1-144) | 19.0 (1-150) | 0.3676 |
| Tissue depth (%) | |||
| Subcutaneous | 64.1 | 67.7 | 0.9048 |
| Fascia | 17.2 | 14.1 | |
| Muscle | 18.8 | 18.3 | |
| Underlying skin disease (%) | |||
| Folliculitis | 38.8 | 40.2 | 1.0000 |
| Atopic dermatitis | 0.05 | 0.01 | 1.0000 |
Clinical response was determined in the clinic for 89% of the 166 subjects eligible for analysis. Of the 14 subjects who did not return for their 1-week follow-up visit, outcome was determined by chart review of hospital records alone (n = 8) and by chart review coupled with a telephone conversation with one of the study investigators (n = 2). Treatment adherence data were available for 113 patients: 72.2% (n = 39) reported taking cephalexin as instructed and 78.0% (n = 46) reported taking placebo as instructed. No major adverse events (events resulting in death, life-threatening situations, or permanent disabilities) were reported for either study group. No disseminated disease such as pneumonia or meningitis was reported.
Clinical outcome.
There was no difference in the clinical cure rate between subjects receiving placebo and those receiving cephalexin (90.5% [n = 76/84] versus 84.1% [n = 69/82]; 95% confidence interval, 0.82 to 0.96 versus 0.74 to 0.91, respectively; difference in the two proportions, 0.0006; 95% confidence interval for this difference, −0.0461 to 0.0472; P = 0.25). Of the 21 subjects in the treatment failure group, four were lost to follow-up (Table 2). Of the 17 subjects who were followed up, 11 from the cephalexin group and six from the placebo group were subsequently prescribed antibiotics. Four from the cephalexin group underwent another surgical procedure, and two, also from the cephalexin group, were hospitalized.
TABLE 2.
Reasons for clinical failures by study group
| Reason | No. of subjects in study group:
|
|
|---|---|---|
| Cephalexin (n = 13) | Placebo (n = 8) | |
| Nurse practitioner thought abscess was not healing as expected | 8 | 3 |
| Patient was prescribed antibiotics for new, nonadjacent abscess | 0 | 2 |
| Patient returned to emergency department complaining of worsening abscess | 2 | 0 |
| Patient started on antibiotics for unrelated medical condition during study period | 0 | 1 |
| Primary care provider urged patient to withdraw from study due to HIV infection | 1 | 0 |
| Loss to follow-up | 2 | 2 |
Patients who were cured and those who were not did not differ with respect to demographics, medical comorbidities, or abscess characteristics (Table 3). Among those with HIV infection, all six of those on placebo and 8/13 on cephalexin were cured (P = 0.128).
TABLE 3.
Characteristics of the 166 subjects, by clinical outcome
| Characteristic | Value for study group
|
|||
|---|---|---|---|---|
| Cure
|
Failure
|
|||
| Placebo | Cephalexin | Placebo | Cephalexin | |
| Shown as no. (%) of subjects | ||||
| Male sex | 61 (80.3) | 47 (68.1) | 7 (87.5) | 12 (92.3) |
| Homeless | 28 (36.8) | 24 (34.8) | 4 (50.0) | 5 (38.5) |
| Race | ||||
| Pacific Islander | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian | 2 (3.3) | 3 (5.6) | 0 (0.0) | 0 (0.0) |
| African-American | 27 (44.3) | 23 (42.6) | 4 (57.1) | 4 (40.0) |
| Caucasian | 31 (50.8) | 28 (51.9) | 3 (42.9) | 6 (60.0) |
| Hepatitis (B or C) | 23 (30.3) | 21 (30.4) | 5 (62.5) | 4 (30.8) |
| Diabetes | 5 (6.6) | 2 (2.9) | 0 (0.0) | 0 (0.0) |
| Intravenous drug use | 38 (50.0) | 32 (46.4) | 4 (50.0) | 5 (38.5) |
| Tissue depth | ||||
| Subcutaneous | 44 (67.7) | 34 (63.0) | 4 (66.7) | 7 (70.0) |
| Fascia | 9 (13.8) | 10 (18.5) | 1 (16.7) | 1 (10.0) |
| Muscle | 12 (18.5) | 10 (18.5) | 1 (16.7) | 2 (20.0) |
| Shown as mean value | ||||
| Age (yr) | 44.2 | 42.5 | 46.5 | 40.9 |
| Mean amt of pus drained (ml) | 20.9 | 29.3 | 14.7 | 44.1 |
| Surface area of abscess (cm2) | 20.2 | 16.8 | 7.1 | 28.0 |
Pathogen distribution and susceptibility.
There was no difference in pathogen distribution between study groups (Table 4). Of the 162 patients for whom cultures were obtained, Staphylococcus aureus was isolated from 114 (114/162 = 70.4%). Of these 114 S. aureus isolates, 99 were tested for antibiotic susceptibilities; 87 (87/99 = 87.8%) were MRSA. Of these 87 MRSA isolates, 86 were tested for the presence of the PVL gene and 80 were positive for PVL (80/86 = 93.0%). Susceptibility data for the isolates tested are shown in Table 5. There was no difference in the distribution as well as clinical cure rates in patients with PVL-positive MRSA between the placebo group and the cephalexin group (Table 6). Of the seven Streptococcus species-only isolates, the three/five patients on placebo were cured and two/two patients on cephalexin were cured.
TABLE 4.
Pathogen distribution by study group
| Organism | No. (%) of subjects by study group:
|
|
|---|---|---|
| Cephalexin (n = 80) | Placebo (n = 82) | |
| Staphylococcus aureus only | 55 (68.8) | 55 (67.1) |
| Streptococcus species only | 2 (2.5) | 5 (6.1) |
| Both Staphylococcus aureus and Streptococcus species | 3 (3.8) | 1 (1.2) |
| Neither species | 20 (25.3) | 21 (25.6) |
TABLE 5.
Antimicrobial susceptibilities of Staphylococcus aureus from abscesses at enrollment, by study groupa
| Antimicrobial agent | No. (%) of isolates for study group:
|
|
|---|---|---|
| Cephalexin (n = 50) | Placebo (n = 49) | |
| Nafcillin | 6 (12.0) | 6 (12.2) |
| Erythromycin | 8 (16) | 7 (14.3) |
| Ciprofloxacin | 25 (50.0) | 30 (61.2) |
| Levofloxacin | 27 (74.0) | 39 (79.6) |
| Tetracycline | 39 (88.0) | 41 (83.7) |
| Clindamycin | 44 (88.0) | 44 (89.8) |
| Rifampin | 49 (98.0) | 49 (100) |
| Gentamicin | 50 (100) | 49 (100) |
| Trimethoprim-sulfamethazole | 50 (100) | 49 (100) |
| Vancomycin | 50 (100) | 49 (100) |
| Linezolid | 50 (100) | 40 (100) |
Of the 110 staphylococcal isolates, antimicrobial susceptibility data were available for 99.
TABLE 6.
Prevalence and cure rates of PVL-producing MRSA, by study group
| Study group and outcome | No. of isolates
|
||
|---|---|---|---|
| MRSA | PVL positive/tested | Cures/PVL positive | |
| Cephalexin (n = 82) | |||
| Cure | 40 | 38/41 | 38/38 |
| Failure | 2 | 2/2 | 0/2 |
| Placebo (n = 84) | |||
| Cure | 39 | 37/39 | 37/37 |
| Failure | 4 | 3/4 | 0/3 |
DISCUSSION
This study compared a commonly used beta-lactam antibiotic, cephalexin, to placebo for the treatment of uncomplicated skin and soft tissue abscesses after incision and drainage in a population with high rates of community-acquired MRSA. Our findings of high clinical cure rates (84 to 90%) and no difference in clinical cure rates for cephalexin compared to placebo indicate that beta-lactam antibiotics probably do not provide an additional benefit over that afforded by incision and drainage in the treatment of cutaneous abscesses. Even though more than 90% of MRSA clinical isolates were positive for PVL genes, this had no apparent effect on outcome.
A possible criticism of the study design is that no active treatment arm was included (assuming that beta-lactams have no clinically useful antimicrobial activity against MRSA). However, given the high cure rates in both the cephalexin and placebo arms—rates which are consistent with those of four recently published studies of SSTIs (88 to 96%) (1, 13, 27, 29)—it is doubtful that an active agent would have performed any better. The one previous placebo-controlled study of cephradine for soft tissue infections, including abscesses, was conducted in 1985 and included 50 subjects (17). That study found no difference in cure rates between the antibiotic and placebo groups; to our knowledge, microbiologic susceptibility was not reported. Observational, retrospective studies have found no difference in outcomes of patients treated with beta-lactam antibiotics for SSTIs caused by MRSA compared to those given an antibiotic to which the isolate was susceptible (11, 14, 20, 30). Our trial is the first interventional study that directly supports this finding. These observational studies in conjunction with our results strongly suggest that antibiotics may not be necessary for treating some SSTIs, including those caused by MRSA.
Clinicians have been reluctant to change the current standard of care, citing the lack of evidence from well-designed randomized control trials and the increasing prevalence of MRSA (7, 10, 21, 26). Even clinicians knowledgeable about antibiotic overuse continue to prescribe beta-lactam antibiotics for soft tissue infections (14, 24). The two main arguments in support of this practice are (i) that these infections may be polymicrobial, containing some streptococcal species sensitive to beta-lactam antibiotics (2, 8, 11), and (ii) that killing the sensitive organisms in the soft tissue infection may tip the balance in favor of host defenses against the organisms that are resistant to the antibiotic given (11).
The strengths of this study include its randomized, double-blind, placebo-controlled design; a well-defined study population; and a 91.6% follow-up rate (97.6% if the additional 10 patients whose follow-up consisted of chart review are included). Nevertheless, our study does have some limitations. Clinical outcomes were assessed at end of treatment, but 10 patients (6%) had their clinical outcomes determined by chart review. Two antibiotic treatment failures were found in emergency department records. When we analyzed the data excluding these 10 patients, it had no effect on study conclusions. Recurrence rates also were not determined, and whether antimicrobial therapy might have a beneficial effect by preventing recurrent infections some weeks or months later is an important question that merits further study. Data on treatment adherence were based on self-report and were not available for all patients. Adherence, therefore, could have been lower than reported. However, given the high cure rate for the placebo alone, this likely had no impact on results.
Our subjects were adults who were recruited from a single clinic, and they may not be representative of the general population. The MRSA rate of 87.8% in our study is higher than what was previously reported at the same clinic (30) and is higher than rates reported from other study sites (26, 30). In a recent survey of MRSA isolates from this clinic, 91% belonged to the PVL-positive epidemic community clone MRSA USA300 (F. Perdreau-Remington, unpublished data), the most prominent community MRSA clone type in the United States and Canada (4, 23) and one that has been implicated as a cause of very severe infection (19). Thus, all or almost all of the PVL-positive clinical isolates probably were USA300. We also had a high rate of injection drug users (48%). Thus, our findings may not be generalizable to children or to patient populations in which MRSA (and USA300 in particular) or injection drug use is not highly prevalent. Although our study criteria permitted enrollment of febrile patients, because only 1 out of the 166 was febrile these study results may not be generalizable to febrile patients. In addition, incision and drainage procedures were performed by attending surgeons; outcomes may not necessarily be the same in clinics run by other health care providers.
We included patients with medical comorbidities such as HIV infection, hepatitis, diabetes, and folliculitis, populations for whom clinicians often prescribe antibiotics (28), but sample sizes were too small to determine whether or not these subgroups might benefit from antibiotics. This area merits further research because clinicians commonly feel compelled to prescribe antibiotics for infections in these populations.
Another population generally thought to require antibiotics is patients with larger abscesses. A previous study cited an abscess greater than 5 cm in diameter as a significant predictor of hospitalization (10). We included patients with abscesses greater than 5 cm in length (n = 28), width (n = 34), or depth (including those down to the muscle) (n = 24). Although the subgroup sample sizes were too small to make definitive conclusions, our overall findings suggest that even patients with large abscesses may not need antibiotics.
In summary, the 90.5% cure rate observed in the placebo arm of this study and good outcomes in cephalexin recipients despite an overall MRSA prevalence of >50% indicate that antibiotics may be unnecessary after surgical drainage of skin and soft tissue abscesses for populations with high rates of MRSA. Perhaps even more important, this study demonstrates that that a placebo control can be safely used in trials of uncomplicated SSTIs and should be considered in future studies of this type. The results of future placebo-controlled trials of SSTIs could have a significant impact on management of community-acquired MRSA infections.
Acknowledgments
We thank the ISIS Clinic surgeons and staff for their assistance with data collection. We also acknowledge T. G. Berger and M. A. Jacobson for their review of the manuscript.
We thank the UCSF School of Medicine Dean's Fund for Research, the Doris Duke Charitable Foundation, and the Department of Surgery for their support (P.M.R.). This work was carried out in part in the General Clinical Research Center at San Francisco General Hospital and supported by grant 5-MO1-RR00083 from the Division of Research Resources, National Institutes of Health, and USPHS grant R01/CCR923381 (H.C.).
Footnotes
Published ahead of print on 10 September 2007.
REFERENCES
- 1.Breedt, J., J. Teras, J. Gardovskis, F. J. Maritz, T. Vaasna, D. P. Ross, M. Gioud-Paquet, N. Dartois, E. J. Ellis-Grosse, and E. Loh. 2005. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob. Agents Chemother. 49:4658-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook, I. 2002. Microbiology of polymicrobial abscesses and implications for therapy. J. Antimicrob. Chemother. 50:805-810. [DOI] [PubMed] [Google Scholar]
- 3.Carleton, H. A., B. A. Diep, E. D. Charlebois, G. F. Sensabaugh, and F. Perdreau-Remington. 2004. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J. Infect. Dis. 190:1730-1738. [DOI] [PubMed] [Google Scholar]
- 4.Diep, B. A., S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367:731-739. [DOI] [PubMed] [Google Scholar]
- 5.Diep, B. A., G. F. Sensabaugh, N. S. Somboona, H. A. Carleton, and F. Perdreau-Remington. 2004. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J. Clin. Microbiol. 42:2080-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Division of Anti-Infective Drug Product, Food and Drug Administration. 1992. Points to consider, 1992. Division of Anti-Infective Drug Product, Food and Drug Administration, Washington, DC.
- 7.Eady, E. A., and J. H. Cove. 2003. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr. Opin. Infect. Dis. 16:103-124. [DOI] [PubMed] [Google Scholar]
- 8.Ebright, J. R., and B. Pieper. 2002. Skin and soft tissue infections in injection drug users. Infect. Dis. Clin. N. Am. 16:697-712. [DOI] [PubMed] [Google Scholar]
- 9.File, T. M., Jr. 1999. Overview of resistance in the 1990s. Chest 115:3S-8S. [DOI] [PubMed] [Google Scholar]
- 10.Frazee, B. W., J. Lynn, E. D. Charlebois, L. Lambert, D. Lowery, and F. Perdreau-Remington. 2005. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann. Emerg. Med. 45:311-320. [DOI] [PubMed] [Google Scholar]
- 11.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 12.Harris, H. W., and D. M. Young. 2002. Care of injection drug users with soft tissue infections in San Francisco, California. Arch. Surg. 137:1217-1222. [DOI] [PubMed] [Google Scholar]
- 13.Jauregui, L. E., S. Babazadeh, E. Seltzer, L. Goldberg, D. Krievins, M. Frederick, D. Krause, I. Satilovs, Z. Endzinas, J. Breaux, and W. O'Riordan. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 41:1407-1415. [DOI] [PubMed] [Google Scholar]
- 14.Lee, M. C., A. M. Rios, M. F. Aten, A. Mejias, D. Cavuoti, G. H. McCracken, Jr., and R. D. Hardy. 2004. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr. Infect. Dis. J. 23:123-127. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman, J. M. 2003. Appropriate antibiotic use and why it is important: the challenges of bacterial resistance. Pediatr. Infect. Dis. J. 22:1143-1151. [DOI] [PubMed] [Google Scholar]
- 16.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 17.Llera, J. L., and R. C. Levy. 1985. Treatment of cutaneous abscess: a double-blind clinical study. Ann. Emerg. Med. 14:15-19. [DOI] [PubMed] [Google Scholar]
- 18.Mah, M. W., and Z. A. Memish. 2000. Antibiotic resistance. An impending crisis. Saudi Med. J. 21:1125-1129. [PubMed] [Google Scholar]
- 19.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 20.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 21.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 22.NCCLS. 1994. NCCLS 2000 methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard, 5th ed. Document M7-A5. NCCLS, Wayne, PA.
- 23.Pan, E. S., B. A. Diep, H. A. Carleton, E. D. Charlebois, G. F. Sensabaugh, B. L. Haller, and F. Perdreau-Remington. 2003. Increasing prevalence of methicillin-resistant Staphylococcus aureus infection in California jails. Clin. Infect. Dis. 37:1384-1388. [DOI] [PubMed] [Google Scholar]
- 24.Rajendran, P. M., D. M. Young, T. Maurer, H. F. Chambers, M. A. Jacobson, and H. W. Harris. 2007. Antibiotic use in the treatment of soft tissue abscesses: a survey of current practice. Surg. Infect. 8:237-238. [DOI] [PubMed] [Google Scholar]
- 25.Rybak, M. J. 2004. Resistance to antimicrobial agents: an update. Pharmacotherapy 24:203S-215S. [DOI] [PubMed] [Google Scholar]
- 26.Salgado, C. D., B. M. Farr, and D. P. Calfee. 2003. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin. Infect. Dis. 36:131-139. [DOI] [PubMed] [Google Scholar]
- 27.Stryjewski, M. E., W. D. O'Riordan, W. K. Lau, F. D. Pien, L. M. Dunbar, M. Vallee, V. G. Fowler, Jr., V. H. Chu, E. Spencer, S. L. Barriere, M. M. Kitt, C. H. Cabell, and G. R. Corey. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 40:1601-1607. [DOI] [PubMed] [Google Scholar]
- 28.Waldrop, R. D., C. Prejean, and R. Singleton. 1998. Overuse of parenteral antibiotics for wound care in an urban emergency department. Am. J. Emerg. Med. 16:343-345. [DOI] [PubMed] [Google Scholar]
- 29.Weigelt, J., K. Itani, D. Stevens, W. Lau, M. Dryden, and C. Knirsch. 2005. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 49:2260-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young, D. M., H. W. Harris, E. D. Charlebois, H. Chambers, A. Campbell, F. Perdreau-Remington, C. Lee, M. Mankani, R. Mackersie, and W. P. Schecter. 2004. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch. Surg. 139:947-951. [DOI] [PubMed] [Google Scholar]