Abstract
Artemisinin is a plant sesquiterpene lactone that has become an important drug for combating malaria, especially in regions where resistance to other drugs is widespread. While the mechanism of action is debated, artemisinin has been reported to inhibit the sarcoplasmic endoplasmic reticulum Ca2+ ATPase (SERCA) in the malaria parasite. Artemisinin is also effective against Toxoplasma in vitro and in vivo, although it is less potent and, hence, is generally not used therapeutically to treat toxoplasmosis. To explore the mechanism of action, we generated chemically derived mutants of Toxoplasma gondii that were resistant to growth inhibition by this compound in vitro. Three artemisinin-resistant (ARTr) mutant clones that differed in their sensitivities in vitro by three- to fivefold compared with that of the wild-type parasites were obtained. ARTr mutants were cross-resistant to other derivatives of artemisinin, the most potent of which was artemisone. Resistance was not due to molecular alterations or differences in the expression of SERCA or other putative targets, such as proteins that code for multidrug resistance or translationally controlled tumor protein. ARTr mutants were resistant to the induction of protein secretion from micronemes, a calcium-dependent process that is triggered by artemisinin. ARTr mutants were not cross-resistant to secretion induced by thapsigargin but were more sensitive and were unable to regulate cytoslic calcium following treatment with this compound. These studies implicate calcium homeostasis in the mechanism of action of artemisinins against apicomplexan parasites.
Artemisinin is a natural product that is produced by the sweet wormwood plant (Artemisia annua). Artemisinin and various related derivatives are potent antimalarial compounds that are used to treat human malaria, especially in regions where resistance to other antimalarial drugs is common (14). Artemisinins contain an essential endoperoxide ring that is thought to be activated by reduced iron (Fe2+) to form reactive intermediates (14). In malaria parasite-infected red blood cells, artemisinin may be activated by heme, which is released from hemoglobin, leading to reactions with secondary targets in the parasite and the restriction of growth (22, 23). Direct labeling studies indicated that one potential target of artemisinin is the translationally controlled tumor protein (TCTP) (5), which binds to artemisinin in Plasmodium falciparum-infected red blood cells. More recent studies suggest an alternative mechanism of action based on the ability of artemisinin to inhibit ATPase6, a sarcoplasmic endoplasmic reticulum-like Ca2+ ATPase (SERCA) of P. falciparum (11). Inhibition of P. falciparum ATPase6 (PfSERCA) could potentially cause growth inhibition by altering calcium homeostasis. Thus, artemisinin may have a mechanism similar to that of thapsigargin, a structurally related compound that is a well-known inhibitor of SERCA (37, 38).
Recent concerns about the possible development of drug resistance have led to the recommended cessation of monotherapy with artemisinin in the field (42). Several potential mechanisms have been proposed to explain the resistance in the malaria parasite. Laboratory reports have indicated that increased numbers of copies of multidrug resistance (MDR) gene 1 (MDR1) are associated with resistance to artemisinin in Plasmodium yoelli (12) or, alternatively, with the increased expression of TCTP (41). Stable resistance to artemisinin has also been developed in the rodent malaria parasite Plasmodium chabaudi; however, it was not associated with mutations in ATPase6 or other suspected targets, such as MDR1 (1). The recent report that mutations in ATPase6 (S769N) are associated with the elevated resistance of P. falciparum parasites to artemether in French Guyana (16) supports the hypothesis that artemisinin and related compounds target SERCA. Other studies reported elevated levels of MDR1 expression in recrudescent or recurrent P. falciparum malaria in Southeast Asia in patients receiving combined therapy with artesunate and mefloquine, although pressure from the latter drug alone may explain this result (2). Hence, there remains some question about the molecular target(s) of artemisinin and about the potential for the development of resistance to this important antimalarial drug.
Artemisinin is also effective against Toxoplasma gondii, although the 50% effective concentrations (EC50s) are ∼50-fold higher than those for malaria parasites (15, 36). This difference in sensitivity may relate to molecular differences in the target(s); differences in activation of the drug; or possibly, differences in efflux mechanisms, such as those for MDR. Toxoplasma gondii is also sensitive to other derivatives, such as artemether, and to several newly synthesized derivatives that are structurally similar to artemisinin (17). Artemisinin is also effective against trypanosomes, where it inhibits Ca2+ ATPase activity in parasite membranes (24), and in inhibiting tumor cells in vitro, where the mechanism of action involves calcium and the induction of apoptosis (28).
The availability of excellent experimental tools for T. gondii has previously been exploited to identify the molecular basis for the actions of drugs that disrupt nucleotide or protein metabolism (30). Combined with techniques for forward genetics (18) and reverse genetics (35), this parasite offers an excellent experimental model with which drug mechanisms and the basis of resistance can be explored. Stepwise selection with increasing concentrations of drug has previously been used to isolate mutants of T. gondii that are resistant to artemisinin (4). However, such mutants are difficult to analyze at the molecular level, as they may arise by multiple alterations in different targets. On the other hand, chemical mutagenesis results in specific point mutations induced by DNA alkylating agents, and this approach has previously been used with T. gondii to map the molecular basis of a variety of specific inhibitors of nucleic acid metabolism (31, 32, 34). In the study described in the present report, we isolated chemically induced mutants of T. gondii that were resistant to artemisinin in order to explore the molecular mode of action of this class of drugs.
MATERIALS AND METHODS
Parasites and culture.
The strains used in this study were T. gondii RH (ATCC 50838); clone 2F (ATCC 50839), which expresses bacterial β-galactosidase (9); and artemisinin-resistant mutant clone A2 (4). They were maintained as tachyzoites in human foreskin fibroblast (HFF) cells grown in Dulbecco's modified Eagle's medium with 10 mM HEPES, 44 mM sodium bicarbonate, 10% fetal bovine serum, 2 mM glutamine, and 10 μg/ml gentamicin.
Establishment of ARTr mutants.
T. gondii clone 2F was used to produce chemically induced mutants that were resistant to artemisinin by previously described procedures (33). Intracellular tachyzoites grown in HFF cells were treated with 100, 200, or 500 μg/ml N-nitroso-N-ethyl-urea (ENU; Sigma-Aldrich, St. Louis, MO) in serum-free medium for 1 h at 37°C. Parasites treated with each dose of ENU were harvested and inoculated into separate T25 flasks containing HFF cells and were selected individually with 2.4, 12, or 300 μM artemisinin. Artemisinin-resistant (ARTr) clones were isolated by single-cell cloning in 96-well plates containing HFF cells.
Parasite growth assay.
Parasites were inoculated into quadruplicate wells per sample in 96-well plates containing monolayers of HFF cells and were treated with artemisinin (Sigma); dihydroartemisinin (LKT Laboratories, Inc., St. Paul, MN); artemether (LKT Laboratories, Inc.); artesunate (LKT Laboratories, Inc.); and 9-epi-10-deoxoartemisinin, 2-deoxyartemisinin, and artemisone (provided by R. Haynes and S. Krishna) at concentrations ranging from 10 nM to 100 μM for 3 days at 37°C. In parallel, mutants were tested for resistance to 5-fluoro-2-deoxyuridine (FUDR; Sigma), or pyrimethamine (Sigma). Following treatment, the culture medium was removed and the monolayers were incubated at 50°C for 10 min in 50 μl of lysis buffer (100 mM HEPES pH 8.0, 1 mM MgSO4, 1% Triton X-100, 5 mM dithiothreitol). After lysis, 160 μl of assay buffer (100 mM phosphate buffer, pH 7.3, 102 mM β-mercaptoethanol, 9 mM MgCl2 [final concentrations]) was added to each well and the plate was further incubated for 10 min at room temperature. The reaction was initiated by addition of 40 μl of 6.25 mM chlorophenol red-β-d-galactopyranoside (Roche Diagnostics, Indianapolis, IN) to each well, and the absorbance (the optical density at 570 nm) was measured. Mean values from three independent experiments were used to estimate the 50% effective concentrations (EC50s) and EC90s by nonlinear regression analysis with the KaleidaGraph program (Synergy Software, Reading, PA).
MIC2 secretion assay.
Microneme (MIC) secretion assays were performed as described previously (8, 21). Briefly, freshly egressed parasites were suspended in assay medium (Dulbecco's modified Eagle's medium with 44 mM sodium bicarbonate, 20 mM HEPES, 2 mM glutamine, 10 μg/ml gentamicin, 3% fetal bovine serum) and were treated with different concentrations of calcium agonists for 5 or 10 min at 18°C. Following the treatments, the parasites were transferred to 37°C for 2 min to allow secretion, chilled on wet ice, and separated into the supernatant and cell pellet by centrifugation at 400 × g. Proteins were resolved by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and Western blotted by using rabbit anti-microneme protein 2 (anti-MIC2) antibody (1:10,000) or mouse anti-β-galactosidase monoclonal antibody 40a-1 (1:300) and horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G (1:10,000) (Jackson Immunoresearch Laboratories, West Grove, PA). Signals were detected by using Super Signal West Pico (Pierce, Rockford, IL).
Intracellular calcium monitoring in live parasites.
Intracellular calcium was monitored by using fura-2-AM {1-[6-amino-2-(5-carboxy-2-oxazolyl)-5-benzofuranyloxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid-pentaacetoxymethyl ester} as described previously (26). Freshly harvested tachyzoites were washed and resuspended in buffer A (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 5.5 mM d-glucose, 50 mM HEPES, pH 7.4) plus 1.5% (wt/vol) sucrose and 5.2 μM fura-2-AM. Parasites were incubated for 30 min at 26°C and then washed twice with buffer A to remove the extracellular dye. The parasites were resuspended in buffer A (final density, 2 × 107 cells/ml), and the fura-2 fluorescence was monitored at room temperature with a Hitachi F-4500 spectrofluorometer (excitation, 340 and 380 nm; emission, 510 nm). The intracellular calcium concentration ([Ca2+]i) was calculated by titration with different concentrations of Ca2+-EGTA buffers by using the ratio of the fluorescence values at 340 and 380 nm after subtraction of the background fluorescence (25). The concentrations of the ionic species and complexes at equilibrium were calculated by using an iterative computer program, as described previously (13). The traces shown are representative of at least three independent experiments conducted with separate cell preparations.
Sequencing of additional Ca2+ ATPases.
Previously identified Ca2+ ATPases in the T. gondii genome (29) were amplified by PCR with gene-specific primers designed to be specific for the draft 3 annotation of the genome (http://ToxoDB.org). Total mRNA was extracted from the RH strain of T. gondii by using the Trizol reagent (Invitrogen, Carlsbad, CA), and cDNAs were generated by using Superscript3 reverse transcriptase (Invitrogen). Full-length cDNAs were sequenced by using gene-specific primers to amplify portions of the gene that were then directly sequenced by using the ABI technology (performed by SeqWright DNA Technology Services, Houston, TX). Consensus sequences were aligned by the using CAP3 program (http://bio.ifom-firc.it/ASSEMBLY/assemble.html), translated into predicted proteins (http://searchlauncher.bcm.tmc.edu/seq-util/Options/sixframe.html), aligned using the ClustalW program (http://www.ebi.ac.uk/clustalw/), and submitted to GenBank.
Real-time qPCR.
PCR primers were designed by using the Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Real-time quantitative PCR (qPCR) was performed with a SmartCycler instrument (Cepheid, Sunnyvale, CA) in a reaction volume of 25 μl containing SYBR GreenER qPCR SuperMix universal (Invitrogen), 0.4 μM each primer, and cDNA reverse transcribed from 3 μg of total RNA. The reaction conditions were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Data analysis was conducted with SmartCycler software (Cepheid). Relative gene expression levels were calculated as the fold change by using the formula 2 − ΔΔCT, where ΔCT is the threshold cycle number (CT) for actin minus the CT for the target gene (see Table 3) and ΔΔCT is the ΔCT for wild-type strain 2F minus the ΔCT for mutant RNAs (20). The housekeeping gene encoding actin (ACT1) was used as a reference control.
TABLE 3.
qPCR analysis of Ca2+ ATPases, MDRs, and TCTP
| NCBI no. | ToxoDB identifiera | Gene | Fold difference for cloneb:
|
||
|---|---|---|---|---|---|
| KN200-1 | KN200-6 | STL500-10A | |||
| AY727534 | 44.m02594 | SERCA | 0.9 ± 0.3 | 1.0 ± 0.6 | 0.7 ± 0.4 |
| EF394334 | 583.m00010 | TgA1 | 1.3 ± 0.1 | 2.7 ± 0.8 | 1.0 ± 0.4 |
| EF394331 | 44.m02812 | TgA2 | 1.0 ± 0.6 | 1.0 ± 0.6 | 0.7 ± 0.5 |
| EF394332 | 65.m01184 | PMR1 | 1.1 ± 0.8 | 1.1 ± 0.3 | 1.7 ± 0.5 |
| EF394333 | 641.m01482 | Golgi-ERc | 0.9 ± 0.6 | 1.1 ± 0.9 | 0.9 ± 0.2 |
| 55.m00137 | MDR1A | 1.2 ± 0.3 | 0.7 ± 0.1 | 0.8 ± 0.2 | |
| 49.m03125 | MDR1B | 1.2 ± 0.1 | 0.7 ± 0.6 | 0.6 ± 0.1 | |
| 59.m03673 | MDR2 | 0.9 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | |
| 50.m03408 | TCTP | 1.1 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.2 | |
Fold differences versus the value for the wild type (strain 2F), which was set equal to 1.0. Values are the averages ± standard deviations of two or three experiments.
ER, endoplasmic reticulum.
Nucleotide sequence accession numbers.
The complete cDNA sequences for the following calcium ATPases from the RH strain were submitted to GenBank: T. gondii SERCA (TgSERCA; draft 3 annotation, 44.m02594; GenBank accession no. AY727534), the plasma membrane calcium ATPase (PMCA)-type Ca2+ ATPase T. gondii plasma membrane calcium ATPase 1 (TgA1;; draft 3 annotation, 583.m00010; GenBank accession no. EF394334), Golgi-endoplasmic reticulum-type Ca2+ ATPase (draft 3 annotation, 641.m01482; GenBank accession no. EF394333), PMR1-like Ca2+ ATPase (draft 3 annotation, 65.m01184; GenBank accession no. EF394332), and the PMCA-type Ca2+ ATPase T. gondii plasma membrane calcium A2 (TgA2; draft 3 annotation, 44.m02812; GenBank accession no. EF394331).
RESULTS
Establishment of ARTr mutants of T. gondii.
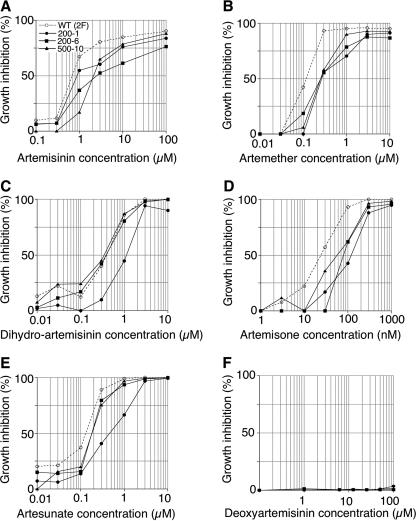
To investigate the mechanism of resistance to artemisinin, we generated resistant clones of T. gondii following chemical mutagenesis. ARTr mutants were selected with three different doses of artemisinin (i.e., 2.4, 12, and 300 μg/ml) following mutagenesis with three different doses of ENU (i.e., 100, 200, and 500 μg/ml). Following repeated screening from different mutagenesis trials, three independent mutants were isolated. Comparison of the growth of these mutants by using the β-galactosidase growth assay indicated that they exhibited approximately two- to threefold increases in the EC50s of artemisinin and approximately fivefold increases in the EC90s of artemisinin compared with those for the wild-type parasites (Fig. 1; Table 1). The level of resistance exhibited by these mutants was independent of the dose of artemisinin used for selection, which ranged from 2 to >100 times greater than the EC50. While the level of resistance exhibited by these mutants is modest, it nonetheless allowed the isolation of clones from a population of wild-type parasites by continued growth in the presence of drug over multiple rounds of passage. The resistance phenotype (EC50 of artemisinin) was stable when the parasites were tested in the presence of artemisinin following repeated passage in the absence of drug and following cryopreservation (data not shown).
FIG. 1.
Isolation of ARTr mutants of T. gondii. The growth of ARTr mutant parasites versus that of the wild-type (WT) parasites in medium containing the indicated compounds is shown. Parasite clones were inoculated in 96-well plates containing HFF cells, and parasite growth was monitored by measurement of β-galactosidase activity following 72 h of incubation with different concentrations of compounds (see Materials and Methods). 200-1, mutant KN200-1; 200-6, mutant KN200-6; 500-10, mutant STL500-10A. The ARTr mutants are defined in Table 1.
TABLE 1.
Selection of ARTr mutants and inhibition by artemisinin, FUDR, and pyrimethaminea
| Clone name | ENU concn (μg/ml)b | ART selection concn (μM)c | Artemisinin
|
EC50 (μM)
|
||
|---|---|---|---|---|---|---|
| EC50 (μM) | EC90 (μM) | FUDR | Pyrimethamine | |||
| Wild type (2F) | 0 | 0 | 0.80 | 1.24 | 0.91 | 0.21 |
| KN200-1 | 200 | 12 | 1.73 | 6.85 | 1.13 | 0.29 |
| KN200-6 | 200 | 2.4 | 1.51 | 8.55 | 1.19 | 0.27 |
| STL500-10A | 500 | 300 | 1.80 | 4.77 | 0.96 | 0.24 |
Estimates were based on the mean of three independent experiments by using quadruplicate wells per sample over a range of concentrations from 10 nM to 100 μM.
The ENU concentration used for mutagenesis.
The artemisinin (ART) concentration used for selection.
Comparison of the growth kinetics of the mutants with those of the wild-type parasites revealed that ARTr mutants of T. gondii were also cross-resistant to a variety of semisynthetic artemisinin derivatives (Fig. 1). Strain KN200-1 was weakly resistant to artemisinin but was slightly more resistant to the other compounds tested (Fig. 1). Interestingly, two of the mutants (KN200-6 and STL-500-10A) did not show resistance to dihydroartemisinin or artesunate, which is rapidly converted to dihydroartemisinin (Fig. 1). Notably, artemisone was ∼30-fold more potent against wild-type parasites (EC50, ∼30 nM) than artemisinin (EC50, 800 nM) (Fig. 1). None of the mutants was resistant to deoxoartemisinin (data not shown), although this compound was also more than 10-fold less potent against wild-type parasites (EC50, ∼10 μM). Neither the wild type nor the mutants showed susceptibility to a derivative not containing an endoperoxide (2-deoxyartemsinin) (Fig. 1F).
The mechanism of resistance in the ARTr mutants of T. gondii does not appear to be due to MDR, as they were still sensitive to the unrelated drugs FUDR and pyrimethamine (Table 1). Moreover, qPCR of several MDR homologues in the T. gondii genome failed to show upregulation of these transcripts (Tables 2 and 3). Previous studies have also suggested that the upregulation of TCTP in P. yoelli may be responsible for artemisinin resistance (41). As such, we checked the parasites for the expression of the mRNA for the TCTP orthologue in T. gondii. qPCR showed no changes in the level of expression of TCTP in the ARTr mutants of T. gondii (Tables 2 and 3), suggesting that the protein encoded by this gene is not responsible for the resistance observed here.
TABLE 2.
Primers used for qPCR
| Gene | Database identifiera | Primer sequence
|
|
|---|---|---|---|
| Forward | Reverse | ||
| TgACT1 | 25.m00007 | 5′-TCCCGTCTATCGTCGGAAAG-3′ | 5′-CCATTCCGACCATGATACCC-3′ |
| TgSERCA | 44.m02594 | 5′-TGATGATCACTGGAGACAACAAGT-3′ | 5′-GAGAGGACTTCTTTCTTCTCTTCAA-3′ |
| TgA1 | 583.m00010 | 5′-AGTTGCAGGAGAAGAACTTCAAAT-3′ | 5′-AACCTACATGTTTGACTTGTGTGAA-3′ |
| TgA2 | 44.m02812 | 5′-GTTTGCTGATTTCTGGAGTTGAAT-3′ | 5′-CACTTCTGTCCAGAGTCTTCTTCTT-3′ |
| PMR1 | 65.m01184 | 5′-GAAAAGGAGTTGAGTTGTCTGCAT-3′ | 5′-CAGCAAATGTAAAAACTTCTGTGAA-3′ |
| Golgi-ERb | 641.m01482 | 5′-AGAACAAGCCTCTAGTCCTCACTCT-3′ | 5′-GAGACCAATGATTTTCACTTTGAAT-3′ |
| MDR1A | 55.m00137 | 5′-TGTCTATGCCTGAAAAAGAAAGTCG-3′ | 5′-GGAAGAACACAGCTATGAATCGAGA-3′ |
| MDR1B | 49.m03125 | 5′-TCTTTCTGGAGTTTGATTTTCGTTG-3′ | 5′-GAAGGAGAGAAATGAGCTTGTAGCC-3′ |
| MDR2 | 59.m03673 | 5′-TTTATCCTTCTTGGAGTTTCGCCTA-3′ | 5′-GACGTCGAGAAGAGAATGAGAAACA-3′ |
| TCTP | 50.m03408 | 5′-ATTGCTGACAATAGCGAGGAAGAC-3′ | 5′-GCTGCATGTAACCTTTGATGTAGGT-3′ |
ER, endoplasmic reticulum.
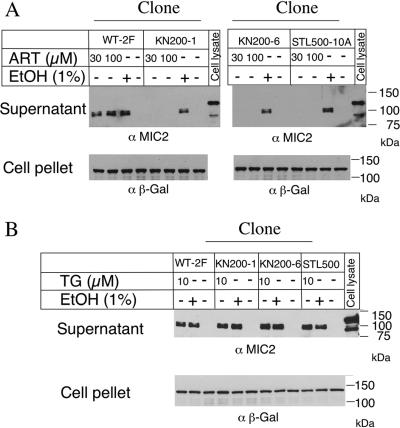
ARTr mutants are resistant to calcium-induced secretion triggered by artemisinin.
We have recently shown that artemisinin induces calcium-dependent secretion of MIC proteins from T. gondii, an effect that is shared by the SERCA inhibitor thapsigargin (29a). MIC2 secretion can easily be detected by the release of the reporter protein MIC2 into the supernatant following stimulation with agonists that raise intracellular calcium levels (7, 21). To determine if ARTr mutants of T. gondii were sensitive to induced secretion, we tested their responses to several calcium agonists. While ARTr mutants responded normally to ethanol, a potent agonist of calcium-mediated secretion (7), they did not secrete MIC2 when they were stimulated with up to 100 μM artemisinin (Fig. 2A). Because wild-type T. gondii secreted MIC2 after treatment with 10 μM artemisinin (Fig. 2A), this result indicates that the mutants were more than 10-fold resistant to artemisinin-induced secretion. It has been suggested that artemisinin may have a mechanism of action similar to that of thapsigargin on the basis of a shared binding site in SERCA (39). Therefore, we tested whether the ARTr mutants were cross-resistant to thapsigargin-stimulated MIC2 secretion. No differences in the sensitivities between the parental wild-type clone (clone 2F) and ARTr mutants were observed (Fig. 2B). Furthermore, we investigated ARTr mutant clone A2, which was previously established by stepwise selection (4). This mutant was also resistant to the induction of MIC2 secretion by artemisinin but responded normally to induction by thapsigargin (data not shown).
FIG. 2.
Induction of MIC2 secretion by calcium agonists in wild-type (WT) and ARTr mutant parasites. (A) MIC2 secretion induced by artemisinin. Wild-type (strain 2F) parasites secreted MIC2 in response to artemisinin, while the mutants (KN200-1, KN200-6, and STL500-10A) were resistant. Parasites were treated with 30 or 100 μM artemisinin (ART) or 1% ethanol (EtOH) for 10 min and incubated at 37°C for 2 min before separation of the supernatant and the cell pellet. Fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with anti-MIC2 (α MIC2)-specific or anti-β-galactosidase (α β-Gal)-specific antibodies. (B) ARTr mutants were not cross-resistant to thapsigargin (TG). Parasites were treated with 10 μM thapsigargin or 1% ethanol for 10 min at 18°C and were transferred to 37°C for 2 min to stimulate secretion. Samples were analyzed as described for panel A.
ARTr mutants have profound defects in calcium homeostasis.
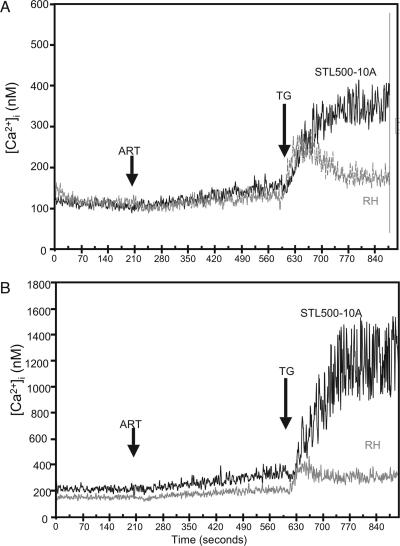
To further explore the physiological basis for the observed resistance, ARTr clones were compared to the wild-type parasites by using fura-2 to monitor [Ca2+]i. ARTr mutants showed elevated [Ca2+]i compared to those in the wild-type parasites both in the absence and in the presence of extracellular calcium (Table 4). The elevations in [Ca2+]i were approximately 2- to 2.5-fold, and while a change of this magnitude is relatively small, such elevations may perturb normal calcium signaling. Treatment of the parasites with artemisinin (20 μM) resulted in only a slight increase in [Ca2+]i levels in either the wild type or the ARTr clones (Fig. 3). As reported previously (26), the treatment of wild-type parasites with thapsigargin (1 μM) resulted in a rapid increase in [Ca2+]i, and in wild-type parasites this response partially recovered toward the baseline over time (Fig. 3). Similar responses were observed in parasites incubated in the absence (Fig. 3A) and the presence of extracellular calcium (Fig. 3B). Surprisingly, when the ARTr clones were stimulated with thapsigargin, [Ca2+]i increased dramatically and did not recover, as shown for clone STL500-10A (Fig. 3). This effect was more pronounced in the presence of extracellular calcium (Fig. 3B) than in its absence (Fig. 3A). The remaining ARTr mutants of T. gondii also showed similar unregulated increases in [Ca2+]i following treatment with thapsigargin (data not shown). Collectively, these results indicate that ARTr clones have elevated resting [Ca2+]i and are unable to regulate intracellular calcium following treatment with thapsigargin.
TABLE 4.
[Ca2+]i in T. gondii, as monitored with fura-2
| Clone | [Ca2+]i (nM)a
|
|
|---|---|---|
| Absence of extracellular calciumb | Presence of extracellular calciumc | |
| Wild type (RH) | 99 ± 23 | 175 ± 29 |
| KN200-1 | 150 ± 44 | 370 ± 171 |
| KN200-6 | 228 ± 50 | 533 ± 153 |
| STL500-10A | 122 ± 30 | 233 ± 31 |
Values are means ± standard deviations from three independent experiments.
1 mM EGTA.
1 mM CaCl2.
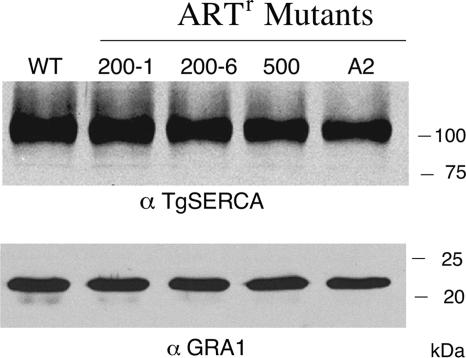
FIG. 3.
Western blot analysis indicates similar levels of expression of TgSERCA in wild-type strain 2F and the ARTr mutants. Cell lysates of the wild type (WT) and the KN200-1 (200-1), KN200-6 (200-6), STL500-10A (500), and A2 (A2) mutants were analyzed by Western blotting with anti-TgSERCA (α TgSERCA) or anti-GRA1 (α GRA1) antibodies.
Expression of TgSERCA and other Ca2+ ATPases.
Previous studies have emphasized that amino acid differences in the putative artemisinin-binding pocket can mediate profound differences in the sensitivity of PfSERCA to inhibition in a heterologous expression system (39). Additionally, field studies have suggested that changes outside the transmembrane domains of SERCA might be responsible for resistance to artemsinins (1). Therefore, we sequenced the entire coding region of the SERCA gene in each of the three mutants listed in Table 1. No differences in amino acid sequences were identified, although the mutant STL-500-10A had a single silent mutation at position 2508 (C was changed to T). We also sequenced the SERCA gene from the previously characterized ARTr A2 clone of T. gondii (4) and found no sequence difference (data not shown). Hence, the differences in artemisinin sensitivity of these clones are not due to mutations in the SERCA gene that would alter the protein. The levels of TgSERCA, as assessed by Western blotting, were also similar between the wild-type strain (the 2F clone) and the ARTr mutants (Fig. 4), indicating that resistance is unlikely to be due to differences in levels of expression.
FIG. 4.
Intracellular calcium levels in T. gondii as monitored with fura-2-AM. Treatment with artemisinin (ART) showed only a modest elevation of the [Ca2+]i in both the wild type (strain RH) and an ARTr mutant (clone STL-500-10A). However, treatment with thapsigargin (TG) induced a rise in [Ca2+]i in wild-type parasites that partially returned to the baseline level. In contrast, treatment of the ARTr mutant STL500-10A with thapsigargin resulted in a prolonged elevation of [Ca2+]i that did not recover. (A) Cells were incubated in the absence of extracellular calcium (plus 1 mM EGTA). (B) Cells were incubated in the presence of extracellular calcium (1 mM). Arrows indicate the addition of artemisinin (20 μM) or thapsigargin (1 μM).
Apicomplexans contain a number of other P-type Ca2+ ATPases (29), suggesting that resistance might arise by alterations in one of these genes. To examine this potential mechanism, we sequenced the full-length cDNAs for four additional candidate genes from both wild-type clone 2F and the three ARTr mutants generated here. Included in this set of genes are the PMCA-type Ca2+ ATPases known as TgA1 and a related gene, TgA2; a second endoplasmic reticulum-type Ca2+ ATPase related to PMR1, and a Golgi-type Ca2+ ATPase. No alterations in the coding regions of these genes were found, although several allelic differences were noted between the type I alleles sequenced here and the type II genome used for reference in the T. gondii genome database (19). Additionally, we analyzed the levels of expression of each of these genes in the mutants compared to those in wild-type strain 2F by qPCR. The levels of expression did not vary by more than approximately twofold, suggesting that resistance was not due to large changes in the levels of expression of these particular Ca2+ ATPases (Tables 2 and 3).
DISCUSSION
The risk for the development of resistance to artemisinins is serious, given the current widespread insensitivity of the malaria parasite to other antimalarial drugs. Hence, defining the mechanism of action and the potential for the development of resistance is a high priority. Laboratory studies can be highly informative about the potential for drug resistance under controlled conditions. Additionally, chemical mutagenesis provides a powerful system for defining the molecular targets in T. gondii, as shown by previous studies with inhibitors of purine and pyrimidine metabolism (32-34) and compounds that disrupt the cytoskeleton (9, 27). Chemical mutagenesis was used here to examine resistance to artemisinin, and while the molecular basis of resistance was not determined precisely, the mutants revealed several unexpected findings that have important implications. ARTr mutants of T. gondii were only rarely isolated, and those mutants that were obtained showed only low-level resistance, although these differences were stable and clearly sufficient to allow selection. This stands in marked contrast to other such screens in which mutants with high-level resistance have been obtained. These features predict that (i) the primary target is essential, (ii) the binding site of the compound is not readily amenable to mutation and/or, (iii) multiple targets may be present. By using a different strategy of stepwise selection, ARTr mutants were obtained in the rodent malaria parasite P. chabaudi, and these mutants also showed low-level resistance (5- to 10-fold). While only low-level resistance to artemisinins has thus far been obtained in laboratory studies, this may still be clinically relevant, as shown by field studies of P. falciparum malaria (16).
The search for chemically stable artemisinins with improved pharmacokinetics and decreased toxicity has led to the development of a large number of derivatives, several of which show increased activities against the malaria parasite (14, 42). The sensitivity of wild-type T. gondii to these different semisynthetic derivatives revealed potencies in the following order: artemisone > artesunate ≈ artemether > dihdyroartemisinin > artemisinin. Similar to reports for the malaria parasite (40), artemisone was more than 10-fold more active than artesunate in inhibiting wild-type T. gondii and almost 30-fold potent than artemisinin. Previous studies have indicated that artemether does not provide protection against toxoplasmosis in the rat model (6). On the basis of the findings of the present study, greater in vivo efficacy might be expected with artemisone treatment of toxoplasmosis.
ARTr mutants of T. gondii were highly stable with continued passage and survived cryopreservation and resuscitation, indicating that they likely have stable genetic changes due to the mutations induced by ENU treatment. However, sequencing of the SERCA gene revealed no molecular differences, nor was there evidence for amplification or overexpression. Moreover, the resistance phenotype was not due to an MDR-like mechanism. Surprisingly, when ARTr cells were treated with thapsigargin, they exhibited extreme elevations in [Ca2+]i and failed to recover to resting levels, unlike wild-type parasites. One potential mechanism that may explain these findings would be if recovery from treatment with thapsigargin is not solely due to the action of SERCA but also is due to another mechanism that is defective in the ARTr mutants. In addition to SERCA, there are four other Ca2+ ATPases in the T. gondii genome, including two plasma membrane Ca2+ ATPases (PMCA type), a second endoplasmic reticulum-type Ca2+ ATPase, and a Golgi-type Ca2+ ATPase (29). However, changes in these other Ca2+ ATPases also do not appear to explain the findings for the mutants, as they did not show differences in sequences or expression levels that would be consistent with a role in resistance. It remains possible that subtle changes in Ca2+ ATPase expression levels may explain the elevated calcium levels and homeostasis defects in the mutants.
Alterations in calcium homeostasis may also arise from disruption of cation transporters other than P-type Ca2+ ATPases. One potential candidate is a plant-like Ca2+/H+ exchanger (T. gondii gene identifier 25.m01788) that is conserved in apicomplexans (29). Although little is known about the role of this transporter in parasites, it may be localized to the acidocalcisome, an intracellular organelle that is important in calcium homeostasis (10). Additionally, previous studies have also shown that disruption of a plasma membrane Na+/H+ exchanger affects calcium homeostasis and alters calcium ionophore-induced egress in T. gondii (3). Alterations of these or alternative cation transporters may affect resting calcium levels and result in resistance to artemisinin by an indirect mechanism. Given the diversity of cation transporters in the genome, a wider range of potential targets needs to be considered in analyzing artemisinin resistance in apicomplexans, including the malaria parasite.
In Plasmodium, the SERCA orthologue PfATPase6 was reported to be a target of the antimalarial drug artemisinin on the basis of heterologous expression studies with Xenopus (11). Consistent with this model, field studies have indicated that the S769N mutation in PfSERCA is associated with increased resistance to artemether (16). Our results with T. gondii are consistent with artemisinin playing a role in calcium homeostasis, possibly by affecting SERCA. The expanded use of artemisinin derivatives for the treatment of malaria makes the identification of its molecular target(s) and resistance mechanisms of high priority (42). Further studies on calcium homeostasis in parasites will be important both for determining the mechanism of action and for understanding the potential mechanisms of resistance to artemisinins.
Acknowledgments
We are grateful to Sanjeev Krishna and Richard Haynes for helpful suggestions and the artemisinin derivatives, Tyler Curiel for providing the A2 mutant, and Julie Nawas and Cuiying Jiang for expert technical assistance. Preliminary genomic DNA sequence data were provided by The Institute for Genomic Research, the Wellcome Trust Sanger Institute, Washington University, and the University of Pennsylvania.
This study was supported by NIH grants AI067051 (to L.D.S.) and AI68467 (to S.N.J.M.) and by the Uehara Memorial Foundation (to K.N.).
Footnotes
Published ahead of print on 13 August 2007.
REFERENCES
- 1.Afonso, A., P. Hunt, S. J. Cheesman, A. C. Alves, C. V. Cunha, V. do Rosario, and P. Cravo. 2006. Malaria parasites can develop stable resistance to artemisinin but lack mutations in the candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrob. Agents Chemother. 50:480-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alker, A. P., P. Lim, R. Sem, N. K. Shah, P. Yi, D. M. Bouth, R. Tsuyuoka, J. D. Maquire, T. Fandeur, F. Ariey, C. Wongsrichanalai, and S. R. Meshnick. 2007. PFMDR1 and in vivo resistance to arteunate-methfloquine in falciparum malaria on the Cambodian-Thai border. Am. J. Trop. Med. Hyg. 76:641-647. [PubMed] [Google Scholar]
- 3.Arrizabalaga, G., F. A. Ruiz, S. Morena, and J. C. Boothroyd. 2004. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J. Cell Biol. 165:653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berens, R. L., E. C. Krug, P. B. Nash, and T. J. Curiel. 1998. Selection and characterization of Toxoplasma gondii mutants resistant to artemisinin J. Infect. Dis. 177:1128-1131. [DOI] [PubMed] [Google Scholar]
- 5.Bhisutthibhan, J., X. A. Pan, P. A. Hoosler, D. J. Walker, C. A. Yowell, J. Carlton, J. B. Dame, and S. R. Meschnick. 1998. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J. Biol. Chem. 273:16192-16198. [DOI] [PubMed] [Google Scholar]
- 6.Brun-Pascaud, M., F. Chau, F. Derouin, and P. M. Girard. 1996. Lack of activity of artemether for prophylaxis and treatment of Toxoplasma gondii and Pneumocystis carinii infections in rat. Parasite 3:187-189. [DOI] [PubMed] [Google Scholar]
- 7.Carruthers, V. B., S. N. J. Moreno, and L. D. Sibley. 1999. Ethanol and acetaldehyde elevate intracellular [Ca2+] calcium and stimulate microneme discharge in Toxoplasma gondii. Biochem. J. 342:379-386. [PMC free article] [PubMed] [Google Scholar]
- 8.Carruthers, V. B., G. D. Sherman, and L. D. Sibley. 2000. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 275:14346-14353. [DOI] [PubMed] [Google Scholar]
- 9.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite Cell 84:933-939. [DOI] [PubMed] [Google Scholar]
- 10.Docampo, R., W. Souza, K. Miranda, P. Rohloff, and S. N. Moreno. 2005. Acidocalcisomes—conserved from bacteria to man. Nat. Rev. Microbiol. 3:251-261. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein-Ludwig, U., R. J. Webb, I. D. A. van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer-Rodriguez, I., J. Perez-Rosado, G. W. Gervais, W. Peters, B. L. Robinson, and A. E. Serrano. 2004. Plasmodium yoelii: identification and partial characterization of an MDR1 gene in an artemisinin-resistant line J. Parasitol. 90:152-160. [DOI] [PubMed] [Google Scholar]
- 13.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 14.Haynes, R. K., and S. Krishna. 2004. Artemisinins: activities and actions. Microb. Infect. 6:1339-1346. [DOI] [PubMed] [Google Scholar]
- 15.Holfels, E., J. McAuley, D. Mack, W. K. Milhous, and R. McLeod. 1994. In vitro effects of artemisinin ether, cycloguanil hydrochloride (alone and in combination with sulfadiazine), quinine sulfate, mefloquine, primaquine phosphate, trifluoperazine hydrochloride, and verapamil on Toxoplasma gondii. Antimicrob. Agents Chemother. 38:1392-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jambou, R., E. Legrand, M. Niang, N. Khim, P. Lim, B. Volney, M. T. Ekala, C. Bouchier, P. Esterre, T. Fandeur, and O. Mercereau-Puijalon. 2005. Resistance of Plasmodium falciparum field isolates to in vitro artemether and point mutations of the SERCA-type Pf-ATPase6. Lancet 366:1960-1963. [DOI] [PubMed] [Google Scholar]
- 17.Jones-Brando, L., J. D'Angelo, G. H. Posner, and R. H. Yolken. 2006. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob. Agents Chemother. 50:4206-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan, A., S. Taylor, C. Su, A. J. Mackey, J. Boyle, R. H. Cole, D. Glover, K. Tang, I. Paulsen, M. Berriman, J. C. Boothroyd, E. R. Pfefferkorn, J. P. Dubey, D. S. Roos, J. W. Ajioka, J. C. Wootton, and L. D. Sibley. 2005. Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii. Nucleic Acids Res. 33:2980-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kissinger, J. C., B. Gajria, L. Li, I. T. Paulsen, and D. S. Roos. 2003. ToxoDB: accessing the Toxoplasma gondii genome. Nucleic Acids Res. 31:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta-delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 21.Lovett, J. L., N. Marchesini, S. N. Moreno, and L. D. Sibley. 2002. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from IP3/ryanodine sensitive stores. J. Biol. Chem. 277:25870-25876. [DOI] [PubMed] [Google Scholar]
- 22.Meschnick, S. R., A. Thomas, A. Ranz, C. M. Xu, and H. Z. Pan. 1991. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49:181-189. [DOI] [PubMed] [Google Scholar]
- 23.Meshnick, S. R. 1998. Artemisinin antimalarials: mechanisms of action and resistance. Med. Trop. (Mars) 58(3 Suppl.):13-17. [PubMed] [Google Scholar]
- 24.Mishina, Y. V., S. Krishna, R. K. Haynes, and J. C. Meade. 2007. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma rhodesiense in vitro growth. Antimicrob. Agents Chemother. 51:1852-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno, S. N., A. E. Vercesi, O. P. Pignataro, and R. Docampo. 1992. Calcium homeostasis in Trypanosoma cruzi amastigotes: presence of inositol phosphates and lack of an inositol 1,4,5-trisphosphate-sensitive calcium pool. Mol. Biochem. Parasitol. 52:251-261. [DOI] [PubMed] [Google Scholar]
- 26.Moreno, S. N. J., and L. Zhong. 1996. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem. J. 313:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrissette, N. S., A. Mitra, D. Sept, and L. D. Sibley. 2004. Dinitroanalines bind alpha-tubulin to disrupt microtubules. Mol. Biol. Cell 15:1960-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu, D., W. Chen, B. Yu, C. Zhang, Y. W. Zhang, and H. Qi. 2007. Calcium and survivin are involved in the induction of apoptosis by dihydroartemisin in human lung cancer SPC-A-1 cells. Methods Fund. Exp. Clin. Pharmacol. 29:33-38. [DOI] [PubMed] [Google Scholar]
- 29.Nagamune, K., and L. D. Sibley. 2006. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the Apicomplexa. Mol. Biol. Evol. 23:1613-1627. [DOI] [PubMed] [Google Scholar]
- 29a.Nagamune, K., W. L. Beatty, and L. D. Sibley. 31 August 2007. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell doi: 10.1128/EC.00262-07. [DOI] [PMC free article] [PubMed]
- 30.Pfefferkorn, E. R. 1990. Cell biology of Toxoplasma gondii, p. 26-50. In D. J. Wyler (ed.), Modern parasite biology. W. H. Freeman, New York, NY.
- 31.Pfefferkorn, E. R., and S. E. Borotz. 1994. Toxoplasma gondii: characterization of a mutant resistant to 6-thioxanthine. Exp. Parasitol. 79:374-382. [DOI] [PubMed] [Google Scholar]
- 32.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1976. Arabinosyl nucleosides inhibit Toxoplasma gondii and allow the selection of resistant mutants. J. Parasitol. 62:993-999. [PubMed] [Google Scholar]
- 33.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1979. Quantitative studies of the mutagenesis of Toxoplasma gondii. J. Parasitol. 65:363-370. [PubMed] [Google Scholar]
- 34.Pfefferkorn, E. R., and L. C. Pfefferkorn. 1977. Toxoplasma gondii: characterization of a mutant resistant to 5-fluorodeoxyuridine. Exp. Parasitol. 42:44-55. [DOI] [PubMed] [Google Scholar]
- 35.Roos, D. S., R. G. K. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:28-61. [DOI] [PubMed] [Google Scholar]
- 36.Sarciron, M. E., C. Saccharin, A. F. Petavy, and F. Peyron. 2000. Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii. Am. J. Trop. Med. Hyg. 62:73-76. [DOI] [PubMed] [Google Scholar]
- 37.Thastrup, O., P. J. Cullen, B. K. Drobak, M. R. Hanley, and A. P. Dawson. 1990. Thapsigargin, a tumor promotor, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 87:2466-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thastrup, O., A. P. Dawson, O. Scharff, B. Foder, P. J. Cullen, B. K. Drobak, P. J. Bjerrum, S. B. Christensen, and M. R. Hanley. 1989. Thapsigargin, a novel molecular probe for studying calcium release and storage. Agents Actions 27:17-23. [DOI] [PubMed] [Google Scholar]
- 39.Uhlemann, A. C., A. Cameron, U. Eckstein-Ludwig, J. Fischbarg, P. Iserovich, F. A. Zuniga, M. East, A. Lee, L. Brady, R. K. Haynes, and S. Krishna. 2005. A single amino acid residue can determine the sensitivity of SERCAs to artemisinins. Nat. Struct. Mol. Biol. 12:628-629. [DOI] [PubMed] [Google Scholar]
- 40.Vivas, L., L. Rattray, L. B. Stewart, B. L. Robinson, B. Fugmann, R. K. Haynes, W. Peters, and S. L. Croft. 2007. Antimalarial efficacy and drug interactions of the novel semi-synthetic endoperoxide artemisone in vitro and in vivo. J. Antimicrob. Chemother. 59:658-665. [DOI] [PubMed] [Google Scholar]
- 41.Walker, D. J., J. L. Pitsch, M. M. Peng, B. L. Robinson, W. Peters, J. Bhisutthibhan, and S. R. Meshnick. 2000. Mechanism of artemisinin resistance in the rodent malaria pathogen Plasmodium yoelii. Antimicrob. Agents Chemother. 44:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodrow, C. J., and S. Krishna. 2006. Antimalarial drugs: recent advances in molecular determinants of resistance and their clinical significance. Cell. Mol. Life Sci. 63:1586-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]