Abstract
The conserved family of AMT/Rh proteins facilitates ammonium transport across animal, plant, and microbial membranes. A bacterial homologue, AmtB, forms a channel-like structure and appears to function as an NH3 gas channel. To evaluate the function of eukaryotic homologues, the human RhCG glycoprotein and the tomato plant ammonium transporter LeAMT1;2 were expressed and compared in Xenopus oocytes and yeast. RhCG mediated the electroneutral transport of methylammonium (MeA), which saturated with Km = 3.8 mM at pHo 7.5. Uptake was strongly favored by increasing the pHo and was inhibited by ammonium. Ammonium induced rapid cytosolic alkalinization in RhCG-expressing oocytes. Additionally, RhCG expression was associated with an alkali-cation conductance, which was not significantly permeable to NH4 + and was apparently uncoupled from the ammonium transport. In contrast, expression of the homologous LeAMT1;2 induced pHo-independent MeA+ uptake and specific NH4 + and MeA+ currents that were distinct from endogenous currents. The different mechanisms of transport, including the RhCG-associated alkali-cation conductance, were verified by heterologous expression in appropriate yeast strains. Thus, homologous AMT/Rh-type proteins function in a distinct manner; while LeAMT1;2 carries specifically NH4 +, or cotransports NH3/H+, RhCG mediates electroneutral NH3 transport.
INTRODUCTION
Ammonium (NH4 +/NH3) is vital for the growth of microorganisms and plants, but it can reach toxic levels when in excess. (The term ammonium is used for the sum of NH4 + and NH3, the chemical symbols are used to denote the molecular species specifically. The same formalism applies to methylammonium [MeA+/MeA]). Most organisms have developed complex ammonium homeostasis mechanisms that comprise ammonium metabolism, import, and export. Proteins involved in ammonium transport were initially identified from yeast and plants and belong to the AMT/MEP/Rh protein family (pfam00909) (Marini et al., 1994; Ninnemann et al., 1994). Later human and animal homologues, the Rh glycoproteins, were identified as ammonium transporters (Marini et al., 2000). Some Rh glycoproteins are involved in the disposal of ammonium in the kidney, probably to maintain acid–base homeostasis (Weiner, 2004).
Plant and yeast AMT/MEPs are involved in the membrane potential–dependent acquisition of the nutrient ammonium (Ninnemann et al., 1994; Marini et al., 1997; Ludewig et al., 2002; Sohlenkamp et al., 2002; Ludewig et al., 2003). This has also been established for many bacterial AMT homologues (Kleiner, 1985; Siewe et al., 1996; Meier-Wagner et al., 2001). An external recruitment site for NH4 + at the pore entrance has been identified in the recently solved crystal structure of the bacterial homologue from Escherichia coli, AmtB (Khademi et al., 2004; Zheng et al., 2004). Based on the rigid high-resolution channel-like structure of AmtB, which represents a milestone in membrane protein structure analysis, Khademi et al. (2004) speculated that AMT/Rh proteins are gas channels that recruit external NH4 + but conduct NH3. This view is supported by recent functional analyses that suggest that AmtB conducts the gas NH3 (Khademi et al., 2004; Javelle et al., 2005). Although an intriguing hypothesis, the gas interpretation may not be valid for eukaryotic members of the family. This “gas” hypothesis is also in opposition to the long-standing view that bacterial ammonium transporters are NH4 + transporters (Kleiner, 1985).
It is of considerable physiological relevance whether neutral NH3 or positively charged NH4 + is transported, since the gradient across the membrane is often opposite for both species. This is the situation at the apical membrane of distal kidney epithelia, where RhCG is coexpressed with the V-type ATPase that actively exports protons into the acidic lumen. Thus, only a small fraction of total luminal ammonium is NH3, which results in an outward NH3 gradient. In contrast, the inside negative membrane potential of the epithelial cells favors NH4 + influx along the electrochemical gradient for NH4 + and, in the presence of a pathway for NH4 +, this will promote accumulation of ammonium in the cytosol. A similar situation is encountered at the plant root epidermis, the site of LeAMT1;2 expression.
The mechanism of ammonium transport is highly controversial for many AMT/Rh homologues. For example, the close AmtB homologue LeAMT1;1 from tomato was suggested to transport NH4 + (Ludewig et al., 2002), and electrogenic NH4 + transport has also been conjectured for the mouse homologue Rhbg (Nakhoul et al., 2005) when expressed in oocytes. In contrast, electroneutral transport has been reported for human RhBG (Ludewig, 2004) and RhAG (Westhoff et al., 2002). However, no ionic current measurements have been shown for RhAG (Westhoff et al., 2002), and the transport of the ammonium analogue MeA/MeA+ has not been studied in Rhbg (Nakhoul et al., 2005). A study on genetic variants of human and mouse erythrocytes and erythrocyte ghosts identified RhAG as a major protein involved in rapid ammonium-dependent alkalinization. It was concluded that RhAG facilitates NH3 diffusion (Ripoche et al., 2004). RhCG was expressed in oocytes and it was suggested that it transports both NH3 and NH4 + (Bakouh et al., 2004). A study on RhAG expression in mammalian cell lines similarly suggested both NH3 and NH4 + transport by RhAG (Benjelloun et al., 2005).
Much of the debate about the mechanism of transport in AMT/Rh proteins originates from the fact that NH4 + transport had been measured in Xenopus oocytes, cells that had been shown to express endogenous NH4 +-inducible currents when exposed to high ammonium concentrations such as 10 mM (Cougnon et al., 1996; Burckhardt and Burckhardt, 1997). However, this view has recently been challenged by several laboratories, and oocytes do not express large endogenous ammonium-inducible currents when exposed to ammonium at <1 mM (Ludewig et al., 2002; Ludewig et al., 2003; Bakouh et al., 2004; Holm et al., 2005).
In this paper we studied two individual eukaryotic AMT/Rh homologues using identical protocols. Human RhCG was chosen as a member of the Rh branch of the family and the plant LeAMT1;2 from Lycopersicon esculentum (tomato) as a representative of the AMT branch (Ludewig et al., 2001). Methylammonium and ammonium transport were compared using current and flux measurements. The data identified remarkable differences between the RhCG glycoprotein and the plant AMT transporter and suggest that RhCG functioned as an electroneutral channel or transporter. Transport may involve external recruitment of NH4 +/NH3, followed by NH3 conduction, as has been suggested for the bacterial homologue AmtB (Khademi et al., 2004; Zheng et al., 2004). Additionally, expression of the RhCG glycoprotein was associated with an ammonium-independent cation conductance in Xenopus oocytes. In contrast, tomato AMT proteins evoked specific, saturable, voltage-dependent, pHo-independent NH4 + (or NH3/H+) currents that were distinct from endogenous ammonium currents. The differences in transport were confirmed by expression in appropriate yeast strains. Thus, despite their sequence similarity and conservation, plant AMT and mammalian Rh glycoproteins are functionally distinct, which may reflect their different physiological roles in ammonium excretion in animals and ammonium acquisition in plants.
MATERIALS AND METHODS
Plasmid Constructs
All ammonium transporter genes were inserted into plasmid pOO2 (Ludewig et al., 2002). Human RhCG was cloned from a kidney cDNA library (CLONTECH Laboratories, Inc.) and checked by sequencing. The RhCG construct contained a 9-bp sequence (Kozak motif: GCCGCCACC) upstream of the ATG. The plasmid pOO2-LeAMT1;2 has been described in a previous study (Ludewig et al., 2003). Capped cRNA was transcribed by SP6 RNA polymerase in vitro using mMessage mMachine (Ambion), after linearization of the plasmid with MluI.
Preparation and Injection of Oocytes
Individual Xenopus frogs were kept separately in water tanks in a plant growth room at 21°C and 10 h light/14 h dark cycle. Oocytes were removed from adult female frogs by surgery and manually dissected. Oocytes (Dumont stage V or VI) were defolliculated using collagenase 10 mg/ml (Boehringer) and trypsin inhibitor 5 mg/ml (Sigma-Aldrich) for 1–2 h and injected with ∼50 nl of cRNA (≈20 ng/per oocyte). Oocytes were kept after injection for 2–3 d at 16°C in ND96 (in mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES (pH 7.4), supplemented with 2.5 mM Na-pyruvate and gentamycin (20 μg/ml). Oocytes were then used for measurements, or were kept at 4°C for another day and then measured.
Electrophysiological Measurements
Standard recording solution was (in mM) 100 NaCl, 2 CaCl2, 2 MgCl2, 4 Tris, pH adjusted to 7.5. In some experiments the pH of this solution was adjusted to pH 5.5, 6.5, or 8.5 with 2-[N-morpholino] ethanesulfonic acid (MES). In potassium- and choline-based solutions, sodium was replaced by the respective chloride salt. In some solutions NaCl was replaced by Na-glutamate and recordings were performed using a ground electrode connected to the recording solution via an agar bridge. Oocytes were placed in a small recording chamber and the gravity-driven solution exchange allowed the full exchange of bath solutions within a few seconds. The oocytes were clamped to −30 mV, which is close to the resting membrane potential of most oocytes used, and the currents were measured after stepping from this holding potential to different test potentials in 20 mV steps for periods of ≤200 ms. Within these short pulses, currents were time independent. At voltages below −120 mV a slowly activating current was observed in many oocyte batches (also in native oocytes). Therefore only these brief voltage pulses were used to monitor endogenous and Rh-associated currents specifically. All recordings were performed at 20–21°C.
For the current–voltage plots, the constant current at each voltage was averaged and plotted against the voltage. When the slowly activating current below −120 mV was observed, the current at the beginning of the pulse was used. A complete recording for a single ammonium concentration included (a) brief voltage steps from +40 to −140 mV in control solution without ammonium, (b) addition of ammonium and the identical pulse protocol, (c) followed by complete wash with standard bath solution and acquisition of the current response to an additional pulse protocol. A full recording was completed within <2 min. These brief applications of ammonium minimized secondary effects due to long incubation in ammonium. Each experimental result was observed in at least three independent batches of oocytes. In more than six batches of oocytes, no differences were observed between noninjected or water-injected oocytes and their respective ammonium and methylammonium transport. Means ± standard deviations are given. In some figures the currents “induced by ammonium” are shown; these were obtained by subtracting the current in the absence of ammonium from the current in the presence of ammonium at each voltage. NH3 and NH4 + concentrations were determined from total ammonium (pKa = 9.25) concentrations using the Henderson-Hasselbalch equation: log10([NH3]/[NH4 +]) = pH−pKa. The pKa = 10.66 was used for calculations involving methylammonium (CH3-NH3 +/CH3-NH2). Before most electrophysiological recordings of RhCG, and all electrophysiological recordings shown in the figures, expression of equally treated oocytes was tested for 14C-methylammonium uptake. All the RhCG expressing oocytes also expressed the RhCG-associated conductance, so that in a few later experiments this current was taken as indicator of RhCG expression. The concentration dependence of currents/uptakes was fitted using the following equation: I = Imax/(1 + Km/c), where Imax is the maximal current/uptake at saturating concentration, Km is the substrate concentration permitting half-maximal currents/uptakes, and c is the experimentally used concentration.
Selection for Healthy Oocytes
Ammonium (1 mM) did not increase the endogenous background currents in oocytes from >30 oocyte batches, as partially reported previously (Ludewig et al., 2002, 2003) and in accordance with observations from other groups (Bakouh et al., 2004; Holm et al., 2005). Oocyte batches with healthy native and H2O-injected oocytes that were used in this study had background currents of less than −80 nA at −140 mV, a background conductance of ≤0.9 μS (between 0 and −100 mV) and a resting membrane potential between −75 and −20 mV. There was no need for preselection of such oocytes, but we noted that some, less healthy appearing oocytes displayed a larger endogenous, more variable, slightly outwardly rectifying background conductance even in the absence of ammonium. These oocytes displayed ammonium (1 mM) and methylammonium (10 mM) inducible currents that were similar to the currents described earlier in native oocytes (Burckhardt and Thelen, 1995; Cougnon et al., 2002; Boldt et al., 2003). The background current of the oocytes used in our study was about half of the endogenous current from oocytes used in laboratories that have recorded and investigated endogenous NH4 +-induced currents (Burckhardt and Thelen, 1995; Cougnon et al., 2002; Boldt et al., 2003). No seasonal differences in endogenous oocyte currents were observed in this study.
Radiotracer Uptake
The ammonium analogue 14C-methylammonium (Amersham Biosciences) was used as radiotracer. Choline- or sodium-based bath solutions were used as indicated for uptake experiments. Batches of >10 oocytes were incubated for 20 min in 200 μl of the respective buffer containing 14C-labeled methylammonium (or at 1/10 dilution; specific activity: 1.85 GBq/mmol) at room temperature. Then the oocytes were carefully washed five times in 1 ml ice-cold buffer containing 10 mM unlabeled methylammonium and separated into three per scintillation vial. After solubilization with 10 μl 5% SDS, 4 ml scintillation buffer was added and activity was analyzed by liquid scintillation counting. For the calculation of the concentration dependence of RhCG, the uptake of water-injected controls was subtracted.
Intracellular pH Changes (pHi)
Intracellular pH changes (pHi) were monitored using the pH-sensitive dye 2',7'-bis-(carboxyethyl)-5(6)-carboxyfluorescein (BCECF) (Sasaki et al., 1992). In brief, several healthy oocytes were placed in uptake buffer (ND96, containing 5 μM BCECF-AM, from a 50 mM stock in DMSO) and incubated at room temperature for 30 min protected from light. Two BCECF-loaded oocytes were placed on a coverslip and mounted on an inverted Leica fluorescence microscope (objective lens ×20). The oocytes were superfused with the standard solution containing 0.5 or 10 mM NH4Cl by gravity flow. Pictures of BCECF fluorescence were taken at maximal rate every 5 s using a spinning wheel fluorescence system with excitation at 410 ± 30 and 470 ± 20 nm and emission at 535 ± 20 nm. The fluorescent ratios were calibrated as in Ludewig (2004).
Expression in Yeast
RhCG and LeAMT1;2 were subcloned into pDR195 and expressed in the ura − Saccharomyces cerevisiae wild-type strain (23344c), the ammonium transporter defective yeast strain (31019b; ΔΔΔmep1;2;3) (Marini et al., 1997) and the alkali metal cation–sensitive strain AB11c (ena1Δ∷HIS3∷ena4; nha1Δ∷LEU2; nhx1Δ∷TRP1) (Maresova and Sychrova, 2005). The plasmid pNHA1-985, harboring the S. cerevisiae Na+/H+ antiporter gene NHA1 under its own promoter was used as a positive control (Maresova and Sychrova, 2005). The growth medium for strain AB11c was YNB (1.7 g/liter yeast nitrogen base w/o amino acid and ammonium sulfate; Difco) supplemented with 3% glucose, 0.1% arginine, 0.01% adenine, 0.01% tryptophane solidified with 2% agar. The pH was adjusted using 50 mM TRIS/MES to pH 5.0 or pH 7.0. The nitrogen-deficient growth medium for strain 31019b was YNB, supplemented with 3% glucose and appropriate NH4Cl concentrations and was adjusted to pH 5.5 using 50 mM MES. The methylammonium toxicity medium contained YNB, 3% glucose, 0.1% arginine, and 125 mM methylammonium.
Online Supplemental Material
Fig. S1 shows the pH dependence of MA uptake by RhCG and concentration dependence of NH4 + currents by LeAMT1;2. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.200509369/DC1.
RESULTS
Ammonium-independent Rh-associated Currents
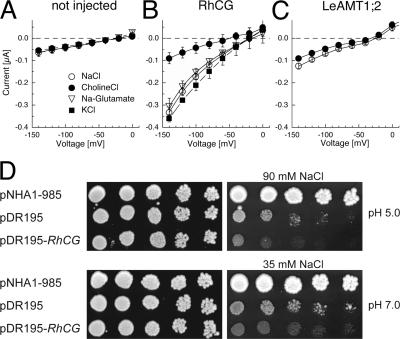
RhCG expression in oocytes was associated with ionic currents in standard solutions, even in the absence of ammonium and methylammonium (Fig. 1, A and B). A small increase in background currents compared with noninjected controls was also seen with LeAMT1;2 expression (Fig. 1, A and C). The currents from native oocytes were not affected by a change to sodium-free choline or potassium solutions, while the RhCG-associated currents diminished in solutions in which sodium was replaced by choline, a large cation that is relatively impermeable to most ion channels (Fig. 1 B). The substitution of sodium by potassium slightly increased the currents in RhCG-expressing oocytes (Fig. 1 B). Although Rh-associated currents were small in choline-based solutions, they were still detectable compared with nonexpressing controls. To test if the inward current was carried by chloride efflux, chloride was partially replaced by glutamate (94%). This did not affect the inward currents and only marginally affected the reversal potential, suggesting that the Rh-associated currents are mainly carried by cations (Fig. 1 B). The maximal RhCG-associated inward currents were ∼−1 μA. This current will be referred to as the Rh-associated conductance throughout the manuscript.
Figure 1.
Voltage-dependent currents associated with RhCG expression are permeable to sodium and potassium. (A) Current–voltage relations of noninjected oocytes, (B) RhCG-expressing oocytes and (C) LeAMT1;2-expressing oocytes at pHo 7.5 (n = 4). Similar data were obtained in oocytes from three batches. (D) RhCG expression increased sodium sensitivity of an alkali metal–sensitive mutant yeast strain. Serial fivefold dilutions of 25-fold diluted saturated cultures were spotted on YNB media with added sodium (right) or without (control, left) and growth was followed for 3 (control plates) or 4 d (plates supplemented with sodium). Yeast expressing the Na+/H+ antiporter NHA1 were insensitive to sodium, but yeast expressing RhCG had increased sodium sensitivity compared with vector-transformed control.
To test if the Rh-associated cation conductance was associated with RhCG expression in another heterologous host, RhCG was expressed in an alkali metal–sensitive S. cerevisiae strain (Δena1-4, Δnha1, Δnhx1) (Maresova and Sychrova, 2005). RhCG increased sodium sensitivity of yeast growth (Fig. 1 D), which may argue that an Rh-associated cation influx is not restricted to RhCG expression in oocytes.
Endogenous Currents Activated by Ammonium at Alkaline pHo
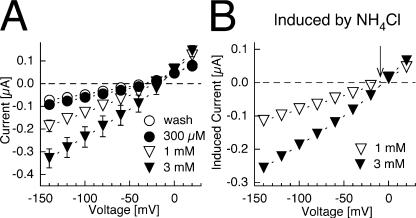
Currents in native oocytes were insensitive to ammonium (<1 mM) at physiological pHo, as reported earlier (Ludewig et al., 2002, 2003; Bakouh et al., 2004; Holm et al., 2005). As in previous studies, ammonium-activated currents were observed at alkaline pHo in native oocytes, which increased in magnitude with elevated pHo and NH3 concentrations (Boldt et al., 2003). Representative current–voltage relations of endogenous ammonium-activated currents at pHo8.5 showed that the currents reversed at ∼0 mV and were outward at positive voltages (Fig. 2).
Figure 2.
Current–voltage relations of endogenous ammonium-induced currents. (A) Currents from native oocytes at pHo 8.5 without ammonium (open circles), by 300 μM (closed circles), 1 mM (open triangle), and 3 mM ammonium (filled triangle), respectively. (B) Ammonium-induced currents (background subtracted, from A) in a typical oocyte batch. Note that ammonium-induced currents have a reversal potential (arrow) and are outward at positive voltages. Data shown are from oocytes from a single batch, similar data were obtained in two other oocyte batches.
Endogenous Transport of Methylammonium Is Electroneutral in Xenopus Oocytes
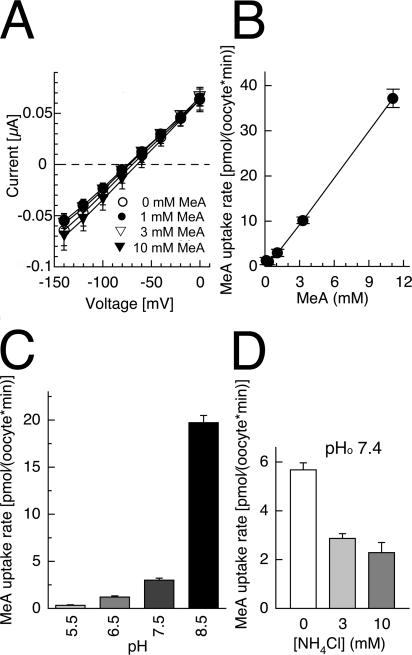
Endogenous background currents were unchanged after addition of the widely used transport analogue methylammonium (10 mM) in all batches tested. Although methylammonium did not affect ionic currents, radiolabeled 14C-methylammonium was taken up (Fig. 3 B). This is compatible with electroneutral MeA transport in native oocytes. The 14C-methylammonium uptake rate depended linearly on concentration (Fig. 3 B) and increased with more alkaline pHo. The increase was less than the 10-fold increase in the free MeA concentration per pH unit (Fig. 3 C). Interestingly, 14C-methylammonium uptake at pHo 7.4 was partially blocked by ammonium (Fig. 3 D), suggesting that 14C-MeA uptake was only partially mediated by lipid diffusion.
Figure 3.
Electroneutral uptake of methylammonium in noninjected control Xenopus oocytes is partially blocked by ammonium. (A) Current–voltage relations without methylammonium (open circles) and with various methylammonium concentrations, 1 mM (filled circles), 3 mM (open triangles), 10 mM (filled triangles) at pHo 7.5. Symbols are partially overlapping. (B) Concentration dependence of 14C-methylammonium uptake rate at pHo 7.5. (C) pHo dependence of 14C-methylammonium uptake rate. (D) Block of 14C-methylammonium uptake (1 mM) by ammonium.
Saturable Methylammonium Transport by RhCG and Inhibition by Ammonium
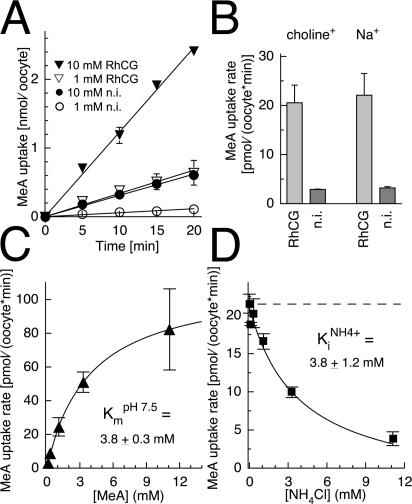
14C-methylammonium uptake into oocytes was increased by RhCG expression (Fig. 4). The uptake of 14C-methylammonium did not saturate within 20 min, either at 1 or 10 mM concentration (Fig. 4 A). RhCG expression increased the 14C-methylammonium transport in oocytes by ∼7–10-fold and was indistinguishable in sodium or choline solutions (Fig. 4 B). Though methylammonium was transported, the addition of methylammonium to RhCG-expressing oocytes did not lead to an increase of the membrane conductance (see below and Fig. 6 D).
Figure 4.
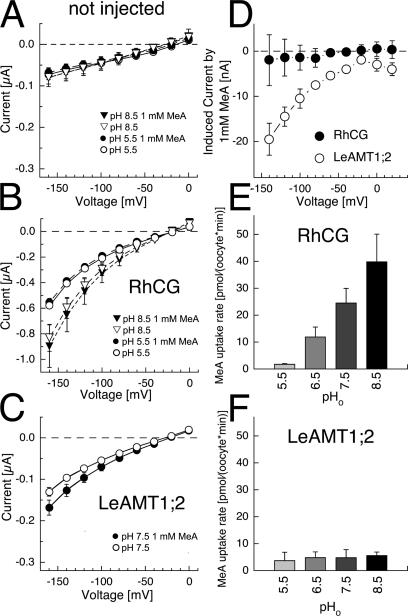
Methylammonium uptake by RhCG-expressing oocytes. (A) Linear MeA uptake by noninjected controls (circles) and RhCG (triangles) at 1 mM (open symbols) and 10 mM (filled symbols) at pHo 7.5. (B) MeA uptake (1 mM) of noninjected and RhCG-expressing oocytes in sodium-free choline (left) and sodium solutions (right). (C) Concentration dependence of RhCG at pHo 7.5. (D) Block of MeA uptake (2 mM) by ammonium, Ki NH4+ = 3.8 ± 1.2 mM at pHo 7.5. Data from four oocyte batches.
Figure 6.
Distinct MeA+ currents and MeA uptake by RhCG and LeAMT1;2. (A) Current–voltage relations from noninjected control oocytes (A) and in RhCG-expressing oocytes (B) in the absence (open symbols) and presence (filled symbols) of methylammonium at pHo 5.5 (circles) and pHo 8.5 (triangles). Symbols are partially overlapping. (C) Current–voltage relations from LeAMT1;2-expressing oocytes in the absence (open circles) and presence (filled circles) of methylammonium at pHo 7.5. (D) Induced current by methylammonium (background currents subtracted) in RhCG (filled circles) and LeAMT1;2-expressing oocytes (open circles). (E) 14C-methylammonium uptake by RhCG at different pHo; background uptake of native oocytes from parallel experiments was subtracted. (F) 14C-methylammonium uptake by LeAMT1;2 at different pHo; background uptake of parallel experiments with native oocytes was subtracted. Note the different current scales in A–C. All experiments in this figure were performed with [methylammonium] = 1 mM, except for panel F: [methylammonium] = 500 μM. Data were collected from six oocyte batches.
The 14C-MeA uptake rates by RhCG were obtained by subtracting the endogenous background uptake at each pHo using equally treated native oocytes from the same batch. The 14C-methylammonium concentration permitting half-maximal transport rate (Km) was ∼3.8 mM at pHo7.5 (Fig. 4 C). The 14C-methylammonium transport was blocked by ammonium, with a Ki = 3.8 mM for NH4 + (or Ki = 18 μM calculated for free NH3 in solution) (Fig. 4 D).
The saturation constant of uptake was determined at two additional pHo, 6.5 and 8.5 (see Fig. S1, available at http://www.jgp.org/cgi/content/full/jgp.200509369/DC1). At pHo 6.5, the concentration permitting half-maximal transport rate was Km pH6.5 = 7.8 ± 2.0 mM, and at pHo 8.5, Km pH8.5 = 1.9 ± 0.5 mM. If calculated for uncharged MeA in solution, the following values were obtained: Km pH6.5 = 0.54 μM and Km pH8.5 = 13 μM.
Saturable MeA+ Currents in LeAMT1;2-expressing Oocytes
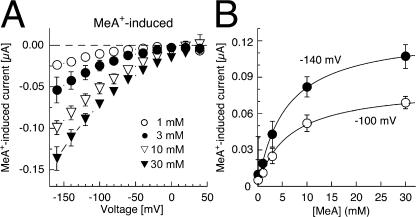
In contrast to the situation with RhCG, the external application of MeA+ induced time-independent inward currents of up to ∼−100 nA in LeAMT1;2-expressing oocytes. A current–voltage plot of the MeA+-induced currents is shown in Fig. 5 A. MeA+-induced currents were determined by subtracting the current without MeA+ from the currents in MeA+-containing solution. The MeA+-induced currents were always inwardly directed, even at positive membrane potentials, as to be expected for an externally applied substrate. The currents saturated in the low mM concentration range and half-maximal currents were 6.1 ± 1.1 mM at −140 mV and 5.6 ± 1.0 mM at −100 mV (Fig. 5 B).
Figure 5.
MeA+ currents by LeAMT1;2. (A) MeA+-induced currents by LeAMT1;2 at pHo 7.5 (n = 4). Background currents were subtracted. Open circles, 1 mM methylammonium; filled circles, 3 mM; open triangles, 10 mM; filled triangles, 30 mM. (B) Concentration dependence of MeA+ currents at −100 mV (open circles, Km = 6.1 ± 1.1 mM) and −140 mV (closed circles, Km = 5.6 ± 1.0 mM) by LeAMT1;2 at pHo 7.5 (n = 4). Data are from three different batches.
Electroneutral MeA Transport by RhCG, but MeA+ Currents by LeAMT1;2
The differences in methylammonium transport by RhCG and LeAMT1;2 were evaluated in detail in Fig. 6. The total methylammonium of all experiments in this figure was 1 mM, except for Fig. 6 F, which was performed with 500 μM. As a control, experiments were also performed with native, noninjected, and H2O-injected oocytes.
In native oocytes, the application of methylammonium did not induce ionic currents at pHo 5.5 and pHo 8.5 (Fig. 6 A). Similarly, currents in RhCG-expressing oocytes were not changed by methylammonium at low and physiological pHo (Fig. 6 B), but a small conductance increase in RhCG-expressing oocytes was observed with methylammonium at alkaline pHo 8.5 (Fig. 6 B). Although negligible currents were observed with methylammonium, 14C-MeA uptake was observed, which strongly increased with more alkaline external pHo (Fig. 6 E). The RhCG uptake rate increase was less than 10-fold per pH unit, but resembled the pHo-dependent MeA uptake by RhAG (Westhoff et al., 2002) and RhBG (Ludewig, 2004).
The methylammonium uptake rate by LeAMT1;2 was distinct and invariant against pHo changes (Fig. 6 F). The 14C-methylammonium uptake by LeAMT1;2-expressing oocytes was paralleled with an MeA+ inward current in the LeAMT1;2-expressing oocytes (Fig. 6 C).
Effect of Ammonium on Ionic Currents by RhCG and LeAMT1;2
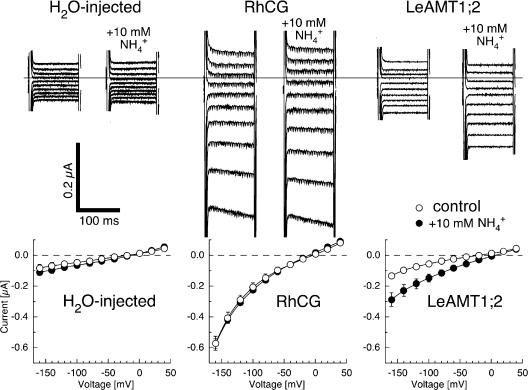
Original current traces and current–voltage plots in the absence and presence of 10 mM ammonium are shown in Fig. 7 for H2O-injected and RhCG- and LeAMT1;2- expressing oocytes. Currents from H2O-injected and RhCG-expressing oocytes were not substantially changed by 10 mM ammonium, although RhCG-expressing oocytes had larger background current overall. In contrast, a large current increase by ammonium was observed in LeAMT1;2-expressing oocytes. The current increase by ammonium was maximal at the most negative voltages tested and was inward even at positive membrane potentials. This is in agreement with the fact that ammonium was only applied externally, and if ammonium does not rapidly equilibrate with the cytosol, specific NH4 + currents are expected to be exclusively inward.
Figure 7.
Ionic currents by ammonium in H2O-injected, RhCG-expressing, and LeAMT1;2-expressing oocytes. Top, original raw current traces without (left) and with 10 mM NH4 + (right). Voltage pulses were given from 40 to −140 mV in steps of −20 mV. Note the current increase by NH4 + in LeAMT1;2 and the large RhCG-associated currents. Bottom, current–voltage plots of currents by ammonium in H2O-injected (n = 4), RhCG-expressing (n = 6), and LeAMT1;2-expressing (n = 5) oocytes with 10 mM NH4Cl (filled circles) and without (open circles). Data shown are from a single oocyte batch, similar data were obtained from two additional oocyte batches.
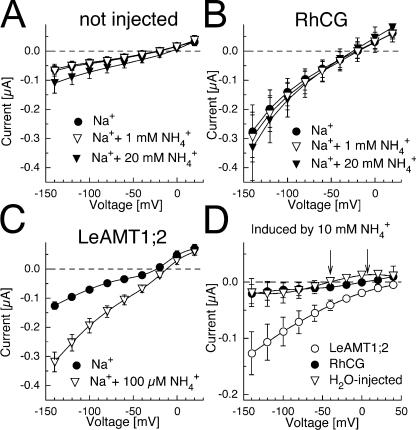
Current–voltage relations with different ammonium concentrations are shown in Figs. 7 and 8. Ammonium did not affect currents in native oocytes at 1 mM, but the addition of 20 mM ammonium increased the conductance, consistent with the fact that the oocytes used in this study were largely impermeable to NH4 + (Fig. 8 A). The currents of RhCG-expressing oocytes were slightly increased by the addition of 1 or 20 mM ammonium (Fig. 8 B). In accordance with Bakouh et al. (2004), the ammonium-induced currents in RhCG-expressing oocytes were larger at more alkaline pHo. In contrast, even low ammonium, such as 100 μM, induced a large, significant current increase in LeAMT1;2-expressing oocytes (Fig. 8, C and D). The ammonium-induced currents were observed in choline as well as sodium solutions (unpublished data). The differences in the current–voltage relations of ammonium-induced currents (the currents in the absence of ammonium subtracted from the currents in the presence of ammonium) for H2O-injected, RhCG-expressing, and LeAMT1;2-expressing oocytes are shown in Fig. 8 D. While the ammonium-induced currents from H2O-injected and RhCG-expressing oocytes were not substantially different, the NH4 + currents by LeAMT1;2 were large (Figs. 7 and 8), saturated with Km (at −100 mV) = 60 μM (Figs. 7 and S1) and were only inward. In contrast, the small ammonium-induced currents by RhCG were inward at negative voltages, reversed at ∼−10 mV and were outward at positive voltages (Fig. 8 D). Interestingly, the magnitude and current–voltage characteristics of the ammonium-induced, RhCG-mediated currents and their pHo dependence were similar to endogenous ammonium-induced oocyte currents (see Fig. 2).
Figure 8.
Voltage dependence of ammonium-induced currents by LeAMT1;2 and RhCG. (A) Current–voltage relations from native oocytes. (B) RhCG-expressing oocytes. Currents were recorded in sodium-based solutions (filled circles) supplemented with 1 mM ammonium (open triangles) and with 20 mM ammonium (filled triangles). (C) Current–voltage relations from LeAMT1;2-expressing oocytes in sodium-based solution without ammonium (filled circles) or with 100 μM ammonium (open triangles). Means ± SD of n = 4–7 oocytes from two batches are shown. Large error bars are mainly due to different background currents. Similar results were recorded in two additional batches. (D) Induced currents (background currents subtracted) by 10 mM ammonium. Open circles, LeAMT1;2; closed circles, RhCG; open triangle, H2O injected. Arrows indicate the reversal of the induced currents. To obtain the induced current means ± SD, the background currents were subtracted from the currents in ammonium in each individual experiment. This reduced the impact of background current magnitude on the error bars, as the resulting currents were insensitive to variable background currents. All experiments were done at pHo 7.5.
Ammonium-induced pHi Alkalinization
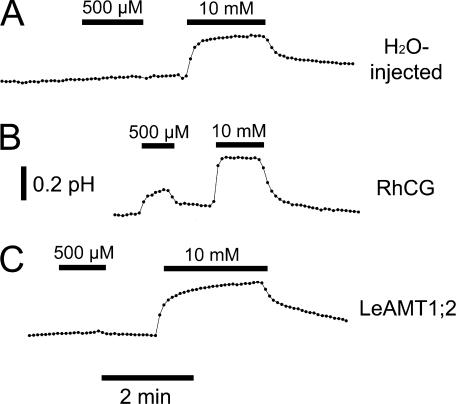
The transport mechanism was further assayed by monitoring intracellular pHi in oocytes using the pH-sensitive dye BCECF. Electroneutral ammonium transport (either as NH3 or NH4 +/H+ exchange) was expected to alkalinize the cytosol. Exposure to 500 μM NH4Cl induced a small, rapid fluorescence ratio change in RhCG-expressing oocytes to a steady-state level, but not in H2O-injected controls or in LeAMT1;2-expressing oocytes (Fig. 9). The application of 10 mM ammonium induced a pHi alkalinization to a similar steady-state level in all oocytes, but alkalinization was more rapid in RhCG-expressing oocytes (Fig. 9). A similar pHi alkalinization by ammonium had been observed with RhBG (Ludewig, 2004). The alkalinization by ammonium in RhCG-expressing oocytes is compatible with NH3 transport by RhCG, while the lack of effect of LeAMT1;2 suggests a different transport mechanism in that transporter.
Figure 9.
Intracellular alkalinization by ammonium in H2O-injected, RhCG-expressing, and LeAMT1;2-expressing oocytes. Representative results of BCECF-loaded oocytes at pH 7.5. Oocytes were exposed to 500 μM and 10 mM NH4Cl for the indicated periods. (A) H2O-injected oocyte. (B) RhCG-expressing oocyte. (C) LeAMT1;2-expressing oocyte. Measured values are indicated by black dots and are connected by thin lines. Upward deflections indicate pHi alkalinization. Similar results were obtained (n = 7, H2O-injected oocytes; n = 11, RhCG; n = 9: LeAMT1;2) in a total of five oocyte batches.
RhCG and LeAMT1;2 Expression in Yeast
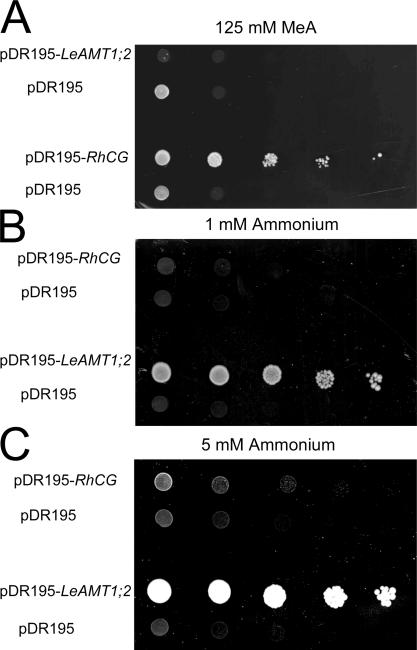
Different transport mechanisms of RhCG and LeAMT1;2 were also identified after expression in another heterologous host, the yeast S. cerevisiae. Wild-type yeast growth was impaired on media supplemented with 125 mM methylammonium, a toxic concentration (Fig. 10). The expression of the MeA+ transporter LeAMT1;2 in wild-type yeast increased the sensitivity to methylammonium further, as may be expected from membrane potential–driven MeA+ import by LeAMT1;2. RhCG-expressing wild-type yeast was more resistant to toxic methylammonium concentrations (Fig. 10 A), as previously shown (Marini et al., 2000). This is not compatible with import and accumulation of MeA+ by RhCG, but may rather suggest RhCG-mediated efflux of H3C-NH2. This suggests that RhCG can function not only in import, but also in the export of methylammonium.
Figure 10.
Opposite effects of RhCG and LeAMT1;2 in yeast. (A) Serial 10-fold dilutions of saturated cultures of the constructs expressed in wild-type yeast. Cells were spotted on YNB media supplemented with 125 mM methylammonium. The picture was taken after 3 d at 28°C. Yeast expressing LeAMT1;2 were more sensitive to toxic methylammonium than vector-transformed controls, while yeast expressing RhCG were more resistant than vector transformed controls. (B and C) Serial 10-fold dilutions of saturated cultures of the constructs expressed in the ΔΔΔmep1,2,3 yeast strain that lacks three endogenous ammonium transporters. LeAMT1;2 rescued the growth on limiting ammonium (1 and 5 mM). The pictures were taken after 5 d of growth. Experiments were repeated four times.
A yeast strain that lacks all endogenous ammonium transporters was unable to acquire sufficient ammonium for growth, but RhCG expression permitted weak growth (Fig. 10, B and C), as had been shown previously (Marini et al., 2000). Since the NH3 gradient was probably unfavorable for uptake on acidic, low-ammonium media, passive influx of NH3 may be suboptimal for growth, although ammonium assimilation may deplete cytosolic NH3 to very low levels. Membrane potential-driven import of NH4 + by LeAMT1;2 was expected to maximize uptake on limiting ammonia. The efficient growth complementation by LeAMT1;2 was compatible with this expectation (Fig. 10, B and C).
DISCUSSION
Ammonium transporters of human and plant origin were functionally compared in oocytes and yeast using parallel, identical tracer flux and current measurements. The saturable ammonium and methylammonium transport by RhCG was electroneutral, while NH4 + (or NH3/H+) co- and MeA+ (or MeA/H+( co-transport by LeAMT1;2 was accompanied by ionic currents.
Rh-associated Cation Conductance in the Absence of NH4 +
Even in the absence of ammonium or methylammonium, the expression of RhCG and RhBG increased the background ionic conductance in oocytes (Ludewig, 2004; Mak et al., 2005). Similarly, a small increase in background conductance was also seen in LeAMT1;2-expressing oocytes. The expression of foreign proteins in oocytes is often associated with unrelated background currents (Weber, 1999). This may suggest that the Rh-associated currents were endogenous to oocytes and not intrinsic to Rh proteins. However, time independence, regulation by alkaline pHo, and their impermeability to NH4 + distinguish these cation currents from other endogenous ionic currents commonly observed by high expression of foreign proteins (Tzounopoulos et al., 1995). In the oocytes used in this study, background currents were not observed with the expression of other foreign proteins, e.g., an iron-phytosiderophore transporter (Schaaf et al., 2004). Furthermore, the expression of RhCG under the control of the strong PMA1 promoter in an alkali metal–sensitive yeast strain affected growth sensitivity, implying cation transport by RhCG in yeast. However, it cannot be excluded that RhCG conferred sodium sensitivity to that strain by an unrelated mechanism.
It is possible that the Rh-associated alkali-cation conductance was endogenous to oocytes and yeast and was activated by Rh expression. We cannot, however, rule out that, e.g., the central pore in the middle of the oligomer of subunits is a pathway for cations. Such a situation is discussed for the, structurally unrelated, aquaporin (water channel) tetramers (Yool and Weinstein, 2002).
Methylammonium Transport
The endogenous uptake of 14C-MeA was linear, electroneutral, and pHo dependent, in accordance with data obtained previously (Westhoff et al., 2002; Ludewig, 2004). Methylammonium did not affect the membrane potential or ionic currents, although MeA and other amines had previously been found to depolarize a subset of Xenopus oocytes (Burckhardt and Thelen, 1995). Seasonal and laboratory differences between oocytes are well established (Weber, 1999). The MeA uptake in native oocytes was probably not completely due to lipid diffusion of the uncharged species, since ammonium partially competed with MeA transport, indicating the presence of endogenous, electroneutral MeA transporters in Xenopus oocytes.
RhCG expression strongly stimulated 14C-MeA uptake. In accordance with data from RhAG (Westhoff et al., 2002), uptake could be monitored for relatively long time periods without saturation phenomena, which might be explained by the large volume of oocytes compared with other cells. It is also possible that some 14C-methylammonium is metabolized inside the cells (Javelle et al., 2005). But even if this is so, it would not mask the qualitative differences between transport in RhCG and LeAMT1;2. RhCG-mediated uptake was electroneutral, uptake rates were pHo dependent and saturated partially within the used concentration range. The 14C-MeA transport characteristics of RhCG were very similar to those of RhAG (Westhoff et al., 2002) and RhBG (Ludewig, 2004), although the saturation by methylammonium occurred at a higher concentration in RhCG. The half maximal saturation of RhCG was lower than in other studies, which may be partially explained by saturation effects (Mak et al., 2005; Zidi-Yahiaoui et al., 2005). Using Avogadro's number (1 pmol = 6.022 × 1011 particles) and the elementary charge (eo = 1.602 × 10−19 As), the hypothetical current that was expected if the charged species MeA+ was transported by RhCG can be calculated: the uptake rate at pHo 8.5 of ∼40 pmol/(oocyte ×(min) would correspond to an electrical current of ∼−64 nA (eo × 40 × 6.022 × 1011/60 s) at the resting potential of ∼0 mV. This current was not observed and suggests that RhCG solely transports the uncharged species. The half maximal uptake rate by RhCG differed fourfold with a 100-fold change in [H+]o and H3C-NH2 concentration, suggesting that mainly H3C-NH3 + is recruited by RhCG.
In contrast, methylammonium induced inwardly directed currents by LeAMT1;2. The uptake of ∼5 pmol/(oocyte*min) (Fig. 6) was associated with an ionic current of roughly ∼7–10 nA (Fig. 3; unpublished data) at the resting potential of methylammonium-exposed oocytes (∼−10 mV). The observed currents matched the expected currents for exclusive MeA+ transport, or MeA/H+ cotransport (−8 nA = eo × 5 × 6.022 × 1011/60 s). MeA+ transport is also compatible with the fact that methylammonium uptake rates were unaffected by pHo.
Ammonium Transport
The electroneutral transport by RhCG was observed for methylammonium, as well as for ammonium. Rapid cytosolic alkalinization is a typical response of many eukaryotic cells to 10 mM ammonium (Roos and Boron, 1981) and has also been observed in oocytes (Sasaki et al., 1992; Burckhardt and Thelen, 1995; Cougnon et al., 1996). In studies that reported a large endogenous NH4 + conductance, however, oocytes were shown to acidify with 10 mM ammonium when measured with pH-sensitive electrodes (Burckhardt and Fromter, 1992; Burckhardt and Thelen, 1995; Cougnon et al., 1996; Boldt et al., 2003). A robust transient alkalinization and subsequent acidification upon addition of 20 mM ammonium has been measured in another study (Holm et al., 2005), suggesting some variability in the ammonium-dependent pHi response in oocytes.
Native oocytes from this study were unaffected by 500 μM ammonium, but alkalinized with 10 mM ammonium, without secondary acidification (Fig. 9). If NH3 entered the cells primarily, the subsequent formation of NH4 + consumed an H+ and lead to cytosolic alkalinization (Roos and Boron, 1981). Rapid alkalinization was observed for RhCG-expressing oocytes, even at low ammonium concentrations (Fig. 9), confirming the electroneutral ammonium transport in Rh glycoproteins. The identical alkalinization was expected for a NH4 +/H+ exchange and a channel-like NH3 transport mechanism (Westhoff et al., 2002; Ludewig, 2004). Though we cannot distinguish between these mechanisms, a channel-like NH3 transport in a hydrophobic pore was suggested from the recently obtained crystal structure in the homologue AmtB (Khademi et al., 2004; Zheng et al., 2004). This mechanism is compatible with the data on Rh glycoproteins. Rapid ammonium-induced alkalinization by RhAG was also found in erythrocyte ghosts (Ripoche et al., 2004) and in kidney cell lines expressing RhBG or RhCG (Zidi-Yahiaoui et al., 2005). The transport mechanism is also compatible with results from RhCG-expressing cell lines (Handlogten et al., 2005) and another study that investigated RhCG and RhBG in oocytes (Mak et al., 2005).
There was no indication for NH4 + currents by RhCG in our study. It remains unclear why others have recorded NH4 + currents associated with RhCG in oocytes (Bakouh et al., 2004; Nakhoul et al., 2005), but it can be speculated that these RhCG-associated currents are related to endogenous currents for the following two reasons. First, the current–voltage relations of ammonium-induced currents are similar for endogenous and RhCG-induced ammonium currents (reversal at ∼0 mV), but differ for NH4 + currents by LeAMT1;2 (only inward). Second, NH3 influx and subsequent internal alkalinization are known to activate endogenous, steeply pH- and NH3-dependent currents in oocytes (Boldt et al., 2003), and NH3 influx by RhCG is expected to activate such currents.
In contrast to the situation with RhCG, low concentrations of ammonium neither alkalinized nor acidified LeAMT1;2-expressing oocytes. With NH4 + transport, some acidification was expected, but at physiological pHi, only a small fraction (∼1%) of the inflowing NH4 + will release H+ by forming NH3. Furthermore, NH4 + depolarizes oocytes that express LeAMT1;2, so NH4 + currents are expected to be exceedingly small in the fluorescence assay. This may explain why no pHi changes with LeAMT1;2 were detected. Despite this, it is of importance that the NH4 + currents by LeAMT1;2 differed from the endogenous currents; LeAMT1;2 currents saturated with Km (at −100 mV)∼60 μM (Ludewig et al., 2003) (see Fig. S1). The currents were observed at acidic, as well as alkaline pHo and had the same magnitude (Ludewig et al., 2003). In addition, LeAMT1;2 carried inwardly rectifying H3C-NH3 + currents at all pHo, and such H3C-NH3 + currents were not observed in native oocytes. The data presented do not exclude that NH4 + transport by LeAMT1;2 occurs molecularly as NH3/H+ cotransport with 1:1 stoichiometry. Though physiologically equivalent, the NH3/H+ cotransport mechanism appears very attractive based on the hydrophobic nature of the pore in AmtB and the conservation of critical pore residues in other AMTs. Furthermore, it can explain the superb selectivity of AMT transporters against alkali cations.
The different transport mechanisms of RhCG and LeAMT1;2 predicted that yeast growth would be differently affected by either transporter on selective media. As expected for a transporter/channel that equilibrates H3C-NH2, RhCG rescued growth of a wild-type strain on toxic methylammonium (due to the acidic exterior and an outwardly directed MeA gradient) (Marini et al., 2000). In contrast, the H3C-NH3 + transporter LeAMT1;2 enhanced methylammonium toxicity, compatible with negative membrane potential–driven H3C-NH3 + accumulation. On limiting ammonium media, RhCG weakly enhanced the growth of a strain that lacked all endogenous ammonium transporters (ΔΔΔmep1,2,3). As the NH3 concentration in these acidic media is extremely low, equilibration of NH3 by RhCG may provide only suboptimal ammonium nutrition to growing cells. Similarly, the NH3 gas channel EcAmtB and human RhAG only weakly rescued the growth of the ΔΔΔmep1,2,3 strain (Marini et al., 2000; Westhoff et al., 2004; Javelle et al., 2005). In contrast, the membrane potential–driven NH4 + transport by LeAMT1;2 was expected to allow efficient ammonium acquisition into the cytosol.
That transporters from the same protein family are functionally distinct is not unprecedented. A recent example comes from the CLC family of anion channels/transporters, which comprises anion channels and rapid 2 Cl−/H+ exchangers (Accardi and Miller, 2004). Although the pathway for anions is well apparent from the atomic CLC structures, it has not been resolved how protons are transported in CLCs. Our data cannot distinguish whether the transport by RhCG and LeAMT1;2 is by a “channel-like” or by a “transporter-like” mechanism. Moreover, it is somewhat surprising that the distantly related bacterial AmtB and Rh glycoproteins appear to share the same electroneutral transport mechanism, while AmtB and LeAMT1;2, which are more closely related on sequence level, are functionally distinct (Ludewig et al., 2001).
Physiological Role of AMT and Rh Ammonium Transporters
The physiological role of human electroneutral NH3 transporters/channels in erythrocytes, liver, and kidney is associated with acid–base balance and homeostasis. Distal kidney epithelia are long known to be little permeable to NH4 +, but mediate net transport of NH3 (Knepper et al., 1989). The acid-secreting intercalated cells of the collecting tubules express basolateral RhBG and apical RhCG (Eladari et al., 2002; Quentin et al., 2003; Verlander et al., 2003). As the major form of acid excreted in the urine is in the form of NH4 +, each transepithelially exported NH3 base binds a proton and NH4 + is finally excreted. Basolateral RhBG and apical RhCG equilibrate the NH3 gradient, thus a >100-fold total ammonium increase can be obtained by the different pH in blood (pH 7.4) and urine (pH 5) across these epithelia.
In contrast, the function of NH4 + transporters in plants is to acquire and accumulate the limiting nutrient ammonium in the strongly negative cytosol. High-affinity electrogenic NH4 + transport and concentrative acquisition of NH4 + has been observed in many plants (Ayling, 1993; Wang et al., 1994) and is probably mediated by AMT proteins.
Supplemental Material
Acknowledgments
We thank Hana Sychrova (Department of Membrane Transport, Prague, Czech Republic) for the AB11c yeast strain and the NHA1-containing plasmid, N. von Wiren for comments on a preliminary version, and Felicity de Courcy for critically reading of the final manuscript.
This work was supported by funding by the Deutsche Forschungsgemeinschaft and a project grant of the University of Tübingen to U. Ludewig.
Lawrence G. Palmer served as editor.
Abbreviations used in this paper: BCECF, 2',7'-bis-(carboxyethyl)-5(6)-carboxyfluorescein; MeA, methylammonium; MES, 2-[N- morpholino]ethanesulfonic acid.
References
- Accardi, A., and C. Miller. 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 427:803–807. [DOI] [PubMed] [Google Scholar]
- Ayling, S.M. 1993. The effect of ammonium ions on membrane potential and anion flux in roots of barley and tomato. Plant Cell Environ. 16:297–303. [Google Scholar]
- Bakouh, N., F. Benjelloun, P. Hulin, F. Brouillard, A. Edelman, B. Cherif-Zahar, and G. Planelles. 2004. NH3 is involved in the NH4 + transport induced by the functional expression of the human RhC glycoprotein. J. Biol. Chem. 279:15975–15983. [DOI] [PubMed] [Google Scholar]
- Benjelloun, F., N. Bakouh, J. Fritsch, P. Hulin, J. Lipecka, A. Edelman, G. Planelles, S.R. Thomas, and B. Cherif-Zahar. 2005. Expression of the human erythroid Rh glycoprotein (RhAG) enhances both NH3 and NH4 + transport in HeLa cells. Pflügers Arch. 450:155–167. [DOI] [PubMed] [Google Scholar]
- Boldt, M., G. Burckhardt, and B.C. Burckhardt. 2003. NH4 + conductance in Xenopus laevis oocytes. III. Effect of NH3 Pflügers Arch. 446:652–657. [DOI] [PubMed] [Google Scholar]
- Burckhardt, B.C., and G. Burckhardt. 1997. NH4 + conductance in Xenopus laevis oocytes. I. Basic observations. Pflügers Arch. 434:306–312. [DOI] [PubMed] [Google Scholar]
- Burckhardt, B.C., and E. Fromter. 1992. Pathways of NH3/NH4 + permeation across Xenopus laevis oocyte cell membrane. Pflügers Arch. 420:83–86. [DOI] [PubMed] [Google Scholar]
- Burckhardt, B.C., and P. Thelen. 1995. Effect of primary, secondary and tertiary amines on membrane potential and intracellular pH in Xenopus laevis oocytes. Pflügers Arch. 429:306–312. [DOI] [PubMed] [Google Scholar]
- Cougnon, M., S. Benammou, F. Brouillard, P. Hulin, and G. Planelles. 2002. Effect of reactive oxygen species on NH4 + permeation in Xenopus laevis oocytes. Am. J. Physiol. Cell Physiol. 282:C1445–C1453. [DOI] [PubMed] [Google Scholar]
- Cougnon, M., P. Bouyer, P. Hulin, T. Anagnostpoulos, and G. Planelles. 1996. Further investigation of ionic diffusive properties and of NH4 + pathways in Xenopus laevis oocyte cell membrane. Pflügers Arch. 431:658–667. [DOI] [PubMed] [Google Scholar]
- Eladari, D., L. Cheval, F. Quentin, O. Bertrand, I. Mouro, B. Cherif-Zahar, J.P. Cartron, M. Paillard, A. Doucet, and R. Chambrey. 2002. Expression of RhCG, a new putative NH3/NH4 + transporter, along the rat nephron. J. Am. Soc. Nephrol. 13:1999–2008. [DOI] [PubMed] [Google Scholar]
- Handlogten, M.E., S.P. Hong, C.M. Westhoff, and I.D. Weiner. 2005. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am. J. Physiol. Renal Physiol. 289:F347–F358. [DOI] [PubMed] [Google Scholar]
- Holm, L.M., T.P. Jahn, A.L. Moller, J.K. Schjoerring, D. Ferri, D.A. Klaerke, and T. Zeuthen. 2005. NH3 and NH4 + permeability in aquaporin-expressing Xenopus oocytes. Pflugers Arch. 450:415–428. [DOI] [PubMed] [Google Scholar]
- Javelle, A., G. Thomas, A.M. Marini, R. Kramer, and M. Merrick. 2005. In vivo functional characterization of the Escherichia coli ammonium channel AmtB: evidence for metabolic coupling of AmtB to glutamine synthetase. Biochem. J. 390:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi, S., J. O'Connell III, J. Remis, Y. Robles-Colmenares, L.J. Miercke, and R.M. Stroud. 2004. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science. 305:1587–1594. [DOI] [PubMed] [Google Scholar]
- Kleiner, D. 1985. Bacterial ammonium transport. FEMS Micobiol. Rev. 32:87–100. [Google Scholar]
- Knepper, M.A., R. Packer, and D.W. Good. 1989. Ammonium transport in the kidney. Physiol. Rev. 69:179–249. [DOI] [PubMed] [Google Scholar]
- Ludewig, U. 2004. Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J. Physiol. 559:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig, U., N. von Wirén, D. Rentsch, and W.B. Frommer. 2001. Rhesus factors and ammonium: a function in efflux? Genome Biol. 2:REVIEWS1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig, U., N. von Wirén, and W.B. Frommer. 2002. Uniport of NH4 + by the root hair plasma membrane ammonium transporter LeAMT1;1. J. Biol. Chem. 277:13548–13555. [DOI] [PubMed] [Google Scholar]
- Ludewig, U., S. Wilken, B. Wu, W. Jost, P. Obrdlik, M. El Bakkoury, A.M. Marini, B. Andre, T. Hamacher, E. Boles, et al. 2003. Homo- and hetero-oligomerization of ammonium transporter-1 NH4 + uniporters. J. Biol. Chem. 278:45603–45610. [DOI] [PubMed] [Google Scholar]
- Mak, D.O., B. Dang, I.D. Weiner, J.K. Foskett, and C.M. Westhoff. 2005. Characterization of ammonia transport by the kidney Rh glyco- proteins, RhBG and RhCG. Am. J. Physiol. Renal Physiol. In press. [DOI] [PMC free article] [PubMed]
- Maresova, L., and H. Sychrova. 2005. Physiological characterization of S. cerevisiae kha1 deletion mutants. Mol. Microbiol. 55:588–600. [DOI] [PubMed] [Google Scholar]
- Marini, A.M., G. Matassi, V. Raynal, B. André, J.P. Cartron, and B. Cherif-Zahar. 2000. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat. Genet. 26:341–344. [DOI] [PubMed] [Google Scholar]
- Marini, A.M., S. Soussi-Boudekou, S. Vissers, and B. André. 1997. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:4282–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini, A.M., S. Vissers, A. Urrestarazu, and B. André. 1994. Cloning and expression of the MEP1 gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J. 13:3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Wagner, J., L. Nolden, M. Jakoby, R. Siewe, R. Kramer, and A. Burkovski. 2001. Multiplicity of ammonium uptake systems in Corynebacterium glutamicum: role of Amt and AmtB. Microbiology. 147:135–143. [DOI] [PubMed] [Google Scholar]
- Nakhoul, N.L., H. DeJong, S.M. Abdulnour-Nakhoul, E.L. Boulpaep, K. Hering-Smith, and L.L. Hamm. 2005. Characteristics of renal Rhbg as an NH4 + transporter. Am. J. Physiol. Renal Physiol. 288:F170–F181. [DOI] [PubMed] [Google Scholar]
- Ninnemann, O., J.C. Jauniaux, and W.B. Frommer. 1994. Identification of a high affinity NH4 + transporter from plants. EMBO J. 13:3464–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin, F., D. Eladari, L. Cheval, C. Lopez, D. Goossens, Y. Colin, J.P. Cartron, M. Paillard, and R. Chambrey. 2003. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J. Am. Soc. Nephrol. 14:545–554. [DOI] [PubMed] [Google Scholar]
- Ripoche, P., O. Bertrand, P. Gane, C. Birkenmeier, Y. Colin, and J.P. Cartron. 2004. The human Rhesus-associated RhAG protein mediates facilitated transport of NH3 into red blood cells. Proc. Natl. Acad. Sci. USA. 101:17222–17227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, A., and W.F. Boron. 1981. Intracellular pH. Physiol. Rev. 61:296–434. [DOI] [PubMed] [Google Scholar]
- Sasaki, S., K. Ishibashi, T. Nagai, and F. Marumo. 1992. Regulation mechanisms of intracellular pH of Xenopus laevis oocyte. Biochim. Biophys. Acta. 1137:45–51. [DOI] [PubMed] [Google Scholar]
- Schaaf, G., U. Ludewig, B.E. Erenoglu, S. Mori, T. Kitahara, and N. von Wiren. 2004. ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J. Biol. Chem. 279:9091–9096. [DOI] [PubMed] [Google Scholar]
- Siewe, R.M., B. Weil, A. Burkovski, B.J. Eikmanns, M. Eikmanns, and R. KrAm. 1996. Functional and genetic characterization of the (methyl)ammonium uptake carrier of Corynebacterium glutamicum. J. Biol. Chem. 271:5398–5403. [DOI] [PubMed] [Google Scholar]
- Sohlenkamp, C., C.C. Wood, G.W. Roeb, and M.K. Udvardi. 2002. Characterization of Arabidopsis AtAMT2, a high-affinity ammonium transporter of the plasma membrane. Plant Physiol. 130:1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos, T., J. Maylie, and J.P. Adelman. 1995. Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys. J. 69:904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlander, J.W., R.T. Miller, A.E. Frank, I.E. Royaux, Y.H. Kim, and I.D. Weiner. 2003. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am. J. Physiol. Renal Physiol. 284:F323–F337. [DOI] [PubMed] [Google Scholar]
- Wang, M.Y., A.D.M. Glass, J.E. Shaff, and L.V. Kochian. 1994. Ammonium uptake by rice roots III. Electrophysiology. Plant Physiol. 104:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, W.M. 1999. Endogenous ion channels in oocytes of Xenopus laevis: recent developments. J. Membr. Biol. 170:1–12. [DOI] [PubMed] [Google Scholar]
- Weiner, I.D. 2004. The Rh gene family and renal ammonium transport. Curr. Opin. Nephrol. Hypertens. 13:533–540. [DOI] [PubMed] [Google Scholar]
- Westhoff, C.M., M. Ferreri-Jacobia, D.O. Mak, and J.K. Foskett. 2002. Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J. Biol. Chem. 277:12499–12502. [DOI] [PubMed] [Google Scholar]
- Westhoff, C.M., D.L. Siegel, C.G. Burd, and J.K. Foskett. 2004. Mechanism of genetic complementation of ammonium transport in yeast by human erythrocyte Rh-associated glycoprotein (RhAG). J. Biol. Chem. 279:17443–17448. [DOI] [PubMed] [Google Scholar]
- Yool, A.J., and A.M. Weinstein. 2002. New roles for old holes: ion channel function in aquaporin-1. News Physiol. Sci. 17:68–72. [DOI] [PubMed] [Google Scholar]
- Zheng, L., D. Kostrewa, S. Berneche, F.K. Winkler, and X.D. Li. 2004. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc. Natl. Acad. Sci. USA. 101:17090–17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidi-Yahiaoui, N., I. Mouro-Chanteloup, A.M. D'Ambrosio, C. Lopez, P. Gane, C. Le van Kim, J.P. Cartron, Y. Colin, and P. Ripoche. 2005. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem. J. 391:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.