Daytime vision in vertebrates initiates with the absorption of light by cone photoreceptors (Rodieck, 1998), which generate signals for color discrimination (Sharpe et al., 1999). In humans, these cells are concentrated in a specialized part of the central retina called the fovea. This region of the eye operates over a wide range of intensities (Aguilar and Stiles, 1954) mediating high temporal (Green, 1970) and spatial visual resolution (Hart, 1987). The importance of the fovea to human vision is most clearly seen in the devastating disease, age-related macular degeneration (Bird, 2003). In line with these observations, cones in lower vertebrates (Normann and Perlman, 1979; Perry and McNaughton, 1991; Burkhardt, 1994) and primates (Schnapf et al., 1990) exhibit faster response kinetics and extended adaptation ranges when compared with rods, although these improved features are accompanied by a loss in light sensitivity.
Given the importance of cone photoreceptors, it is problematic that there is not yet a broad understanding of their unique features. This deficiency is due to the lack of an experimental system that provides a physiologically suitable cell preparation that can be manipulated genetically to modulate gene expression. This elusive goal in phototransduction research has recently been reached by E. Pugh and colleagues (Daniele et al., 2005; Nikonov et al., 2005; and on p. 359 of this issue), who have crossed over the hurdle by establishing a robust way to record light responses from murine cones. In this first glimpse of cone responses, these investigators have uncovered some unique properties of cone physiology and opened the way for further explorations using genetic manipulation.
Lessons from Rods
Enormous progress in terms of understanding rod phototransduction has come from many labs in studies using the suction electrode recording technique pioneered by Baylor and colleagues in the late 1970s (Baylor et al., 1979). In combination with genetically manipulated mice, a quantitative description of the rod photoresponse has been established (Lamb and Pugh, 1992; for review see Arshavsky et al., 2002). These studies established that amplification, a measure of the gain of the transduction cascade, occurs in three stages and elucidated the molecular basis for each of these stages. In the first stage, gain is achieved through the activation of many transducin (GT) molecules by a light-activated rhodopsin molecule (R*). In the second stage, many cGMP molecules are hydrolyzed by activated cGMP phosphodiesterase (PDE). Finally, the cGMP-gated channels have a cooperativity in cGMP binding, leading to an addition gain step. The rising phase of the response to a brief flash of light is determined by the combined effects of the three stages and can be described by a parabolic equation ( Lamb and Pugh, 1992):
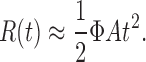 |
(1) |
R(t) is the normalized response, Φ is the number of photoisomerizations of rhodopsin, and A is the amplification constant. The amplification constant can be quantitatively understood in biochemical terms:
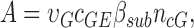 |
(2) |
where υG is the rate of transducin activation per R*, cGE is the coupling efficiency from G* activation to PDE activation, βsub is related to the rate of cGMP hydrolysis per PDE subunit, and ncG is the Hill coefficient of cGMP channel opening. A satisfying aspect of this framework is that all parameters are linked to measured properties. For example, βsub identifies the contribution of a PDE subunit to the amplification of the photoresponse and is defined using the enzymatic properties of PDE:
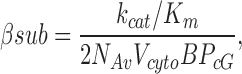 |
(3) |
where kcat/Km is the apparent second order rate constant for free PDE and cGMP reactions, Vcyto is the volume of the cytoplasm, NA is Avogadro's number, and BPcG is the cytoplasmic cGMP buffering power. Biochemical and physiological experiments (for review see Arshavsky et al., 2002) have shown that changes in the concentration of effector molecules in the outer segment leads to alterations in the photoresponse. For example, large protein translocations into and out of the outer segment can reduce the amplification of the response (Sokolov et al., 2002).
Response termination is not quite as well understood quantitatively (Hamer et al., 2005). It involves mechanisms that inactive each of the integrating stages. First, disruption of GT activation by light-activated rhodopsin occurs via phosphorylation of rhodopsin by GRK1 and arrestin binding (Arshavsky, 2002). Second, hydrolysis of GTP bound to activated GT α subunit is accelerated by the RGS9-Gb5L-R9AP complex (Chen et al., 2000; Arshavsky et al., 2002). This is the slowest step in rod recovery from saturating flashes. Finally, cGMP levels are restored to reestablish circulating current via multiple mechanisms including calcium-dependent activation of guanylate cyclase via GCAP proteins (Arshavsky et al., 2002; Korenbrot and Rebrik, 2002; Palczewski et al., 2004). It has also been recently shown that developmental changes in calcium feedback through increasing concentrations of calcium-binding proteins can change the functioning of rod vision in amphibians (Solessio et al., 2004). The impressive progress in a quantitative understanding of rod phototransduction has framed the issues of what molecular mechanisms determine the unique properties of cones.
Cones Preserve Responsiveness in Strong Light
Cone and rod photoreceptors contain similar types of proteins for phototransduction, though they often are encoded in distinct genes. Physiologically, however, their response properties are quite different. Although the rising phase appears to be quite similar in both rods and cones, the responses terminate much more quickly in cones and overshoots the dark current level (Baylor, 1987). This may be related to quantitative differences in the level of expression of proteins involved in inactivating GT (Cowan et al., 1998), activating guanylate cyclase (Palczewski et al., 2004), or calcium homeostasis (Korenbrot and Rebrik, 2002). More significantly, cones do not saturate in response to background illumination but remain responsive over more than seven orders of magnitude (Perlman and Normann, 1998). Rods saturate (i.e., become unresponsive to incremental flashes) at much lower photobleaching levels.
How do cones maintain their sensitivity? There appears to be two different realms in cone physiology: a high-intensity range where pigment depletion dominates the response sensitivity and a low-intensity range where mechanisms involving other adaptive methods must be active (Burkhardt, 1994; Perlman and Normann, 1998). To further understand the adaptive properties of cones, we need to understand how cones terminate their responses so quickly and their behavior in bright lights. Until now, however, it has not so far been possible to investigate these questions in mice cones, where genetic manipulations are enormously powerful.
Recording from Mouse Cones
Recording from mouse cones has proved challenging because they are only a small fraction of the total photoreceptor population in this rod-dominated retina (Carter-Dawson and LaVail, 1979). A breakthrough was reported by Pugh and colleagues last year, who took advantage of the Nrl knockout mouse (Mears et al., 2001) in which photoreceptor cell fate was drastically altered (Daniele et al., 2005; Nikonov et al., 2005).
Pugh and colleagues showed quite convincingly that photoreceptors in the Nrl−/− mouse closely resemble cone photoreceptors, using both morphological and molecular techniques (Daniele et al., 2005). They also studied the functional properties of Nrl−/− cone cells at the single cell level. Because of their abundance, it was possible to improve techniques for long and stable recordings. It turned out that the suction pipette approach, which had been successful for mouse rods, was not tolerated well by the more fragile cone outer segments. By drawing the inner segments of the cones into the recording pipette, Nikonov et al. (2005) determined that the responses had faster kinetics and reduced sensitivity compared with wild-type mouse rods. In a twist from many other species, mouse cones coexpress both S- and M-cone opsin. Thus, the authors characterized the dim flash responses from cells obtained from mice lacking both Nrl and Grk1 function. These cells exhibit differences in the recoveries to stimuli that activate the M- and S-pigment; only the M-pigment–driven responses are slowed down in the double knockout. This is a surprising and very significant result as it reveals an unexpected complexity in the light responses of cones. One possible explanation is that there is an additional inactivation mechanism (e.g., another kinase) that is specific for S-opsin. Another possibility is that S- and M-opsin–activated states have different stabilities (Vought et al., 1999). To have sensitivity in the ultraviolet range (λmax ∼360 nm), the retinylidene Schiff base linkage apparently must be unprotonated (Babu et al., 2001; Kusnetzow et al., 2004). Thus, the different inactivation mechanism for S-opsin may be a tradeoff for stability of the activated form in order to achieve spectral tuning.
In the article published in this issue, Nikonov et al. applied their novel recording approach to study cones in a wild-type retina. One complication not present in the Nrl−/− retina is that the cells are not isolated from other photoreceptors (e.g., rods), so background illumination is required to isolate the cone responses. In a further refinement of the experimental design, the authors studied cone responses in the GNAT1−/− mouse, in which rod transduction has been specifically disabled. The combination of WT and KO mice shows convincingly that reliable cone responses can be recorded from many cells, permitting a thorough quantitative analysis of the photoresponses under dim light and stronger background illumination. The findings from this initial characterization will form the basis for future mechanistic explorations of the shape and size of the photoresponse. For now, the amplification constants were two- to threefold lower for cones than rods, indicating either a reduced efficiency of transducin activation (υG) or differences in cone PDE properties (kcat/KM). The dominant time constant for recovery from a bright flash is much faster in mouse cones than rods; and most interestingly, the circulating currents recover substantially in both S- and M-type cones following a flash of light that bleaches a substantial fraction (>50%) of the pigment. This immunity is in stark contrast to rods, which do not recover significantly. In salamanders, it has been proposed that the apoprotein (the bleached pigment) has an activity that may act somewhat like light (Cornwall and Fain, 1994; Cornwall et al., 1995) and thus play an adaptive role in desensitizing the photoresponse. Perhaps this is not an important mechanism in mouse cone responses to bright backgrounds, which could point to key differences in terms of setting the dynamic range for phototransduction. As stated above, one of the key features of cones is that they adapt to a wide range of light intensities, which is the most central property required by these cells to function in bright light. We can look forward to more mechanistic information in this powerful animal model now that the technical challenges have been met.
Acknowledgments
The authors are supported by the National Institutes of Health grants EY-11256 and EY-12975 (B.E. Knox), Research to Prevent Blindness (unrestricted grant to SUNY UMU Department of Ophthalmology) and Lions of CNY.
Abbreviation used in this paper: PDE, phosphodiesterase.
References
- Aguilar, M., and W.S. Stiles. 1954. Saturation of the rod mechanism of the retina at high levels of stimulation. Opt. Acta (Lond.). 1:59–65. [Google Scholar]
- Arshavsky, V.Y. 2002. Rhodopsin phosphorylation: from terminating single photon responses to photoreceptor dark adaptation. Trends Neurosci. 25:124–126. [DOI] [PubMed] [Google Scholar]
- Arshavsky, V.Y., T.D. Lamb, and E.N. Pugh Jr. 2002. G proteins and phototransduction. Annu. Rev. Physiol. 64:153–187. [DOI] [PubMed] [Google Scholar]
- Babu, K.R., A. Dukkipati, R.R. Birge, and B.E. Knox. 2001. Regulation of phototransduction in short-wavelength cone visual pigments via the retinylidene Schiff base counterion. Biochemistry. 40:13760–13766. [DOI] [PubMed] [Google Scholar]
- Baylor, D.A. 1987. Photoreceptor signals and vision. Proctor lecture. Invest. Ophthalmol. Vis. Sci. 28:34–49. [PubMed] [Google Scholar]
- Baylor, D.A., T.D. Lamb, and K.W. Yau. 1979. The membrane current of single rod outer segments. J. Physiol. 288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Bird, A.C. 2003. The Bowman lecture. Towards an understanding of age-related macular disease. Eye. 17:457–466. [DOI] [PubMed] [Google Scholar]
- Burkhardt, D.A. 1994. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J. Neurosci. 14:1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson, L.D., and M.M. LaVail. 1979. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 188:245–262. [DOI] [PubMed] [Google Scholar]
- Chen, C.K., M.E. Burns, W. He, T.G. Wensel, D.A. Baylor, and M.I. Simon. 2000. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 403:557–560. [DOI] [PubMed] [Google Scholar]
- Cornwall, M.C., and G.L. Fain. 1994. Bleached pigment activates transduction in isolated rods of the salamander retina. J. Physiol. 480(Pt 2):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall, M.C., H.R. Matthews, R.K. Crouch, and G.L. Fain. 1995. Bleached pigment activates transduction in salamander cones. J. Gen. Physiol. 106:543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, C.W., R.N. Fariss, I. Sokal, K. Palczewski, and T.G. Wensel. 1998. High expression levels in cones of RGS9, the predominant GTPase accelerating protein of rods. Proc. Natl. Acad. Sci. USA. 95:5351–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele, L.L., C. Lillo, A.L. Lyubarsky, S.S. Nikonov, N. Philp, A.J. Mears, A. Swaroop, D.S. Williams, and E.N. Pugh Jr. 2005. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest. Ophthalmol. Vis. Sci. 46:2156–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D.G. 1970. Regional variations in the visual acuity for interference fringes on the retina. J. Physiol. 207:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, R.D., S.C. Nicholas, D. Tranchina, T.D. Lamb, and J.L. Jarvinen. 2005. Toward a unified model of vertebrate rod phototransduction. Vis. Neurosci. 22:417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, W. 1987. The temporal responsiveness of vision. In Adler's Physiology of the Eye, Clinical Application. R.A. Moses and W.M. Hart, editors. The CV Mosby Company, St. Louis. 429–457.
- Korenbrot, J.I., and T.I. Rebrik. 2002. Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones. Adv. Exp. Med. Biol. 514:179–203. [DOI] [PubMed] [Google Scholar]
- Kusnetzow, A.K., A. Dukkipati, K.R. Babu, L. Ramos, B.E. Knox, and R.R. Birge. 2004. Vertebrate ultraviolet visual pigments: protonation of the retinylidene Schiff base and a counterion switch during photoactivation. Proc. Natl. Acad. Sci. USA. 101:941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, T.D., and E.N. Pugh Jr. 1992. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449:719–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears, A.J., M. Kondo, P.K. Swain, Y. Takada, R.A. Bush, T.L. Saunders, P.A. Sieving, and A. Swaroop. 2001. Nrl is required for rod photoreceptor development. Nat. Genet. 29:447–452. [DOI] [PubMed] [Google Scholar]
- Nikonov, S.S., L.L. Daniele, X. Zhu, C.M. Craft, A. Swaroop, and E.N. Pugh Jr. 2005. Photoreceptors of Nrl −/− mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. J. Gen. Physiol. 125:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann, R.A., and I. Perlman. 1979. The effects of background illumination on the photoresponses of red and green cones. J. Physiol. 286:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski, K., I. Sokal, and W. Baehr. 2004. Guanylate cyclase-activating proteins: structure, function, and diversity. Biochem. Biophys. Res. Commun. 322:1123–1130. [DOI] [PubMed] [Google Scholar]
- Perlman, I., and R.A. Normann. 1998. Light adaptation and sensitivity controlling mechanisms in vertebrate photoreceptors. Prog. Retin. Eye Res. 17:523–563. [DOI] [PubMed] [Google Scholar]
- Perry, R.J., and P.A. McNaughton. 1991. Response properties of cones from the retina of the tiger salamander. J. Physiol. 433:561–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck, R.W. 1998. The First Steps in Seeing. First edition. Sinauer Associates, Sunderland, MA. 562 pp.
- Schnapf, J.L., B.J. Nunn, M. Meister, and D.A. Baylor. 1990. Visual transduction in cones of the monkey Macaca fascicularis. J. Physiol. 427:681–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe, L., A. Stockman, H. Jagle, and J. Nathans. 1999. Opsin genes, cone photopigments, color vision, and color blindness. In Color Vision: From Genes to Perception. K. Gegenfurtner and L. Sharpe, editors. Cambridge University Press, New York. 3–53.
- Sokolov, M., A.L. Lyubarsky, K.J. Strissel, A.B. Savchenko, V.I. Govardovskii, E.N. Pugh Jr., and V.Y. Arshavsky. 2002. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 34:95–106. [DOI] [PubMed] [Google Scholar]
- Solessio, E., S.S. Mani, N. Cuenca, G.A. Engbretson, R.B. Barlow, and B.E. Knox. 2004. Developmental regulation of calcium-dependent feedback in Xenopus rods. J. Gen. Physiol. 124:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel, K.J., P.V. Lishko, L.H. Trieu, M.J. Kennedy, J.B. Hurley, and V.Y. Arshavsky. 2005. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J. Biol. Chem. 280:29250–29255. [DOI] [PubMed] [Google Scholar]
- Vought, B.W., A. Dukkipatti, M. Max, B.E. Knox, and R.R. Birge. 1999. Photochemistry of the primary event in short-wavelength visual opsins at low temperature. Biochemistry. 38:11287–11297. [DOI] [PubMed] [Google Scholar]


