Abstract
TRPM7 is a Ca2+- and Mg2+-permeable cation channel that also contains a protein kinase domain. While there is general consensus that the channel is inhibited by free intracellular Mg2+, the functional roles of intracellular levels of Mg·ATP and the kinase domain in regulating TRPM7 channel activity have been discussed controversially. To obtain insight into these issues, we have determined the effect of purine and pyrimidine magnesium nucleotides on TRPM7 currents and investigated the possible involvement of the channel's kinase domain in mediating them. We report here that physiological Mg·ATP concentrations can inhibit TRPM7 channels and strongly enhance the channel blocking efficacy of free Mg2+. Mg·ADP, but not AMP, had similar, albeit smaller effects, indicating a double protection against possible Mg2+ and Ca2+ overflow during variations of cell energy levels. Furthermore, nearly all Mg-nucleotides were able to inhibit TRPM7 activity to varying degrees with the following rank in potency: ATP > TTP > CTP ≥ GTP ≥ UTP > ITP ≈ free Mg2+ alone. These nucleotides also enhanced TRPM7 inhibition by free Mg2+, suggesting the presence of two interacting binding sites that jointly regulate TRPM7 channel activity. Finally, the nucleotide-mediated inhibition was lost in phosphotransferase-deficient single-point mutants of TRPM7, while the Mg2+-dependent regulation was retained with reduced efficacy. Interestingly, truncated mutant channels with a complete deletion of the kinase domain regained Mg·NTP sensitivity; however, this inhibition did not discriminate between nucleotide species, suggesting that the COOH-terminal truncation exposes the previously inaccessible Mg2+ binding site to Mg-nucleotide binding without imparting nucleotide specificity. We conclude that the nucleotide-dependent regulation of TRPM7 is mediated by the nucleotide binding site on the channel's endogenous kinase domain and interacts synergistically with a Mg2+ binding site extrinsic to that domain.
INTRODUCTION
TRPM7 is a widely expressed member of the melastatin-related subfamily of TRPM proteins (for recent reviews see Fleig and Penner, 2004; Harteneck, 2005) that combines structural elements of both an ion channel and a protein kinase (Nadler et al., 2001; Runnels et al., 2001; Ryazanova et al., 2001; Yamaguchi et al., 2001). It represents the only ion channel known that is essential for cellular viability (Nadler et al., 2001). Significant efforts have been made to characterize the properties of both the channel and kinase activities of TRPM7 as well as their possible interdependence in mediating physiological responses. However, contrasting accounts of practically every biophysical property and the regulatory mechanisms that control channel activity have been presented.
For example, Clapham and colleagues have classified TRPM7 as a nonselective cation channel that is activated by ATP via its kinase domain (Runnels et al., 2001), whereas our laboratory has described it as a highly selective divalent cation channel conducting both Mg2+ and Ca2+ ions that is constitutively active at low levels and regulated by intracellular levels of free Mg2+ and Mg-nucleotides (Nadler et al., 2001). Subsequent work has established that TRPM7 is indeed a divalent-specific cation channel that conducts essentially all physiological as well several toxic divalent cations (Monteilh-Zoller et al., 2003). It is now also clear that free intracellular Mg2+ can regulate TRPM7 activity (Schmitz et al., 2003; Takezawa et al., 2004; Matsushita et al., 2005), and the reported ATP-dependent activation of TRPM7 (Runnels et al., 2001) was in fact due to the use of Na·ATP, which reduced free Mg2+. However, the regulation by Mg2+-nucleotides proposed by our laboratory has been challenged by Cahalan and colleagues. While they confirmed the original observation that TRPM7 can be inhibited by Mg·ATP in the presence of EGTA, they also reported that this inhibition is lost when using a strong Mg2+ chelator, HEDTA, leading them to conclude that the Mg2+-nucleotide effect can be explained by free Mg2+ alone (Kozak and Cahalan, 2003).
A further point of contention is the role of the kinase domain in regulating channel activity. Clapham and colleagues suggested that the kinase domain is essential for channel activation, since two point mutations designed to disrupt the kinase's phosphotransferase activity resulted in nonfunctional channels (Runnels et al., 2001). However, based on the crystal structure of TRPM7's kinase domain (Yamaguchi et al., 2001), one of the mutations was considered to be inconsequential for phosphotransferase activity and the other mutation was found to express a fully functional channel protein (Schmitz et al., 2003; Matsushita et al., 2005), suggesting that a functional kinase is not essential for channel activity. In fact, we found that even a full deletion of TRPM7's kinase domain still produced functional ion channels (Schmitz et al., 2003), although another study using a different truncation mutant failed to observe any channel activity (Matsushita et al., 2005). While our previous work suggests that the phosphotransferase-deficient forms of the protein do affect TRPM7 channel activity by rendering them less sensitive to inhibition by Mg2+ and Mg·ATP (Schmitz et al., 2003; Takezawa et al., 2004), Nairn and colleagues suggested that the kinase domain of TRPM7 is completely ineffectual in modulating channel activity (Kozak and Cahalan, 2003; Matsushita et al., 2005).
Finally, there is a debate about the reported modulation of TRPM7 by phospholipids. Clapham and colleagues proposed that TRPM7's channel activity requires phosphatidylinositol bisphosphate (PIP2) and its depletion via PLC-mediated hydrolysis renders it inactive. Our own work challenged this interpretation in that we observed no signs of PLC-mediated breakdown of PIP2 in cells that overexpress TRPM7, consistent with and presumably due to the fact that TRPM7 binds to PLC (Runnels et al., 2002). Instead, we proposed that TRPM7 is up-regulated via the PKA pathway and this modulation requires a functional TRPM7 kinase domain (Takezawa et al., 2004).
In the present study, we have attempted to resolve some of the controversial aspects of TRPM7 regulation by a comprehensive assessment of the inhibitory effects of free Mg2+ and Mg-nucleotides, both individually and in combination, on wild-type and phosphotransferase-deficient channels. Our results suggest a synergistic inhibition of TRPM7 activity by both Mg2+ and Mg-nucleotides that is mediated by two distinct sites, where the Mg2+ binding site is extrinsic and the nucleotide binding site is intrinsic to the TRPM7 kinase domain.
MATERIALS AND METHODS
Cells
HEK-293 cells stably transfected with the HA-tagged human TRPM7 construct as previously described (for details see Schmitz et al., 2003) were grown in DMEM medium supplemented with 10% FBS, blasticidin (5 μg/ml), and zeocin (0.4 mg/ml). TRPM7 expression was induced 1 d before use by adding 1 μg/ml tetracycline to the culture medium. Patch-clamp measurements were performed 16–24 h post-induction (for details see Nadler et al., 2001). The phosphotransferase activity-deficient point mutants of TRPM7, K1648R, and G1799D, as well as the Δ-kinase mutant were based on the human TRPM7 construct (for details see Schmitz et al., 2003). TRPM7 mutant expression was induced the day preceding the experiment by adding 1 μg/ml tetracycline to the culture medium. Patch-clamp measurements were performed 12–16 h post-induction for the point mutants and 18–24 h post-induction for the Δ-kinase mutant.
Solutions
Cells grown on glass coverslips were transferred to the recording chamber and kept in a standard modified Ringer's solution of the following composition (in mM): NaCl 140, KCl 2.8, CaCl2 1, MgCl2 2, glucose 10, HEPES·NaOH 10, pH 7.2, with osmolarity typically ranging from 295 to 325 mOsm. Intracellular pipette-filling solutions contained (in mM) Cs-glutamate 140, NaCl 8, Cs-BAPTA 10, HEPES·CsOH 10, pH 7.2 adjusted with CsOH. In some experiments, BAPTA was omitted and in others it was replaced by HEDTA and/or EGTA. Since TRPM7 activity is affected by both extra- and intracellular pH (Gwanyanya et al., 2004; Jiang et al., 2005; Kozak et al., 2005), particular care was taken to adjust pH. Mg-adenosine nucleotide concentrations and free intracellular [Mg2+]i were calculated with WebMaxC standard (12/31/2003, http://www.stanford.edu/∼cpatton/webmaxcS.htm; the binding constants are derived from the NIST database at http://www.nist.gov/srd/nist46.htm and their values are listed at http://www.stanford.edu/∼cpatton/xlsconstants.htm). All other Mg-nucleotides were used at concentrations matching the corresponding Mg-adenosine nucleotide concentration. The solutions were made using appropriate mixtures of magnesium chloride and sodium-nucleotides. All chemicals used were purchased from Sigma-Aldrich. For a detailed list of all ingredients for the various intracellular solutions used in this study, see online supplemental material (available at http://www.jgp.org/cgi/content/full/jgp.200509410/DC1).
Patch-clamp Experiments
Patch-clamp experiments were performed in the tight-seal whole-cell configuration at 21–25°C. High-resolution current recordings were acquired by a computer-based patch-clamp amplifier system (EPC-9, HEKA, Lambrecht). Patch pipettes had resistances between 2 and 4 MΩ after filling with the standard intracellular solution. Immediately following establishment of the whole-cell configuration, voltage ramps of 50 ms duration spanning the voltage range of −100 to +100 mV were delivered from a holding potential of 0 mV at a rate of 0.5 Hz over a period of 600 s. All voltages were corrected for a liquid junction potential of 10 mV between external and internal solutions because of glutamate use as intracellular anion. Currents were filtered at 2.9 kHz and digitized at 100-μs intervals. Capacitive currents and series resistance were determined and corrected before each voltage ramp using the automatic capacitance compensation of the EPC-9. The low-resolution temporal development of membrane currents was assessed by extracting the current amplitude at +80 mV from individual ramp current records. Where applicable, statistical errors of averaged data are given as means ± SEM with n determinations and statistical significance was assessed by Student's t test. The currents were normalized with the capacitance measurement immediately after break-in in order to remove cell size variations. Dose response curves were calculated using the function f(x) = (Ymax * (1/(1 + (IC50/x)n))), where Ymax is the maximal normalized current, IC50 is the Mg2+ or Mg-nucleotide concentration at which inhibition is half maximal, x is the Mg2+ or Mg-nucleotide concentration, and n is the Hill coefficient. The Hill coefficient for all dose responses was very close to 2. It was systematically fixed at this value in order to compare the different dose responses. Dose–response curves were normalized to unity by dividing by Ymax.
Online Supplemental Material
The online supplemental material elaborates on the exact intracellular solution compositions used, sorted by figure (available at http://www.jgp.org/cgi/content/full/jgp.200509410/DC1).
RESULTS
TRPM7 Is Inhibited by Free Mg2+, ATP, and ADP, but Not by AMP
In an earlier study, we have shown that Mg·ATP and free Mg2+ both inhibit TRPM7 channels (Nadler et al., 2001). To further assess the regulation of TRPM7-mediated divalent transport, we performed whole-cell patch-clamp experiments in HEK-293 cells overexpressing human TRPM7 in which we introduced various concentrations of free Mg2+ and nucleotides. In a first set of control experiments, we established a dose–response curve for free Mg2+ by perfusing cells with intracellular solutions containing defined levels of free Mg2+ in the absence of any added nucleotides. As can be seen in Fig. 2 A, increasing concentrations of Mg2+ caused a dose-dependent inhibition of TRPM7 currents. The half-maximal inhibition derived from the dose–response curve depicted in Fig. 2 B was 720 ± 79 μM (n = 6–10). In a second set of control experiments we tested various adenosine nucleotides for inhibition of TRPM7. With 6 mM Na-ATP, Na-ADP, and Na-AMP in the internal solution and no added Mg2+, TRPM7 currents activate quickly and reach a plateau after 100–200 s (Figs. 1, A–D). Whole-cell currents were large for all three nucleotides, but there was a decrease in the peak amplitudes obtained with ADP (136 ± 32 pA/pF; n = 4) and AMP (110 ± 25 pA/pF; n = 5) compared with ATP (173 ± 25 pA/pF; n = 9). This order of potency correlates with the magnesium-complexing strength of the nucleotide species, indicating that, although no Mg2+ was present in the pipette solution, intracellular Mg2+ levels may be slightly different due to intracellular Mg2+ mobilization or Mg2+ influx through TRPM7 itself. Thus, Mg2+ binding efficacy of the nucleotides may affect TRPM7 activity via buffering free Mg2+, with ATP being the most and AMP being the least efficient.
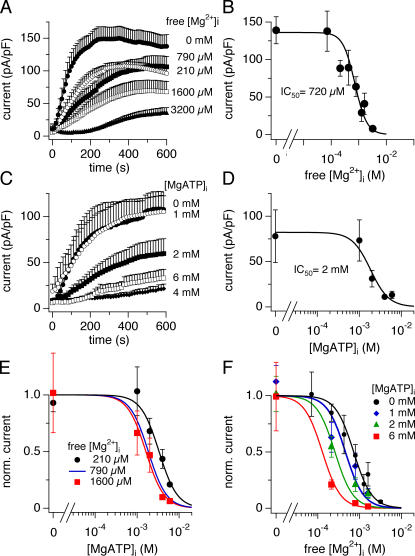
Figure 2.
Inhibition of TRPM7 by Mg·ATP and free Mg2+. Whole-cell currents were recorded in HEK-293 cells induced to overexpress human TRPM7. Cs-BAPTA was used as buffer. (A) Cells were perfused with increasing concentrations of free Mg2+ (n = 6–10). (B) Dose–response curve for the inhibition of TRPM7 current by increasing free Mg2+ concentrations (0, 70, 210, 780, 1,270, 1,600, and 3,200 μM). Data points correspond to average and normalized current amplitudes measured at + 80 mV after 200 s of whole-cell recording, plotted as a function of free Mg2+ concentration (n = 6–10). The best fit was obtained by a Hill coefficient close to 2 and was therefore fixed at that value. The calculated IC50 value for free Mg2+ concentration inhibition of TRPM7 current is 720 ± 79 μM. (C) Cells were perfused with increasing concentrations of Mg·ATP (in this example, the free Mg2+ concentration was fixed to around 780 μM; n = 4–7). (D) Dose–response curve for the inhibition of TRPM7 current by increasing Mg·ATP concentrations (0, 1, 2, 4, and 6 mM Mg·ATP, free Mg2+ concentrations were fixed in this example around 790 μM). Data points correspond to average and normalized current amplitudes measured at +80 mV after 200 s of whole-cell recording, plotted as a function of Mg·ATP concentration (n = 4–7). The best fit was obtained by a Hill coefficient close to 2 and was therefore fixed at that value. The calculated IC50 value for ATP concentration inhibition of TRPM7 current under physiological free Mg2+ (790 μM) is 2 ± 0.7 mM. (E) Comparison of dose–response curves as a function of Mg·ATP concentrations at different fixed free Mg2+ concentrations (210, 790, and 1,600 μM). Data points from the 790 μM dose–response curve were omitted for clarity as they are presented in B. Each dose–response was calculated as in B and normalized to 1 by dividing with Ymax (n = 4–12). (F) Comparison of dose–response curves as a function of free Mg2+ concentrations at different fixed Mg·ATP concentrations (0, 1, 2, 4, and 6 mM). Each dose–response curve was calculated as in Fig. 1 F and normalized to 1 by dividing with Ymax (n = 4–8). All the Hill coefficients of the dose–response curves in C and D were fixed to 2.
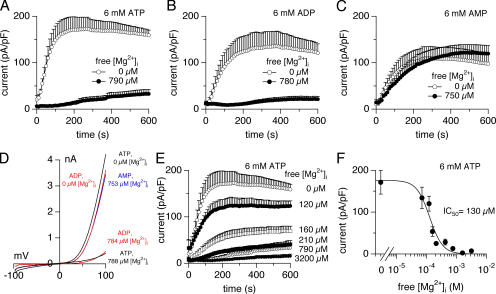
Figure 1.
Inhibition of TRPM7 by ATP, ADP and free Mg2+, but not by AMP. Whole cell currents were recorded in HEK-293 cells induced to overexpress human TRPM7. Cs-BAPTA was used as intracellular calcium buffer. (A) Cells were perfused with 6 mM ATP and no Mg2+ (n = 4) or 6 mM Mg·ATP and 790 μM free Mg2+ (n = 7). (B) Cells were perfused with 6 mM ADP and no Mg (n = 5) or 6 mM Mg·ADP and 780 μM free Mg2+ (n = 6). (C) Cells were perfused with 6 mM AMP and no Mg (n = 9) or 6 mM AMP and 750 μM free Mg2+ (n = 5). (D) Representative current–voltage (I/V) relationships for TRPM7, in the conditions described above (A–C). All I/V relationships were taken at 200 s and were derived from a high-resolution current record in response to a voltage ramp of 50 ms duration that ranged from −100 mV to +100 mV. (E) Inhibition of TRPM7 currents by 6 mM Mg·ATP and increasing free Mg2+ concentrations (n = 4–8). (F) Dose–response curve of TRPM7 current inhibition by 6 mM Mg·ATP and increasing free Mg2+ concentrations (0, 70, 120, 160, 210, 420, 790, 1,600, and 3,200 μM) at 200 s (n = 4–8). The best fit was obtained with a Hill coefficient close to 2 and was therefore fixed at that value.
When intracellular solutions were buffered to physiological levels of Mg (∼800 μM), ATP and ADP both strongly inhibited TRPM7 currents (Fig. 1, A and B), whereas AMP was completely ineffective at doing so (Fig. 1 C). These findings suggest that in the presence of free Mg2+, Mg·ATP and Mg·ADP, but not AMP (which does not complex Mg2+), strongly affect TRPM7 activity. This inhibition by Mg-nucleotides appears to be synergistic with free Mg2+, since the same level of free Mg2+ in combination with AMP produces only a moderate reduction in current amplitude compared with the combined presence of ATP/ADP and free Mg2+.
To assess the inhibition of TRPM7 ion channels by Mg·ATP and free Mg2+ quantitatively, we perfused cells with a fixed Mg·ATP concentration of 6 mM and varied the free Mg2+ concentration. As illustrated in Fig. 1 (E and F), TRPM7 currents were progressively inhibited by free Mg2+ concentrations of 70 μM or higher. The inhibition of the current was already nearly complete at 210 μM free Mg2+. The calculated IC50 for free Mg2+ in the presence of a fixed Mg·ATP concentration of 6 mM was 130 ± 18 μM (Fig. 1 F). By comparison, in the absence of Mg·ATP, the free Mg2+ concentration necessary to block TRPM7 currents by 50% is >700 μM (Fig. 2 B). These results suggest that a cross-talk exists between Mg-nucleotides and free intracellular Mg2+.
Mg·ATP and Free Mg2+ Regulate TRPM7 Ion Channels in Synergy
In a further set of experiments, we determined how both Mg·ATP and free Mg2+ coregulate TRPM7 channel activity. We measured the inhibition of TRPM7 currents under increasing Mg·ATP concentrations while maintaining the free Mg2+ concentration fixed to low, physiological, or high levels (210, 790, or 1600 μM, respectively). As demonstrated in Fig. 2 C, increasing Mg·ATP concentrations strongly inhibited TRPM7 currents when free intracellular Mg2+ was fixed to ∼800 μM (n = 4–7) with an IC50 of 2 ± 0.76 mM (Fig. 2 D). The biggest changes were seen over physiological Mg·ATP concentrations between 2 and 4 mM Mg·ATP. This behavior was also evident at lower and higher Mg2+ concentration (Fig. 2 E). In summary, the calculated IC50 values for Mg·ATP inhibition of TRPM7 currents decreased with increasing levels of free Mg2+ from 3.33 ± 0.49 mM (n = 5–12) at ∼200 μM free Mg2+ to 2 ± 0.76 mM (n = 4–7) at physiological free Mg2+ of ∼800 μM and down to 1.62 ± 0.18 mM (n = 5–7) at ∼1,600 μM free Mg2+ (Table I and Fig. 2 E). All dose response curves showed a Hill coefficient very close to 2, suggesting the need of two cooperative binding sites to cause inhibition of the channel.
TABLE I.
Inhibition of TRPM7 by Mg·ATP and Free Mg2+
| Nucleotide | IC50 (μM free Mg2+) | [Free Mg2+] | IC50 (mM Mg·ATP) |
|---|---|---|---|
| None | 720 ± 79 (n = 6–10) | 210 μM | 3.33 ± 0.49 (n = 5–12) |
| 1 mM MgATP | 457 ± 206 (n = 5–15) | 790 μM | 2.0 ± 0.76 (n = 4–7) |
| 2 mM MgATP | 256 ± 66 (n = 5) | 1600 μM | 1.62 ± 0.18 (n = 5–7) |
| 4 mM MgATP | 110 ± 7 (n = 4–8) | ||
| 6 mM MgATP | 128 ± 18 (n = 4–8) |
Comparison of half maximal inhibitory concentrations (IC50) of free Mg2+ at fixed Mg·ATP concentrations (left two columns) and IC50 values of Mg·ATP at fixed free Mg2+ concentrations (right two columns). The whole-cell currents were recorded in HEK-293 cells induced to overexpress human TRPM7.
We should point out that we have prepared dose–response curves at different time points (100, 200, 300, 400, and 600 s) and, while their IC50 varied <10% without affecting the apparent cooperativity (unpublished data), we decided that the most adequate time point was at 200 s for the following reasons. The most important consideration is based on the equilibration time constant of the relevant molecules after going whole cell, which is estimated to be ∼6 s for Mg2+, ∼90 s for ATP, and ∼120 s for BAPTA/HEDTA (Pusch and Neher, 1988). 200 s would allow most factors supplied by the pipette solution to be close to equilibrium. Later time points could be less reliable, since the perfusion of the cytosol may lead to washout of larger proteins or factors that may affect TRPM7 (e.g., cytoskeleton, G proteins, enzymes, calmodulin, polyamines). In fact, we often observe a slow change of TRPM7 currents after ∼300 s that manifests itself in slow inactivation when currents are large or an increase when currents are strongly inhibited (see e.g., Fig. 1, A and B, and Fig. 2 A). Thus, it seems that the large increase in current that occurs after 200 s in Fig. 2 A at 3,200 μM free Mg2+ is unrelated to changes in Mg2+ or Mg-nucleotide levels (both of which we believe to have achieved nearly steady-state levels by this time), but instead reflects some other factor that has not yet been identified.
We next performed complementary experiments in which we increased free Mg2+ concentrations (210, 780, 1,600, and 3,200 μM) at fixed levels of Mg·ATP (0, 1, 2, 4, or 6 mM). As described above, TRPM7 currents were inhibited by free Mg2+ in the absence of ATP (Fig. 2, A and B) with an IC50 of 720 ± 79 μM (n = 6–10). Addition of Mg·ATP gradually increased the efficacy of Mg2+ to inhibit TRPM7 up to 4 mM Mg·ATP (Fig. 2 F), where the IC50 of inhibition by free Mg2+ was decreased sevenfold (110 ± 7 μM; n = 5–7). Higher concentrations of Mg·ATP did not further decrease the IC50 of inhibition by free Mg2+. The individual IC50 values under the various experimental conditions are listed numerically in Table I. From these results, it would seem that free Mg2+ can inhibit TRPM7 without requiring Mg·ATP, whereas Mg·ATP does require the additional presence of free Mg2+. In physiological terms, however, where free Mg2+ levels are relatively constant at 0.5–1 mM, Mg·ATP would likely play a dominant role in setting TRPM7 magnitude, since physiological levels of Mg·ATP around 2–4 mM would block >80% of TRPM7 current. In any case, Mg·ATP and free Mg2+ are both likely to regulate TRPM7 ion channels in synergy.
Mg·ATP Inhibits TRPM7 Independently of the Ca2+/Mg2+ Chelator
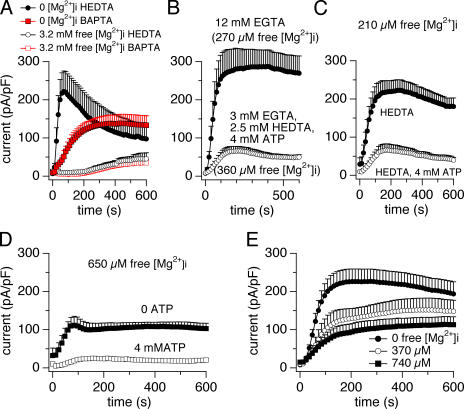
An important question regarding the efficacy of Mg·ATP to suppress TRPM7 was raised by a report that found when using HEDTA to buffer free Mg2+ to 270 μM, even high concentrations of 5 mM Mg·ATP were completely ineffective in suppressing TRPM7 above and beyond the inhibition seen when Mg2+ was buffered to the same level with EGTA (Kozak and Cahalan, 2003). Since our previous experiments were all performed with BAPTA, we first performed experiments in which BAPTA was replaced by HEDTA. We observed that HEDTA in the absence of added Mg2+ caused a rapid and massive activation of TRPM7 that initially surpassed the activation seen with BAPTA (Fig. 3 A), which was not unexpected given the stronger Mg2+ chelation of HEDTA. Interestingly, however, the current inactivated after reaching a peak and eventually decayed to levels below those seen with BAPTA. Addition of Mg2+ at a concentration of 3.2 mM strongly inhibited TRPM7 activity to very low levels and average currents observed with BAPTA and HEDTA were essentially indistinguishable (Fig. 3 A).
Figure 3.
Mg2+ and Mg·ATP inhibition of TRPM7 currents is independent of the buffer. Whole-cell currents were recorded in HEK-293 cells induced to overexpress human TRPM7. The currents were normalized by the capacitance of the cells recorded at break-in. (A) Comparison of HEDTA and BAPTA on TRPM7 current inhibition by Mg2+. Cells were perfused with 10 mM of either buffer without added Mg2+ or with 3.2 mM free Mg2+ (n = 4–8). (B) Mg·ATP block of TRPM7 current using the conditions published by Kozak and Cahalan (2003). The cells were perfused with 12 mM EGTA and 0.5 mM MgCl2 (270 μM free Mg2+; n = 5) or with 2.5 mM HEDTA, 3 mM EGTA, 4 mM Mg·ATP, 360 μM free Mg2+ (n = 6). Mg·ATP block is clearly shown. Left axis labeling same as in A. (C) Comparison of HEDTA and BAPTA on TRPM7 current inhibition by Mg·ATP. Cells were perfused with 10 mM of either buffer with a fixed concentration of 210 μM free Mg2+ with or without 4 mM Mg·ATP (n = 5–8). Left axis labeling same as in A. (D) Typical example of Mg·ATP block of TRPM7 current in the absence of any Mg2+ or Ca2+ chelator and under physiologically relevant magnesium concentrations. The cells were perfused with a fixed concentration of 650 μM free Mg with or without 4 mM Mg·ATP (n = 6). (E) Mg2+ inhibition of TRPM7 current with EGTA buffering. Cells were perfused with 10 mM of EGTA and 2.5 mM Ca2+ (≈55 nM free) without added Mg2+ or with 0.5 or 1 mM MgCl2 (370 and 740 μM free Mg2+, respectively (n = 7–11).
We next sought to replicate the experimental results obtained by Kozak and Cahalan (2003), who reported the complete loss of Mg·ATP sensitivity of TRPM7 when using a mixture of 3 mM EGTA and 2.5 mM HEDTA compared with 12 mM EGTA, where both solutions were adjusted to a free Mg2+ of 270 μM. They observed that both solutions suppressed TRPM7 by ∼70–75% compared with solutions that lack Mg2+ and nucleotides and interpreted this as evidence that Mg·ATP does not provide any additional inhibition beyond that induced by Mg2+ alone. Fig. 3 B shows the average currents we observed when using intracellular solutions as used by Kozak and Cahalan (2003). In reasonable agreement with the above study, we saw ∼80% inhibition of TRPM7 currents by the internal solutions containing 3 mM EGTA and 2.5 mM HEDTA and the additional presence of 5 mM Mg·ATP. However, we were not able to suppress TRPM7 to the same degree when using 12 mM EGTA to buffer free Mg2+ at 270 μM (see also our dose–response curve in Fig. 2 B). It should be noted that the Kozak and Cahalan study used the same software as we do to calculate free Mg2+. However, software versions before 10/2004 were afflicted by a problem with binding constants involving ATP (for details see http://www.stanford.edu/∼cpatton/mcn072802.htm), and therefore the ATP-containing solution used by Kozak and Cahalan was not buffered at calculated levels of 270 μM free Mg2+ as the EGTA solution, but rather at ∼360 μM. We decided to further probe the inhibitory effect of Mg·ATP by increasing HEDTA concentrations to 10 mM, reducing free Mg2+ to 210 μM, and using physiological levels of Mg·ATP of 4 mM. As illustrated in Fig. 3 C, and consistent with Fig. 3 B, the low concentration of 210 μM free Mg2+ by itself had no significant inhibitory effect on the maximal TRPM7 current, being similar to that observed at 0 Mg2+ (compare Fig. 3 A). Remarkably, though, the presence of some free Mg2+ prevented the marked inactivation we observed in 0 Mg2+ conditions, resulting in a sustained activation of TRPM7 current. This might suggest that Mg2+, while acting as an inhibitor of TRPM7, is also required to maintain a sustained current. The additional presence of 4 mM Mg·ATP, even under these more stringent experimental conditions, clearly produced a strong suppression of TRPM7. We therefore cannot support the results and interpretation put forward by Kozak and Cahalan and we conclude that TRPM7 is in fact subject to Mg-nucleotide–dependent regulation.
Regardless of whether or not the inhibition of TRPM7 by Mg·ATP is dependent on the Ca2+/Mg2+ chelator used, a more relevant question is whether the effect occurs under physiological conditions, i.e., in the absence of any exogenous chelators. Fig. 3 D demonstrates that this is indeed the case. Here, the intracellular solution contained no chelator and free Mg2+ was set to near physiological levels of 650 μM. The addition of physiological levels of 4 mM Mg·ATP caused a strong suppression of TRPM7 currents, suggesting that Mg·ATP represents a physiological regulator of TRPM7 activity.
A recent study reported a surprisingly low efficacy of free Mg2+ to inhibit TRPM7 (Hermosura et al., 2005). The authors found that 1 mM added free Mg2+ reduced TRPM7 currents by <20%, whereas all published work on either heterologous or native TRPM7 demonstrates that this concentration of free Mg2+ inhibits the current by at least 50%. However, the experimental conditions in all of these studies are slightly different and may account for the discrepancy. Since Hermosura et al. used the very same TRPM7-overexpressing HEK-293 cells we used in the present study, we determined the efficacy of free Mg2+ in blocking TRPM7 using the same experimental conditions the authors of the previous study employed. However, as illustrated in Fig. 3 E, we were unable to reproduce and confirm the experimental results of Hermosura et al. regarding the Mg2+-mediated inhibition of WT TRPM7. In our hands, perfusing cells with 1 mM added Mg2+ in the presence of 10 mM EGTA plus 2.5 mM Ca2+ (free [Ca2+]i ≈ 55 nM) produced a 50% inhibition of TRPM7 currents compared with the 0 Mg2+ control, which is very similar to the degree of inhibition we and others have observed under a variety of experimental conditions.
NTP and NDP Inhibit TRPM7 Currents with Varying Efficacy
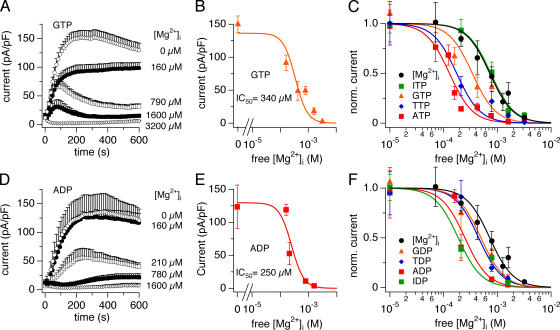
We next asked whether the inhibitory effect was specific for adenosine nucleotides and tested for the ability of other nucleotides for possible regulation of TRPM7. We first perfused cells with guanosine nucleotides (6 mM Mg·GTP) and increasing free Mg2+ concentrations. As illustrated in Fig. 4 A, this nucleotide produced a strong inhibition of TRPM7 currents (n = 5–12). At physiological free Mg2+ concentrations, the response to GTP was biphasic in that the nucleotide seemed to first potentiate the current before blocking it. The calculated IC50 of free Mg2+ in the presence of 6 mM Mg·GTP was 340 ± 95 μM (Fig. 4 B). We then extended this analysis to other purine- and pyrimidine-based triphosphates (ITP, UTP, TTP, and CTP). Except for ITP, each of these nucleotides showed a potentiation of TRPM7 inhibition by free Mg2+, with ATP being the most potent. Based on the IC50 values for 6 mM Mg·NTP, the sequence of potency was as follows: ATP > TTP > CTP ≥ GTP ≥ UTP > ITP ≈ free Mg2+ alone (Fig. 4 C and Table II).
Figure 4.
Di- and triphosphate nucleotides inhibit TRPM7 currents. Whole cell currents were recorded in HEK-293 cells induced to overexpress human TRPM7. Cs-BAPTA was used as intracellular calcium buffer. (A) Typical example of TRPM7 current inhibition by NTP. Cells were perfused with 6 mM Mg·GTP and increasing free Mg2+ concentrations (n = 5–12). (B) Dose–response curve of TRPM7 current inhibition by 6 mM Mg·GTP and increasing free Mg2+ concentrations (0, 160, 420, 790, 1,600, and 3,200 μM). Data points correspond to average and normalized current amplitudes measured at +80 mV after 200 s of whole-cell recording, plotted as a function of free Mg2+ concentration (n = 5–12). The calculated IC50 value for free Mg2+ concentration in the presence of 6 mM Mg·GTP is 340 ± 95 μM. (C) Comparison of dose–response curves as a function of free Mg concentrations for different NTP. Each dose response was calculated as in B and normalized to 1 by dividing with Ymax. (D) Typical example of TRPM7 current inhibition by ADP. Cells were perfused with 6 mM Mg·ADP and increasing free Mg2+ concentrations (n = 6–15). (E) Dose–response curve of TRPM7 current inhibition by 6 mM Mg·ADP and increasing free Mg2+ concentrations (0, 160, 210, 780, and 1,600 μM). Data points correspond to average and normalized current amplitudes measured at +80 mV after 200 s of whole-cell recording, plotted as a function of free Mg2+ concentration (n = 6–15). The calculated IC50 value for free Mg2+ concentration in the presence of 6 mM Mg·ADP is 250 ± 81 μM. (F) Comparison of dose–response curves as a function of free Mg2+ concentration for different NDP. Each dose–response was calculated as in E and normalized to 1 by dividing with Ymax. All the Hill coefficients of the dose–response curves were fixed to 2.
TABLE II.
Inhibition of TRPM7 by Di- and Triphosphate Nucleotides
| Nucleotide | IC50 (μM) | Nucleotide | IC50 (μM) |
|---|---|---|---|
| ATP | 128 ± 18 (n = 4–8) | ADP | 249 ± 81 (5–6) |
| GTP | 342 ± 95 (n = 5–12) | GDP | 479 ± 96 (n = 5–8) |
| ITP | 758 ± 60 (n = 5–19) | IDP | 187 ± 73 (n = 5–8) |
| CTP | 278 ± 103 (n = 5–11) | CDP | 874 ± 309 (n = 5–6) |
| TTP | 175 ± 42 (n = 4–8) | TDP | 466 ± 89 (n = 6–15) |
| UTP | 375 ± 25 (n = 5–10) | UDP | 655 ± 76 (n = 6–7) |
| None | 720 ± 79 (n = 6–10) |
Comparison of half maximal inhibitory concentrations (IC50) of free Mg2+ at fixed 6 mM NTP and NDP. The whole-cell currents were recorded in HEK-293 cells induced to overexpress human TRPM7.
We then assessed the effect of 6 mM nucleotide diphosphates within the same range of free Mg2+ concentrations. All NDP tested enhanced the block of TRPM7 currents by free Mg2+, except CDP. Fig. 4 D shows the ADP effect as an example. However, each NDP was somewhat less potent than its corresponding NTP, with the exception of IDP. Based on the IC50 values for 6 mM Mg·NDP, the sequence of potency was as follows: IDP ≥ ADP > TDP = GDP > UDP ≥ CDP ≈ free Mg alone (Fig. 4 F and Table II). The dose response behavior of IDP, however, may not be entirely accurate. The current amplitudes measured with 6 mM Mg·IDP and 210 μM free Mg2+ conditions were very low compared with higher free Mg2+ concentrations. As commercial IDP is produced from ADP, it is possible that ADP contaminations were present in the solution used. Considering that we had to add very high Na·IDP concentrations (∼31 mM) to obtain 210 μM free Mg2+ and 6 mM Mg·IDP, an ADP contamination of just 2–3% would amount to ∼1 mM of ADP in the solution and could therefore account for the observed inhibition. From these experiments, we propose that ATP and ADP are the most potent nucleotides as inhibitors of TRPM7 currents. As most nucleotides tested showed an increase in Mg2+ inhibition efficacy, we conclude that ATP, ADP, and free Mg2+ act in synergy to regulate TRPM7. The cellular levels of these three players will therefore regulate very precisely the entry of divalents via TRPM7 channels.
Mg·ATP Inhibition of TRPM7 Requires a Functional Kinase Domain
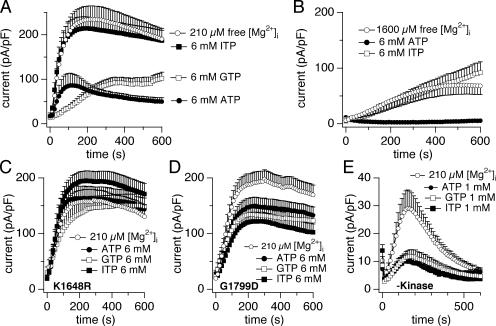
The difference of TRPM7 current inhibition between Mg·ATP and Mg·ITP (Fig. 4 C) came as a surprise, since these molecules are chemically very similar. It is not likely due to differences in free Mg2+ concentration, since increasing free Mg2+ concentration to 1,600 μM still exhibited a difference in the suppression of the current between 6 mM Mg·ATP and 6 mM Mg·ITP (Fig. 5 B). The fact that TRPM7 can discriminate between the two nucleotides suggests a particularly specific inhibitory action of Mg·ATP that might be conferred by the channel's endogenous kinase domain. As we already demonstrated that phosphotranferase activity-deficient mutants of TRPM7 exhibit altered Mg2+ and Mg·ATP-dependant suppression of basal channel activity (Schmitz et al., 2003), we wondered if this difference between nucleotides would still occur in cells overexpressing the phosphotransferase activity-deficient protein. We tested the effect of Mg-nucleotides on three different HEK-293 cell lines overexpressing proteins that had been modified by point mutations K1648R and G1977D as well as a truncation mutant with a complete deletion of the kinase domain (Δ-kinase). The first point mutation targets a strictly conserved lysine residue in α-kinases and protein kinases that interacts with the α and β phosphates of ATP. This lysine is absolutely essential for catalytic activity (Yamaguchi et al., 2001). The second mutation is directed toward a conserved COOH-terminal glycine-rich motif that is thought to be involved in substrate recognition. It is located within the region corresponding to the activation loop of classical kinases (Yamaguchi et al., 2001). All three mutants produced membrane currents that showed the same characteristic current–voltage relationships as wild-type TRPM7. While the two point mutants also had similar current amplitudes as wt TRPM7, the Δ-kinase construct yielded significantly smaller currents that inactivated relatively quickly, as previously described (Schmitz et al., 2003).
Figure 5.
Phosphotransferase-deficient point mutants of TRPM7 affect NTP inhibition. Whole cell currents were recorded in HEK-293 cells induced to overexpress human TRPM7 WT or human TRPM7 mutants. The intracellular calcium buffer was Cs-BAPTA. (A) HEK-293 cells induced to overexpress human TRPM7 WT. Comparison of TRPM7 current inhibition by 6 mM Mg·ATP, Mg·GTP, Mg·ITP, and no nucleotide. In all conditions, free Mg2+ concentration was clamped at 210 μM (n = 5–20). (B) Comparison of TRPM7 current inhibition by 6 mM Mg·ATP and Mg·ITP with free Mg2+ concentration clamped at 1,600 μM (n = 7–8). (C) HEK-293 cells induced to overexpress human TRPM7 point mutation K1648R. Comparison of TRPM7 current inhibition by 6 mM Mg·ATP, Mg·GTP, Mg·ITP, or no nucleotide. In all conditions, free Mg2+ concentration was clamped at 210 μM (n = 6–11). (D) HEK-293 cells induced to overexpress human TRPM7 point mutation G1977D. Comparison of TRPM7 current inhibition by 6 mM Mg·ATP, Mg·GTP, Mg·ITP, or no nucleotide. In all conditions, free Mg2+ concentration was clamped at 210 μM (n = 6–12). (E) HEK-293 cells induced to overexpress human TRPM7 Δ-kinase mutation. Comparison of TRPM7 current inhibition by 1 mM Mg·ATP, Mg·GTP, Mg·ITP, or no nucleotide. In all conditions, free Mg2+ concentration was clamped at 210 μM (n = 6–17).
When exposed to the various nucleotides, the K1648R mutation clearly changed the NTP's ability to regulate TRPM7 (Fig. 5 C). In this mutant, there was no longer a difference between ATP, GTP, and ITP or the control solution containing 210 μM free Mg2+ alone. This point mutation therefore is no longer susceptible to selective inhibition of channel activity by either Mg·ATP or Mg·GTP. Performing the same experiment in cells overexpressing the mutant TRPM7 protein with the second point mutation (G1799D) produced slightly different results. As illustrated in Fig. 5 D, the NTPs tested showed a similar, relatively small inhibition of the current compared with the control solution containing 210 μM free Mg2+ alone. As in the other mutant, however, there was no significant difference between Mg·ATP, Mg·GTP, and Mg·ITP. The Δ-kinase mutant, on the other hand showed a very pronounced block of TRPM7 in the presence of nucleotides compared with the same free magnesium concentration alone (Fig. 5 E). However, the block was similar for all three nucleotides tested. These results indicate that the inhibitory action of the nucleotides is primarily mediated via binding to the channel's endogenous kinase domain, which does discriminate between nucleotide species. The complete removal of the kinase domain reenables nucleotides to inhibit TRPM7, which may be explained by exposure of a previously inaccessible binding site extrinsic to TRPM7 kinase domain that does not discriminate between nucleotide species.
DISCUSSION
The results of the present study firmly establish that both free Mg2+ and Mg-nucleotide complexes (but not free nucleotides) regulate the channel activity of TRPM7. Either of these factors individually can suppress TRPM7 currents, and their combined presence causes a mutually synergistic inhibition by shifting the respective dose–response curves of either molecule. Normal physiological levels of Mg2+ and Mg-nucleotides can largely account for the reduced basal activity of TRPM7 channels in resting cells, and reduction in either one will lead to up-regulation of channel activity. We also observed significant differences in the efficacy of various species of Mg-nucleotides, with the physiologically most relevant adenosine and guanosine nucleotides being the most potent ones. Both nucleotide tri- and diphosphates, but not monophosphates, were effective in regulating TRPM7 channels. The Mg-nucleotide inhibition is likely mediated by a nucleotide binding site on the TRPM7 kinase domain, since point mutations that affect phosphotransferase activity of the kinase domain by interfering with nucleotide binding suppressed the Mg-nucleotide–mediated inhibition of channel activity.
Regulation of TRPM7 via Mg2+ and Mg·ATP
The inhibition of TRPM7 by Mg·ATP has been debated since the discovery of this protein. Our group proposed an inhibition by both free Mg2+ and Mg·ATP (Nadler et al., 2001; Schmitz et al., 2003), while a parallel study postulated activation by increasing intracellular ATP concentrations (Runnels et al., 2001). This issue has been resolved and the ATP-mediated activation of TRPM7 was ultimately due to a decrease in free Mg2+ caused by Na·ATP. The ability of Mg·ATP to suppress TRPM7 has been challenged by Kozak and Cahalan (2003) based on experiments in which free Mg2+ was maintained at low levels and EGTA and HEDTA were used to buffer free Mg2+. That study presented two datasets where free Mg2+ was buffered to 270 μM with either 12 mM EGTA or by EGTA (3 mM) + HEDTA (2.5 mM) + Mg·ATP (5 mM). Both solutions caused a very similar inhibition of TRPM7 by ∼75%, so the authors concluded that inhibition is via Mg2+ alone and that Mg·ATP is ineffective when a strong Mg2+ chelator such as HEDTA is used. This conclusion rests entirely on the accuracy of these two data points. We therefore replicated the exact experimental conditions and we find that the solution containing EGTA (3 mM) + HEDTA (2.5 mM) + Mg·ATP (5 mM) indeed produces ∼80% inhibition of the current, which is similar to the block Kozak and Cahalan report under those conditions. However, we do not observe a significant inhibition of TRPM7 currents by 270 μM free Mg2+ buffered with 12 mM EGTA. Since the 12 mM EGTA control solution, which Kozak and Cahalan use as a reference point, produces a ∼75% block compared with 0 Mg2+, it would correspond to an IC50 of ∼150 μM for the Mg2+-dependent inhibition of TRPM7. In the absence of a full dose–response curve that would confirm such a very low IC50, we point out that our previous studies (Nadler et al., 2001; Schmitz et al., 2003), as well as the data presented here in Fig. 2 B, consistently place the IC50 of Mg2+-dependent TRPM7 block at ∼700 μM, a value that is in excellent agreement with the IC50 of 660 μM obtained by others (Nadler et al., 2001; Kozak et al., 2002; Prakriya and Lewis, 2002; Schmitz et al., 2003). Importantly, Mg·ATP can inhibit TRPM7 in the absence of any chelator, which provides a compelling argument for a key role of this nucleotide in shaping TRPM7 channel activity in physiologically more relevant circumstances. Further support for the nucleotide-mediated regulation of TRPM7 comes from a recent study in human retinoblastoma cells, where Mg·ATP appears to be more effective than Mg2+ alone in inhibiting TRPM7 whole-cell currents (Hanano et al., 2004).
Nevertheless, the HEDTA experiments offer additional insight into TRPM7 regulation by free Mg2+. With HEDTA and no added Mg2+, we observed in the first 100 s both accelerated activation kinetics as well as larger peak currents of TRPM7 currents compared with BAPTA, which would be consistent with HEDTA's more effective chelation of cytosolic Mg2+. This suggests that in the absence of a strong Mg2+ chelator, cells can counteract the decrease in Mg2+ imposed by a Mg2+-free pipette solution, at least temporarily, by mobilizing Mg2+ from intracellular stores. By adding HEDTA, this Mg2+ would be effectively captured and result in a faster and larger increase in TRPM7 current. Interestingly, however, the current subsequently decays to levels comparable to or even below those seen at steady state with BAPTA. Since addition of just 200 μM free Mg2+ prevents this HEDTA-mediated decay without significantly reducing the peak current magnitude, it is conceivable that some Mg2+ may be required for proper channel function, while higher levels of Mg2+ inhibit TRPM7. Alternatively, this effect may be due to a pharmacological effect of the buffers where either HEDTA enhances and/or BAPTA inhibits TRPM7 directly. Another possibility could be related to the fact that TRPM7 has a Zn2+ binding site. Since HEDTA is a very efficient buffer for this ion, the removal of Zn2+ might compromise TRPM7's molecular structure.
Our results demonstrate that free Mg2+ alone, in the absence of added Mg·ATP, is capable of suppressing TRPM7 currents, whereas free ATP alone is without effect. In fact, free ATP will effectively chelate any free Mg2+ and thereby cause activation of TRPM7 via a decrease in free Mg2+. We established the efficacy of free Mg2+ in the absence of any added ATP and arrived at an IC50 of 720 μM. Due to its Mg2+ binding, it is impossible to test the effects of Mg·ATP in isolation, since the complexed nucleotide will be in equilibrium with free Mg2+ according to its dissociation constant. We determined the efficacy of Mg·ATP at the lowest possible levels of free Mg2+ (∼200 μM) to be 3.3 mM. When putting these values into a physiological context, where free Mg2+ is thought to be in the range of 0.5–1 mM and Mg·ATP in the range of 3–4 mM in most cells, it is obvious that neither free Mg2+ alone nor Mg·ATP alone can account for the constitutive suppression of TRPM7 currents. We estimate that TRPM7 activity in a resting cell is suppressed by ∼93% based on the ratio of peak currents in the absence of Mg2+ and Mg·ATP (150 pA/pF, n = 9, 10 mM BAPTA; see Fig. 2 A) and the basal current obtained immediately following break-in (14 pA/pF, n = 48). When perfused with highly inhibitory solutions (high Mg2+ and/or high Mg·ATP), this basal current first decreases, but in time TRPM7 currents increase again and reach higher values. Thus, assuming physiological levels of Mg2+ (0.8 mM) and Mg·ATP (4 mM), their individual contributions would account for 34% and 51% inhibition, respectively (see Fig. 2, B and D, for the dose responses), and the combination of both factors would yield 85%. We therefore assume that the combined effects of the two regulators may account for most of the suppression experienced by TRPM7 under resting conditions, but there may well be additional factors (e.g., GTP and GDP, spermine, etc.) that further reduce TRPM7 activity to arrive at 93%.
Synergy between Mg2+ and Mg·ATP
Mg·ATP is the essential nucleotide complex that binds to the active site of all known protein kinases and the presence of both Mg2+ and ATP is necessary to support phosphorylation (Adams, 2001). The TRPM7 kinase domain is atypical, but the analysis of its crystal structure revealed a striking resemblance to classical protein kinases (Yamaguchi et al., 2001). The present investigation offers some insight into the interactions between free Mg2+ and Mg·ATP in regulating TRPM7 activity. We established dose–response curves of TRPM7 current inhibition by increasing the Mg·ATP concentration at fixed low (∼200 μM), physiological (∼800 μM), or high (∼1600 μM) free Mg2+ concentrations. There was only a modest shift in the IC50 for Mg·ATP-mediated inhibition of TRPM7 from 3.3 ± 0.5 mM (at 200 μM free Mg2+) to 2.0 ± 0.8 mM at 800 μM free Mg2+ and no significant further sensitization at 1.6 mM free Mg2+. While this amounts to a twofold shift in the IC50 for Mg·ATP, it is important to note that this shift occurs at relatively low concentrations of free Mg2+ and is essentially complete at 800 μM free Mg2+. This suggests that slight variations in free Mg2+ around physiological levels may not have a major impact on the nucleotide sensitivity of TRPM7. Conversely, the efficacy of free Mg2+ is shifted about one order of magnitude when increasing Mg·ATP from 0 to 6 mM. Even for physiologically relevant Mg·ATP concentrations between 1 and 4 mM, the shift in IC50 for Mg2+ was fourfold. Therefore, it appears that free Mg2+ and Mg·ATP are acting in synergy (or cooperatively) to inhibit TRPM7, since they differentially affect each other's inhibitory efficacy.
Mono-, Di-, and Triphosphate Nucleotide Effects
When testing for different adenosine phosphates, we were surprised that Mg·ADP inhibited TRPM7 currents with a very similar efficacy as the triphosphate nucleotide. Both Mg·ATP and Mg·ADP at 6 mM were able to enhance the inhibition of TRPM7 current by free Mg2+ (5.6- and 3-fold, respectively). However, equivalent AMP concentrations in the presence of physiological levels of free Mg2+ showed no effect on TRPM7 current. Since AMP does not complex Mg2+, this result suggests that only the full Mg-nucleotide complex can act as an inhibitor of TRPM7.
These results also indicate that a simple decrease in Mg·ATP levels would not be sufficient to cause a massive activation of TRPM7 as long as the total amount of di- and triphosphate species remains constant. One of the conserved features between TRPM7 kinase and classical protein kinases is a strictly conserved lysine residue (Lys-72 in PKA, Lys-1648 in human TRPM7), which interacts with the α- and β-phosphates of ATP (Yamaguchi et al., 2001). It is therefore conceivable that this lysine residue could also interact and stabilize ADP binding for a similar inhibitory effect. As a consequence of this dual regulation, ADP may serve as a safeguard that prevents excessive TRPM7 activation when ATP levels fluctuate under normal physiologic conditions. This safeguard system may fail in pathophysiological situations, such as hypoxia or hypoglycemia, and may contribute to cellular calcium overload and cell death (Nadler et al., 2001; Aarts et al., 2003).
While ATP and ADP were the most potent of all the nucleotides tested to block TRPM7 channels, nearly all other Mg·NTP and Mg·NDP tested were also effective to varying degrees (see Fig. 4). Although the physiological concentration of Mg·ATP can reach up to 6 mM, the physiological concentrations of the purine and pyrimidine nucleotides are of course lower. The average values for total intracellular nucleotide triphosphates in mammalian cells (Traut, 1994) were found to be 3.152 ± 1.698 mM, 468 ± 224 μM, 567 ± 460 μM, and 278 ± 242 μM for ATP, GTP, UTP, and CTP, respectively. The free Mg2+ concentration was estimated at 1.1 mM and complexed Mg at 8 mM. The initial potentiation of TRPM7 that is followed by inactivation with increasing GTP concentrations is likely due to the G protein regulation of TRPM7 (Takezawa et al., 2004). The rather low potency of ITP block compared with ATP is striking, although a previous study made a similar observation on rat heart KATP channels, where ATP, ADP, and CTP strongly inhibited the channels but ITP was much less effective (Lederer and Nichols, 1989). The molecules are very similar (the OH group of adenosine is replaced by NH2 at the 6′ position in inosine), and it is quite surprising that they exhibit vastly different behavior in suppressing TRPM7 currents, whereas structurally more distinct nucleotides like CTP and TTP were relatively potent inhibitors. ATP and ITP also complex Mg2+ with similar potency, making a difference at that level unlikely. There may be nucleotide-specific conformational changes in TRPM7 molecule that influence the closing of the channel, possibly due to differences in hydrogen bonding that stabilize the nucleotide at the binding site.
The Kinase Domain Mediates the Nucleotide-dependent Regulation of Channel Function
Nucleotides are known to affect the phosphotransferase activity of protein kinases, but an important unresolved question pertaining to the TRPM7 ion channel is whether the kinase component can regulate the channel (or vice versa). One model of TRPM7 function proposed that the kinase domain is essential for gating the channel (Runnels et al., 2001). In contrast, we previously demonstrated that mutant TRPM7 channels without phosphotransferase activity still exhibit channel activation comparable to wt TRPM7 and are still regulated by Mg2+ and Mg·ATP (Schmitz et al., 2003). We also found that the cAMP/PKA signaling pathway can regulate TRPM7 activity through the channel's kinase domain, as phosphotransferase-deficient mutants are no longer regulated by that pathway (Takezawa et al., 2004). A recent study argues for a complete dissociation of TRPM7 channel function and regulation by Mg2+ or autophosphorylation by kinase activity (Matsushita et al., 2005). Our results in the present study show the loss of Mg·ATP and Mg·GTP block in the phosphotransferase-deficient mutant K1648R. The lysine residue mutated is implicated in catalysis and is conserved in classical kinases (Yamaguchi et al., 2001; Matsushita et al., 2005). In the second mutant G1799D, some regulation remains, even though phosphotransferase activity is lost. This second mutation may be involved in peptide substrate binding or orientation of the substrate-binding loop (Yamaguchi et al., 2001; Matsushita et al., 2005).
An important question relates to whether the nucleotide binding alone is sufficient to regulate TRPM7 channel activity or whether kinase activity per se also plays a role, since this domain can autophosphorylate the channel. The primary residue of this phosphorylation is a matter of controversy, since some groups observe phosphorylation of serine (Ryazanova et al., 2004; Matsushita et al., 2005), while another identified threonine as a target (Hermosura et al., 2005). TRPM7 has also been identified to phosphorylate other proteins such as annexin (Dorovkov and Ryazanov, 2004). Ryazanova et al. (2004) found that the TRPM7 kinase domain cannot use Mg·GTP as a phosphate donor for phosphorylation reactions. It was speculated that one of the GTP oxygens would repel E1718 in the nucleotide binding site. Our data here argue that Mg·GTP might in fact be able to bind to the nucleotide-binding site, as this nucleotide clearly is efficient in suppressing current activity. This observation and our kinase mutant data support the notion that the inhibitory effects seen by Mg·NTPs are largely independent of the channel's kinase activity and that Mg-nucleotide binding itself is sufficient.
At variance with other reports, Hermosura et al. (2005) recently identified threonin-1428 as a significant locus for TRPM7 autophosphorylation. This study also reported that disabling this phosphorylation site by a T1428I point mutation not only reduced the phosphate incorporation by 30% but also changed the sensitivity of the channel toward inhibition by free Mg2+. The authors found that 1 mM added free Mg2+ reduced the currents of the T1428I mutant by ∼50% compared with Mg2+-free solutions. This value on its own would not suggest an overly sensitive channel, since it is in fact very similar to the degree of Mg2+-induced inhibition observed in numerous studies on heterologous (Nadler et al., 2001; Schmitz et al., 2003) or native TRPM7 (Kozak et al., 2002; Prakriya and Lewis, 2002), including the IC50 observed in the present study (see Fig. 1 and 2). However, in the context of that study, the same concentration of Mg2+ produced a surprisingly small inhibition of wt TRPM7 of <20%. Our attempts to reproduce those data under the same experimental conditions in the same HEK-293 cells overexpressing wt TRPM7 were not successful (see Fig. 3 D), and we therefore cannot conclude that the phosphorylation state of that particular threonine residue is important for shaping Mg2+ and/or Mg-nucleotide sensitivity. It should be noted that a sensitization would be rather unexpected and in apparent conflict with the reduced Mg2+ and Mg·ATP sensitivity reported for phosphotransferase-deficient mutants, which would likely also result in a dephosphorylated protein.
Multiple Binding Sites for Mg2+ and Mg·ATP Inhibition
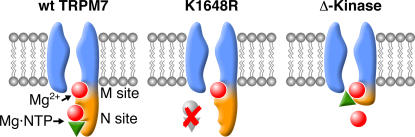
Magnesium is the only intracellular divalent cation, present in vivo at concentrations >1 μM, that has a strong affinity for polyphosphate ions (da Silva and Williams, 2001). Therefore, NTP4− are complexed nearly exclusively to Mg2+ in cells, and protein kinases use the Mg2+ complex of ATP as a substrate rather than free ATP4− anion. In most protein kinases, the apparent Km for Mg·ATP decreases with increasing concentrations of free Mg2+. Given the predicted similarity in magnesium coordination between different Ser/Thr protein kinases and the striking resemblance of structure (but not of sequence) between the TRPM7 kinase domain and typical protein kinases (Sigel et al., 2001; Kim et al., 2005), it is tempting to suggest that TRPM7 also possesses a magnesium binding site. Especially since mutant TRPM7 proteins whose nucleotide binding site has been mutated retain Mg2+ sensitivity, and truncation mutants without the entire kinase domain are even more sensitive to Mg2+ inhibition (Schmitz et al., 2003). Fig. 6 represents the simplest hypothetical model describing the Mg2+ and Mg-nucleotide regulation of TRPM7.
Figure 6.
Hypothetical model of Mg2+ and Mg-nucleotide regulation of TRPM7. The model depicts the binding of Mg2+ and Mg·NTP to the wt TRPM7 channel, the phosphotransferase-deficient point mutant K1648R, and the Δ-kinase truncation mutant. The wt TRPM7 channel provides two distinct sites (M and N sites) that jointly confer intermediate sensitivity to Mg2+ and Mg·NTP. The functional kinase domain (highlighted in orange) harbors the N site and awards specificity for various nucleotide species. The phosphotransferase-deficient mutant lacks the functional N site and is primarily regulated by the M site, but the loss of the synergistic N site interaction results in a channel with reduced susceptibility to Mg2+ block. Finally, the Δ-kinase truncation mutant completely eliminates the kinase domain and the N site, but exposes the M site, which previously was only accessible to Mg2+, to NTP binding. This results in a channel with enhanced sensitivity to both Mg2+ and NTP, but does not impart nucleotide specificity, suggesting that the Mg·NTP may simply function as a Mg donor to the M site.
As illustrated in the model, the wt TRPM7 channel may possess two distinct binding sites for Mg2+ and Mg·NTP, the latter being located within the channel's endogenous kinase domain and the former upstream, possibly close to inner mouth of the channel. Our data suggest that both sites cooperatively mediate the synergistic inhibition of TRPM7 by free Mg2+ and Mg-nucleotides. The sensitivity of the channel toward these inhibitors is relatively high, resulting in >90% inhibition at physiological levels of free Mg2+ and Mg·NTP. The modification of TRPM7 by point mutations that prevent significant nucleotide binding or coordination to the kinase domain essentially removes the nucleotide-mediated component and only leaves the Mg2+-dependent regulatory site. This effectively disrupts the synergy between the two sites and renders the channel largely insensitive to nucleotide regulation at low levels of Mg2+ and also lowers the sensitivity to block by free Mg2+ itself. We cannot rule out that the point mutants retain some small capacity of nucleotide binding that could become more obvious at higher levels of free Mg2+. This could explain why these point mutants can be inhibited by very high concentrations of Mg·ATP in the presence of 500 μM free Mg2+ (Schmitz et al., 2003). Finally, when removing the kinase domain entirely, we see the opposite effect, in that kinase deletion mutants are more sensitive to both free Mg2+ and Mg-nucleotides. We hypothesize that the truncation of the COOH-terminal kinase domain exposes the Mg2+ binding site and converts it into a high-affinity site that can be blocked by very low levels of free Mg2+. In addition, this site now becomes available for binding Mg-nucleotides. However, since the kinase deletion mutant does not discriminate between nucleotide species, we assume that it simply accepts the Mg-phosphate moiety as a binding partner. This model considers Mg2+ and Mg-nucleotide binding as primary regulators of channel activity, although the phosphorylation status of the channel may contribute to its sensitivity toward these inhibitors (Schmitz et al., 2003).
Conclusions
The present study resolves two controversial issues regarding the relative roles of Mg-nucleotides and the endogenous kinase domain in regulating TRPM7 channel activity. While in contrast to a conflicting report by Cahalan and colleagues that disputes the ability of Mg-nucleotides to regulate TRPM7 (Kozak and Cahalan, 2003), the comprehensive analysis presented here provides unambiguous evidence that Mg·nucleotides do in fact regulate TRPM7 channels by inhibiting channel activity in synergy with free Mg2+. This extends and corroborates the original observation of the Mg-nucleotide regulation (Nadler et al., 2001) and reaffirms the aptness of the originally proposed term MagNuM (for magnesium-nucleotide regulated metal ion current) put forward in the first two published accounts of heterologous and native TRPM7 currents (Nadler et al., 2001; Hermosura et al., 2002). The nucleotide-dependent regulation of TRPM7 appears to be mediated by a binding site within the endogenous kinase domain and therefore reaffirms that the kinase domain is linked to channel function and not completely separate as proposed by Cahalan and colleagues (Matsushita et al., 2005). This regulation is seen with nearly all NTPs and NDPs, and nucleotide specificity is imparted by the nucleotide binding site as demonstrated by phosphotransferase-deficient point mutants. These data complement our previous demonstration of the kinase domain's participation in the receptor-mediated regulation of TRPM7 via cAMP and PKA (Takezawa et al., 2004).
Supplemental Material
Acknowledgments
We thank Carolyn E. Oki and Ka'ohimanu L. Dang for expert technical assistance and Carsten Schmitz (University of Denver, Denver, CO) for providing the mutant TRPM7 TrexHEK293 cell lines.
This work was supported by National Institutes of Health grant R01-GM065360 to A. Fleig, and R01-NS040927 to R. Penner.
Olaf S. Andersen served as editor.
References
- Aarts, M., K. Iihara, W.L. Wei, Z.G. Xiong, M. Arundine, W. Cerwinski, J.F. MacDonald, and M. Tymianski. 2003. A key role for TRPM7 channels in anoxic neuronal death. Cell. 115:863–877. [DOI] [PubMed] [Google Scholar]
- Adams, J.A. 2001. Kinetic and catalytic mechanisms of protein kinases. Chem. Rev. 101:2271–2290. [DOI] [PubMed] [Google Scholar]
- da Silva, J.J.R.F., and R.J.P. Williams. 2001. The Biological Chemistry of the Elements: the Inorganic Chemistry of Life. Second edition. Oxford University Press, Oxford. 575 pp.
- Dorovkov, M.V., and A.G. Ryazanov. 2004. Phosphorylation of annexin I by TRPM7 channel-kinase. J. Biol. Chem. 279:50643–50646. [DOI] [PubMed] [Google Scholar]
- Fleig, A., and R. Penner. 2004. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol. Sci. 25:633–639. [DOI] [PubMed] [Google Scholar]
- Gwanyanya, A., B. Amuzescu, S.I. Zakharov, R. Macianskiene, K.R. Sipido, V.M. Bolotina, J. Vereecke, and K. Mubagwa. 2004. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation. J. Physiol. 559:761–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano, T., Y. Hara, J. Shi, H. Morita, C. Umebayashi, E. Mori, H. Sumimoto, Y. Ito, Y. Mori, and R. Inoue. 2004. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J. Pharmacol. Sci. 95:403–419. [DOI] [PubMed] [Google Scholar]
- Harteneck, C. 2005. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch. Pharmacol. 371:307–314. [DOI] [PubMed] [Google Scholar]
- Hermosura, M.C., M.K. Monteilh-Zoller, A.M. Scharenberg, R. Penner, and A. Fleig. 2002. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J. Physiol. 539:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura, M.C., H. Nayakanti, M.V. Dorovkov, F.R. Calderon, A.G. Ryazanov, D.S. Haymer, and R.M. Garruto. 2005. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. USA. 102:11510–11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J., M. Li, and L. Yue. 2005. Potentiation of TRPM7 inward currents by protons. J. Gen. Physiol. 126:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C., N.H. Xuong, and S.S. Taylor. 2005. Crystal structure of a complex between the catalytic and regulatory (RIα) subunits of PKA. Science. 307:690–696. [DOI] [PubMed] [Google Scholar]
- Kozak, J.A., and M.D. Cahalan. 2003. MIC channels are inhibited by internal divalent cations but not ATP. Biophys. J. 84:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak, J.A., H.H. Kerschbaum, and M.D. Cahalan. 2002. Distinct properties of CRAC and MIC channels in RBL cells. J. Gen. Physiol. 120:221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak, J.A., M. Matsushita, A.C. Nairn, and M.D. Cahalan. 2005. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J. Gen. Physiol. 126:499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer, W.J., and C.G. Nichols. 1989. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J. Physiol. 419:193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita, M., J.A. Kozak, Y. Shimizu, D.T. McLachlin, H. Yamaguchi, F.Y. Wei, K. Tomizawa, H. Matsui, B.T. Chait, M.D. Cahalan, and A.C. Nairn. 2005. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J. Biol. Chem. 280:20793–20803. [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller, M.K., M.C. Hermosura, M.J. Nadler, A.M. Scharenberg, R. Penner, and A. Fleig. 2003. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler, M.J., M.C. Hermosura, K. Inabe, A.L. Perraud, Q. Zhu, A.J. Stokes, T. Kurosaki, J.P. Kinet, R. Penner, A.M. Scharenberg, and A. Fleig. 2001. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 411:590–595. [DOI] [PubMed] [Google Scholar]
- Prakriya, M., and R.S. Lewis. 2002. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J. Gen. Physiol. 119:487–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch, M., and E. Neher. 1988. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 411:204–211. [DOI] [PubMed] [Google Scholar]
- Runnels, L.W., L. Yue, and D.E. Clapham. 2001. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 291:1043–1047. [DOI] [PubMed] [Google Scholar]
- Runnels, L.W., L. Yue, and D.E. Clapham. 2002. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 4:329–336. [DOI] [PubMed] [Google Scholar]
- Ryazanova, L.V., M.V. Dorovkov, A. Ansari, and A.G. Ryazanov. 2004. Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J. Biol. Chem. 279:3708–3716. [DOI] [PubMed] [Google Scholar]
- Ryazanova, L.V., K.S. Pavur, A.N. Petrov, M.V. Dorovkov, and A.G. Ryazanov. 2001. Novel type of signaling molecules: protein kinases covalently linked with ion channels. Mol. Biol. 35:271–283. [PubMed] [Google Scholar]
- Schmitz, C., A.L. Perraud, C.O. Johnson, K. Inabe, M.K. Smith, R. Penner, T. Kurosaki, A. Fleig, and A.M. Scharenberg. 2003. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 114:191–200. [DOI] [PubMed] [Google Scholar]
- Sigel, H., E.M. Bianchi, N.A. Corfu, Y. Kinjo, R. Tribolet, and R.B. Martin. 2001. Stabilities and isomeric equilibria in solutions of monomeric metal-ion complexes of guanosine 5′-triphosphate (GTP4-) and inosine 5′-triphosphate (ITP4-) in comparison with those of adenosine 5′-triphosphate (ATP4-). Chemistry. 7:3729–3737. [DOI] [PubMed] [Google Scholar]
- Takezawa, R., C. Schmitz, P. Demeuse, A.M. Scharenberg, R. Penner, and A. Fleig. 2004. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc. Natl. Acad. Sci. USA. 101:6009–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut, T.W. 1994. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140:1–22. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, H., M. Matsushita, A.C. Nairn, and J. Kuriyan. 2001. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol. Cell. 7:1047–1057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.