Abstract
Bestrophins are a newly discovered family of Cl− channels, some members of which are activated by intracellular Ca2+. So far, all studies were carried out with whole-cell recordings from plasmid-transfected cultured cells, so it is unclear whether Ca2+ activates bestrophin through a metabolic mechanism or in a more direct way. We report here experiments that addressed this question with excised, inside-out membrane patches. We chose human bestrophin-4 (hBest4) for heterologous expression because it gave particularly large Cl− currents when expressed, thus allowing detection even in excised membrane patches. hBest4 gave a negligible Cl− current in a Ca2+-free solution on the cytoplasmic (bath) side, but produced a Cl− current that was activated by Ca2+ in a dose-dependent manner, with a K 1/2 of 230 nM. Thus, Ca2+ appears to activate the bestrophin Cl− channel without going through a freely diffusible messenger or through protein phosphorylation. Because the activation and deactivation kinetics were very slow, however, we cannot exclude the involvement of a membrane-associated messenger.
INTRODUCTION
Bestrophin is the protein product of VMD2, a gene that when defective causes juvenile-onset, autosomal-dominant, vitelliform macular dystrophy (VMD/Best disease) in the retina (Petrukhin et al., 1998; Marquardt et al., 1998). The human genome codes for four members of the bestrophin family, with no obvious homology to any other human protein (Sun et al., 2002; Tsunenari et al., 2003). A number of recent studies using heterologous expressions have shown that bestrophins form Cl− channels (Sun et al., 2002; Tsunenari et al., 2003; Qu et al., 2003, 2004; Qu and Hartzell, 2004; Fischmeister and Hartzell, 2005). Disease-associated point mutations in bestrophin have also been found to result in severely inhibited Cl− currents when heterologously expressed (Sun et al., 2002; Qu et al., 2003).
Bestrophin has been localized to the basolateral membrane of the retinal pigment epithelial (RPE) cells (Marmorstein et al., 2000). These cells mediate the transfer of water, ions, and metabolites between the photoreceptors and the heavily vascularized choroid behind the RPE. Because Cl− flux is generally associated with the transepithelial transport of substances, it is reasonable to think that bestrophin, with its purported location, has an important role in such a function of RPE cells. In the electrooculogram, which is a mass voltage signal rather similar to the common electroretinogram but recorded on a longer time scale, there is a component (the “light peak”) that reaches peak in 6–9 min after light onset (Francois et al., 1967; Deutman, 1969; Gallemore et al., 1998b). In patients with Best disease, this light peak is substantially reduced even before the onset of disease symptoms, indicating that the light peak reflects a physiological entity the defect of which is intrinsically associated with (and possibly causes) the disease rather than a consequence of degenerative processes in the course of the disease (Francois et al., 1967; Deutman, 1969; Gallemore et al., 1998b). Moreover, electrophysiological evidence suggests that the light peak reflects an increase in the basolateral Cl− conductance of RPE cells (Steinberg et al., 1985; Gallemore et al., 1998a). The correlative information from these studies thus corroborates the conclusion from heterologous expression that bestrophin is a Cl− channel.
A putative agent controlling the Cl− conductance associated with the electrooculogram light peak is intracellular Ca2+ (Steinberg et al., 1985; Gallemore et al., 1998a). Indeed, the Cl− channel formed by heterologously expressed human bestrophin is opened by a rise in intracellular Ca2+ concentration (Sun et al., 2002). Xenopus and mouse bestrophin homologues were found to be Ca2+ sensitive in a similar way (Qu et al., 2003, 2004). However, all studies so far were performed with the whole-cell, patch-clamp recording method, so the question remains whether the effect of Ca2+ on bestrophin goes through an enzymatic reaction such as protein phosphorylation, or is more direct. At the same time, it is difficult to study with the whole-cell recording method the kinetics of activation and deactivation of the channel in response to Ca2+ changes, and to examine more than one intracellular Ca2+ concentration in a single experiment. Accordingly, we have turned to excised, inside-out patch recording. We have chosen human bestrophin-4 (hBest4) for these experiments because, among the gene products of the four human bestrophin genes, hBest4 shows by far the highest whole-cell Cl− current in transfected cell lines (Tsunenari et al., 2003). A large Cl− current is important for obtaining any measurable current from excised membrane patches. Our experiments indicate that Ca2+ is able to activate hBest4 in a cell-free environment, and provide a clearer dose dependence of the opening of the bestrophin channel on Ca2+.
MATERIALS AND METHODS
Electrophysiology
CHO-K1 cells or HEK293 cells were cotransfected with the hBest4 and EGFP expression plasmids (both in the pRK5 vector) at a 4:1 or 10:1 ratio, by using 3–6 μl FuGENE 6 (Roche Applied Science) at 1–2.5 μg of hBest4 plasmid DNA per 35-mm dish (containing four or five 12-mm circular coverslips plated with cells). The EGFP plasmid (0.1–0.5 μg) alone was also used as a mock-transfection control. Within 3 d after transfection, inside-out patches of plasma membrane were excised from transfected cells identified by EGFP fluorescence and recorded at room temperature (23–25°C). The data were acquired with a DIGIDATA1200 and pClamp6 software (Molecular Devices Corporation). Solutions containing different buffered Ca2+ concentrations were applied with a rapid solution changer (RSC-200, Bio-Logic Science Instruments). Liquid junction potentials were <5 mV and have not been compensated. With respect to nomenclature, hBest4 is the human bestrophin family member with the first 12 residues being MTVSYTLKVAEA (NCBI accession no. AAR99657; also see Fig. 1 of Tsunenari et al., 2003).
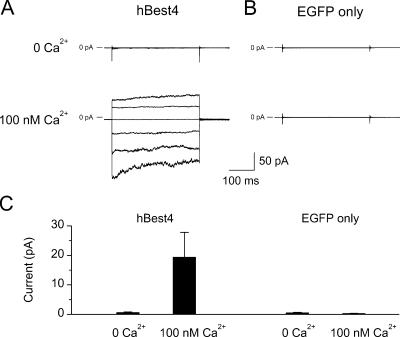
Figure 1.
Cl− current of hBest4 in inside-out membrane patches excised from transfected CHO-K1 cells. (A) Cell cotransfected with hBest4 plasmid and EGFP plasmid. (B) Cell transfected with EGFP plasmid alone (negative control). In each case, the same patch was alternatively exposed to near-zero Ca2+ and 100-nM Ca2+ in the bath. The current traces were produced by 350-ms voltage steps from a holding potential of 0 mV to voltages between −120 and +80 mV in 40-mV increments. Both of the patch pipette solution and the bath solution contained NMDG+ and Cl− as major ions. (C) Collected results of experiments shown in A and B, measured at the end of the 350-ms voltage to +80 mV (mean ± SEM). Left, nine patches; right; seven patches.
Solutions
Standard bath solution contained (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 Na-HEPES, pH 7.4. The patch-pipette solution contained (in mM) 150 NMDG-Cl, 1 EGTA, and 20 NMDG-HEPES, pH 7.4. The compositions of the various bath solutions are summarized in Table I; in addition, they all contained 20 mM NMDG-HEPES, pH 7.2. The free Ca2+ concentration was calculated with the WINMAXC2.5 software (Patton et al., 2004).
TABLE I.
Compositions of Bath Solutions
| Ca2+ solutions
| |||||||
|---|---|---|---|---|---|---|---|
| NMDG-Cl | Methanesulfonate | CaCl2 | EDTA | EGTA | HEDTA | NTA | |
| mM | mM | mM | mM | mM | mM | mM | |
| Near-zero Ca2+ | 135 | 10 | |||||
| 20 nM Ca2+ | 128.5 | 3.24 | 10 | ||||
| 40 nM Ca2+ | 125.2 | 4.9 | 10 | ||||
| 100 nM Ca2+ | 127.6 | 3.7 | 10 | ||||
| 300 nM Ca2+ | 122.2 | 6.4 | 10 | ||||
| 1 μM Ca2+ | 132.4 | 1.3 | 10 | ||||
| 10 μM Ca2+ | 128.8 | 3.1 | 10 | ||||
| 100 μM Ca2+ | 131.4 | 1.82 | 5 | ||||
| Low Cl− | 20 | 111.4 | 1.82 | 5 | |||
All solutions contained 20 mM HEPES, and pH was adjusted to 7.2 with NMDG. HEDTA, N-hydroxyethylethylenediaminetriacetic acid; NTA, nitrilotriacetic acid.
RESULTS
When expressed in CHO-K1 cells, hBest4 gave whole-cell currents that often exceeded 20 nA (versus, for example, hBest1, which typically gave currents no more than 1 nA). In the steady presence of 100 nM free Ca2+ on the cytoplasmic side, the Cl− current (measured with NMDG+ as the cation on both sides of the membrane; see Materials and Methods) recorded from a membrane patch with a voltage step resembled in time course the whole-cell current of hBest4 previously reported (cf. Fig. 2 of Tsunenari et al., 2003); a −120-mV pulse induced a rapid inward current that decreased by ∼30% over several hundred milliseconds, and a +80-mV pulse induced an outward current that slowly increased over the same duration (Fig. 1 A, bottom, and collected results in Fig. 1 C; 9 cells). These current relaxations probably reflected a mild voltage dependence of the Ca2+-activated Cl− current. The same patch in the absence of Ca2+ showed negligible current (Fig. 1 A, top, and collected results in Fig. 1 C). Experiments on cells mock transfected with the EGFP plasmid alone gave negligible currents in the absence or presence of cytoplasmic Ca2+ (Fig. 1, B and C; 7 cells).
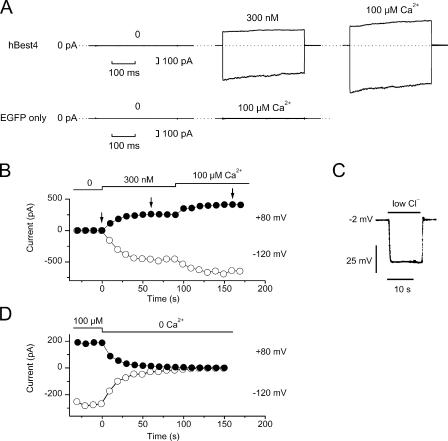
Figure 2.
Ca2+ dependence of hBest4 Cl− current. (A) Top, currents recorded from an excised patch of hBest4-transfected HEK293 cell at free Ca2+ concentrations of near-zero, 300 nM, and 100 μM. In each panel, the membrane voltage was stepped from 0 to −120 and +80 mV for 350 ms. Bottom, control experiment on an excised patch from a HEK293 cell mock-transfected with EGFP plasmid alone. (B) Complete recordings of the same patch as shown in the top of A to indicate the time course of activation of hBest4 current by Ca2+. Each point was derived from a set of measurements as shown in the top of A, showing the current amplitude at the end of the 350-ms pulse to −120 mV (open symbol) and +80 mV (closed symbol). The arrows indicate the time points at which the measurements in the top of A were obtained. The current activation time course at 300 nM Ca2+ in B could be described by a single exponential with time constant of 19 s at −120 mV and 10 s at +80 mV. (C) Solution-exchange time course measured with the same patch shown in A and B. The solution exchange was relatively fast. Membrane voltage was recorded in zero-current clamp mode. A high-Cl− solution (135 mM Cl−, E Cl = −2.7 mV) containing 100 μM Ca2+ was replaced by a low-Cl− solution (23.6 mM Cl−, E Cl = −47 mV) containing the same free [Ca2+]. No compensation for liquid-junction potential has been made in the trace. After compensation, the initial voltage would correspond to −1.5 mV, and reach −45 mV within 1–2 s after the onset of the solution exchange. (D) Same kind of experiment as in B, but from a different patch and showing the decline time course of the current.
For dose–response experiments, we used HEK293 cells for expression because these gave substantially larger Cl− currents with hBest4 than CHO-K1 cells. In Fig. 2 A, top, which shows one such experiment, there was no detectable current at negative or positive voltages in the absence of Ca2+ on the cytoplasmic side (left trace, 10 mM EDTA and no added Ca2+; see also Fig. 2, B and D). Adding 300 nM Ca2+ activated a current that gradually increased over ∼1 min (Fig. 2 A, middle trace in top panel, and Fig. 2 B). Increasing the Ca2+ concentration to 100 μM activated more current, again with a slow time course. This slowness was not due to a slow solution exchange, which was near completion within 1 s (Fig. 2 C and legend). The disappearance of the current upon removing Ca2+ had a similarly slow time course (Fig. 2 D). In 24 patches, the mean current at +80 mV was 1.2 ± 0.6 (mean ± SD) pA at near-zero Ca2+ and 168 ± 136 pA at 100 μM Ca2+. Control experiments with mock-transfected HEK293 cells using the EGFP plasmid alone gave a mean current at +80 mV of 0.6 ± 0.5 pA (6 patches) at near-zero Ca2+ and 2.0 ± 1.9 pA (11 patches) at 100 μM Ca2+ (see Fig. 2 A, bottom, for example). Thus, any endogenous Ca2+-activated Cl− current in HEK293 cells should have little effect on the measurements of the bestrophin Cl− current.
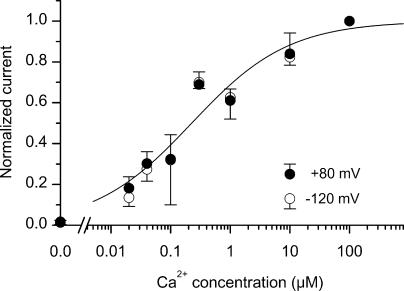
The collective dose dependence of the Cl− current on Ca2+ concentration from multiple experiments is shown in Fig. 3. For each patch, three different Ca2+ concentrations were used in the following order: near-zero, an intermediate concentration, and 100 μM. The currents were normalized against that at 100 μM Ca2+, which was saturated. We were unable to test more than one intermediate Ca2+ concentration for a given patch because of the slow onset and offset of the current (see Fig. 2, B and D), as well as the general instability of the patches. A high Ca2+ concentration such as 100 μM also produced a desensitization-like phenomenon, in that a subsequent exposure to the intermediate concentration of Ca2+ elicited a smaller current than before. No apparent difference in the relation between positive and negative voltages was observed. In both cases, the relation can be described by the Hill equation, with a K1/2 of 230 nM Ca2+ and a Hill coefficient, n, of 0.53. The small Hill coefficient means that the activation of the Cl− current spans over a 1,000-fold change in free Ca2+ concentration; significant activation occurred at 20-nM Ca2+, but the activation still did not reach maximum at 10 μM Ca2+.
Figure 3.
Dependence of hBest4 Ca2+-activated Cl− current on free Ca2+ concentration. Same procedure as in Fig. 2 B, including the order of solution application: near-zero Ca2+ followed by an intermediate Ca2+ concentration, and then by 100 μM Ca2+. The plotted currents have been normalized with respect to the current at 100 μM Ca2+. Filled circles represent mean values (±SD) at +80 mV, and open circles represent mean values at −120 mV (3–5 patches each). The smooth curve is the Hill equation with K1/2 = 230 nM and a Hill coefficient of 0.53.
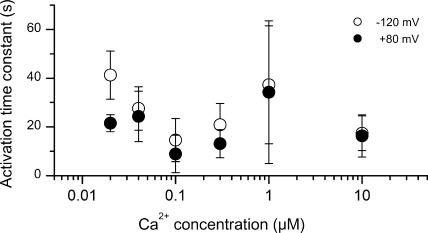
As mentioned earlier, the time course of current onset in response to Ca2+ application was slow (Fig. 2 B). This time course showed no obvious dependence on voltage or Ca2+ concentration (Fig. 4). At +80 mV, for example, the current onset could be described by a single-exponential function with a time constant of 21.5 ± 3.5 s at 20 nM Ca2+ (mean ± SD, 3 patches), versus 16.3 ± 8.7 s (4 patches) at 10 μM Ca2+, i.e., a less than twofold change in time course for a 500-fold change in Ca2+ concentration. The deactivation of the current when switching the Ca2+ concentration from 100 μM to near zero was comparably slow (Fig. 2 D), with a single-exponential time constant of 18.0 ± 7.9 s at +80 mV (n = 17) (and 21.5 ± 7.2 s at −120 mV).
Figure 4.
Time constant of current activation at different Ca2+ concentrations. Same patches as in Fig. 3. The values are mean ± SD.
DISCUSSION
Based on the extensive evidence reported so far, it appears that bestrophins are bona fide chloride channels (Sun et al., 2002; Tsunenari et al., 2003; Qu et al., 2003, 2004; Qu and Hartzell, 2004; Fischmeister and Hartzell, 2005). Recently, hBest1 has also been reported to influence the kinetics and voltage dependence of endogenous L-type Ca2+ channels when hBest1 was transiently expressed in an RPE cell line (Rosenthal et al., 2006), though what this means remains unclear.
In this paper, we have demonstrated that hBest4 Cl− channels on excised membrane patches can be activated by free Ca2+ on the cytoplasmic side. These experiments allowed us to obtain a fairly well-defined dose dependence of channel activation on free Ca2+ concentration. The K1/2 value for the activation was 230 nM Ca2+. Previously, by comparing whole-cell currents elicited with different intrapipette Ca2+ concentrations across bestrophin-transfected HEK293 cells, others have estimated the K1/2 to be 210 nM for xBest2a and 228 nM for xBest 2b (Xenopus), and 230 nM for mBest2 (mouse) (Qu et al., 2003, 2004). These K1/2 values are remarkably close to our estimate here, but this agreement should be taken with caution because other bestrophin channels were used in these other studies, and the experimental approach was different. Generally speaking, the dose–response relations derived from whole-cell recordings (Qu et al., 2003, 2004) are somewhat indirect, because they involved comparisons across cells and also assumed perfect Ca2+ buffering by the pipette solution dialyzed into the cells. Qu et al. (2003, 2004) used EGTA to buffer Ca2+ up to the several-micromolar range, at the upper end of which EGTA might not be very efficient (Patton et al., 2004). One difference between our findings and those of Qu et al. (2003, 2004) is that the Hill coefficient we measured is less than unity, versus 5–7, as can be estimated from the data of Qu et al. (2003, 2004). A Hill coefficient of <1 is rather unusual, typically indicating negative cooperativity. On the other hand, a Hill coefficient of 5–7 seems unusually high as well. Ideally, the excised-patch experiments described here should be repeated with the other bestrophin family members (including those used by Qu et al.), although low levels of functional expression might make this difficult.
We also tested the effect of divalent cations other than Ca2+, and found that Sr2+ was about as effective as Ca2+, but Ba2+ was only very weakly effective, and Mg2+ was practically ineffective (unpublished data). This selectivity conforms with the property of other Ca2+-activated Cl− channels (e.g., Reisert et al., 2003).
Another unusual feature with hBest4 is that the current activates and deactivates very slowly in response to changes in Ca2+ concentration, with a time constant as long as 10–20 s. Previous studies on Ca2+-activated Cl− currents in excised patches of native membrane of Xenopus oocytes and other cell types gave activation and deactivation kinetics that are orders of magnitude faster (e.g., Gomez-Hernandez et al., 1997; Reisert et al., 2003). Because the activation time course of hBest4 shows hardly any dependence on Ca2+ concentration, and the deactivation kinetics are comparably slow, the underlying rate-limiting step does not appear to be dominated by Ca2+ binding/unbinding. One possibility is that the opening/closing transitions of hBest4 after Ca2+ binding are very slow. However, the voltage-dependent relaxations of the current were only in the range of hundreds of milliseconds (see Fig. 1; also Fig. 2 in Tsunenari et al., 2003), suggesting that the opening/closing transitions were not rate limiting. The slow kinetics may reflect an effect of Ca2+ on the channel that is indirect. We can probably rule out the involvement of a kinase as an intermediate because no ATP was required for the channel activation in our experiments. Nonetheless, the action of Ca2+ may still be indirect and act through, for example, a membrane-associated messenger such as lipid or a membrane-associated regulatory protein. Finally, an intermediate scenario is, in principle, also possible; namely, Ca2+ binds directly to bestrophin, but this binding indirectly controls the opening/closing of the channel through another entity. A complex activation mechanism may also explain the sublinear dose–response relation. Interestingly, among the various bestrophins that we have studied, heterologously expressed hBest1, hBest2, and dmBest1 (Drosophila) showed no obvious voltage-dependent relaxations of the current, whereas hBest3 and ceBest1 (Caenorhabditis elegans), like hBest4, both showed very slow (in the range of a second or hundreds of milliseconds) voltage-dependent current relaxations (Sun et al., 2002; Tsunenari et al., 2003). Thus, there is heterogeneity in channel kinetics among the bestrophin homologues. Whether this implies heterogeneity in the gating mechanism for bestrophins, as apparently is the case for the TRP channel superfamily (Clapham, 2003; Nilius et al., 2005), is unclear.
If Ca2+ indeed binds directly to bestrophin, where is the binding site? In the case of the large-conductance Ca2+-activated K+ channel (BK channel), a high-affinity Ca2+-binding site (the Ca2+ bowl) has been identified between the S9 and S10 domains, corresponding to the sequence TELVNDTNVQFLDQDDDDDPDTELYLTQ (residues 883–910, with the negatively charged glutamates and aspartates in bold face) near the cytoplasmic COOH terminus of mSlo1 (Schreiber and Salkoff, 1997; Schreiber et al., 1999). This sequence has 10 negative charges, 5 of which are consecutive. Interestingly, all members of the human bestrophin family also have five consecutive negative charges, together with three nearby negative charges, in the cytoplasmic COOH terminus (for hBest4: 306AEQIINPFGEDDDDFETNQLIDRNLQV332; for hBest1–3: residues 291–317, which are highly homologous to hBest4). We have noticed that Xenopus and mouse bestrophins also have a similar sequence: residues 306–332 for Xenopus bestrophin 2 (Qu et al., 2003) and residues 291–317 for mouse bestrophin 2 (Krämer et al., 2004; Qu and Hartzell, 2004). Five of the disease-associated human mutations in hBest1 previously studied are in fact situated in this region (Q293K, G299E, E300D, D301E, and T307I; see Sun et al., 2002). All of these mutants produced substantially reduced whole-cell currents in transfected HEK293 cells, suggesting that this region is crucial for channel function. Whether this region indeed has a role in the Ca2+ sensitivity of bestrophin Cl− channels remains to be determined.
Acknowledgments
We would like to thank members of the Yau laboratory for discussions on the experiments.
This work was supported by NIH grants EY06837 (K.-W. Yau) and EY14367 (J. Nathans) and an award from the Ruth and Milton Steinbach Fund (K.-W. Yau).
Olaf S. Andersen served as editor.
Abbreviations used in this paper: hBest, human bestrophin; mBest, mouse bestrophin; xBest, Xenopus bestrophin; RPE, retinal pigment epithelial.
References
- Clapham, D.E. 2003. TRP channels as cellular sensors. Nature. 426:517–524. [DOI] [PubMed] [Google Scholar]
- Deutman, A.F. 1969. Electro-oculography in families with vitelliform dystrophy of the fovea. Detection of the carrier state. Arch. Ophthalmol. 81:305–316. [DOI] [PubMed] [Google Scholar]
- Fischmeister, R., and H.C. Hartzell. 2005. Volume sensitivity of the bestrophin family of chloride channels. J. Physiol. 562:477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois, J., A. De Rouck, and D. Fernandez-Sasso. 1967. Electro-oculography in vitelliform degeneration of the macula. Arch. Ophthalmol. 77:726–733. [DOI] [PubMed] [Google Scholar]
- Gallemore, R.P., B.A. Hughes, and S.S. Miller. 1998. a. Light-induced responses of the retinal pigment epithelium. In The Retinal Pigment Epithelium. M.F. Marmor and T.J. Wolfensberger, editors. Oxford University Press, Oxford. 175–198.
- Gallemore, R.P., F. Maruiwa, and M.F. Marmor. 1998. b. Clinical electrophysiology of the retinal pigment epithelium. In The Retinal Pigment Epithelium. M.F. Marmor and T.J. Wolfensberger, editors. Oxford University Press, Oxford. 199–223.
- Gomez-Hernandez, J.-M., W. Stühmer, and A.B. Parekh. 1997. Calcium dependence and distribution of calcium-activated chloride channels in Xenopus oocytes. J. Physiol. 502:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer, F., H. Stöhr, and B.H.F. Weber. 2004. Cloning and characterization of the murine Vmd2 RFP-TM gene family. Cytogenet. Genome Res. 105:107–114. [DOI] [PubMed] [Google Scholar]
- Marmorstein, A.D., L.Y. Marmorstein, M. Rayborn, X. Wang, J.G. Hollyfield, and K. Petrukhin. 2000. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 97:12758–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt, A., H. Stöhr, L.A. Passmore, F. Krämer, A. Rivera, and B.H.F. Weber. 1998. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum. Mol. Genet. 7:1517–1525. [DOI] [PubMed] [Google Scholar]
- Nilius, B., K. Talavera, G. Owsianik, J. Prenen, G. Droogmans, and T. Voets. 2005. Gating of TRP channels: a voltage connection? J. Physiol. 567:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, C., S. Thompson, and D. Epel. 2004. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 35:427–431. [DOI] [PubMed] [Google Scholar]
- Petrukhin, K., M.J. Koisti, B. Bakall, W. Li, G. Xie, T. Marknell, O. Sandgren, K. Forsman, G. Holmgren, S. Andreasson, et al. 1998. Identification of the gene responsible for Best macular dystrophy. Nat. Genet. 19:241–247. [DOI] [PubMed] [Google Scholar]
- Qu, Z., R.W. Wei, W. Mann, and H.C. Hartzell. 2003. Two bestrophins cloned from Xenopus laevis oocytes express Ca2+-activated Cl− currents. J. Biol. Chem. 278:49563–49572. [DOI] [PubMed] [Google Scholar]
- Qu, Z., R. Fischmeister, and C. Hartzell. 2004. Mouse bestrophin-2 is a bona fide Cl− channel: identification of a residue important in anion binding and conduction. J. Gen. Physiol. 123:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Z., and C. Hartzell. 2004. Determinants of anion permeation in the second transmembrane domain of the mouse bestrophin-2 chloride channel. J. Gen. Physiol. 124:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert, J., P.J. Bauer, K.-W. Yau, and S. Frings. 2003. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J. Gen. Physiol. 122:349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal, R., B. Bakall, T. Kinnick, N. Peachey, S. Wimmers, C. Wadelius, A. Marmorstein, and O. Strauss. 2006. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J. 20:178–180. [DOI] [PubMed] [Google Scholar]
- Schreiber, M., and L. Salkoff. 1997. A novel calcium-sensing domain in the BK channel. Biophys. J. 73:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, M., A. Yuan, and L. Salkoff. 1999. Transplantable sites confer calcium sensitivity to BK channels. Nat. Neurosci. 2:416–421. [DOI] [PubMed] [Google Scholar]
- Steinberg, R.H., R.A. Linsenmeier, and E.R. Griff. 1985. Retinal pigment epithelial cell contributions to the electroretinogram and electrooculogram. Prog. Retinal Res. 4:33–66. [Google Scholar]
- Sun, H., T. Tsunenari, K.-W. Yau, and J. Nathans. 2002. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl. Acad. Sci. USA. 99:4008–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunenari, T., H. Sun, J. Williams, H. Cahill, P. Smallwood, K.-W. Yau, and J. Nathans. 2003. Structure-function analysis of the bestrophin family of anion channels. J. Biol. Chem. 278:41114–41125. [DOI] [PMC free article] [PubMed] [Google Scholar]