Uniquely among the major intracellular second messengers, calcium is an element; an obvious statement, but one with profound consequences for its signaling functions. Being an element, cells cannot synthesize or degrade calcium, they can only actively move it from one place to another, allow it to move passively down concentration gradients, and let it bind to things. The active moving is done by pumps and transporters that establish enormous (>10,000-fold) concentration gradients of Ca2+ ions between the very low basal free [Ca2+] in the cytosol (∼50–100 nM) and the much higher concentrations in the extracellular fluid and reservoirs sequestered in intracellular organelles, principally the ER or SR and mitochondria. Opening of Ca2+-permeable channels in the plasma or intracellular membranes can then evoke large and extremely rapid increases in local cytosolic [Ca2+] as Ca2+ ions flow passively down their electrochemical gradient (Hille, 2001). Moreover, Ca2+ signals may be spatially, as well as temporally, restricted (Marchant and Parker, 2000). This is because diffusion becomes a relatively slow process at distances greater than a few micrometers, and because diffusion of Ca2+ ions in the cytosol is further restricted by binding to immobile buffers; although an interesting wrinkle is that the signal carried by Ca2+ (“message”) actually travels faster then the ions themselves (the “messengers”) (Pando et al., 2006). Cells have thus evolved a diverse and complex repertoire of Ca2+ signals, which are more tightly localized in space and time than is possible with “molecular” messengers such as cyclic nucleotides that diffuse more readily and are kinetically limited by enzyme turnover rates of synthesis and degradation (Allbritton et al., 1992). Such local intracellular Ca2+ signals have been a subject of much interest over the last decade or more; not least because Ca2+ is the only second messenger we can presently image with micrometer and millisecond resolution.
The best examples to date are provided by Ca2+ signals generated by liberation of Ca2+ ions from the ER and SR through inositol trisphosphate receptors (IP3R) and ryanodine receptors (RyR). Both of these receptors form Ca2+-permeable channels that have the notable property that their opening is promoted by cytosolic Ca2+ itself, leading to a regenerative mechanism of Ca2+-induced Ca2+ release (CICR). To prevent this process getting out of hand and generating all-or-none whole cell responses, IP3R and RyR are typically arranged in discrete clusters, permitting a hierarchical generation of signals ranging from opening of individual channels (generically termed “fundamental” events), through the concerted opening of several channels in a cluster by local CICR (“elementary” events), to global waves that propagate in a saltatory fashion between clusters by successive cycles of Ca2+ release, diffusion, and CICR (Berridge, 1997). The elementary events, including “puffs” mediated by IP3R (Parker and Yao, 1991) and “sparks” mediated by RyR (Cannell et al.,1993), serve as the basic building blocks of intracellular Ca2+ signals in many cell types. For example, sparks form the basis for the local control model of graded cardiac muscle contraction (Cannell et al., 1993), and permit a spatially regulated activation of plasma membrane Ca2+-dependent K+ channels (Jaggar et al., 1998).
In the case of Ca2+ influx across the plasma membrane, localized Ca2+ transients are well known to arise at specialized areas of high channel density, such as the active zones of neurotransmitter release at synapses (Neher, 1998). However, signals analogous to the elementary sparks and puffs (i.e., involving concerted local activation of small numbers of channels within a widely distributed population) have hitherto not been observed. This may not appear surprising because, unlike the CICR that provides positive feedback to orchestrate neighboring RyR and IP3R, the numerous types of voltage- and ligand-gated plasma membrane channels are generally either insensitive to cytosolic Ca2+ or are actually inhibited (Hille, 2001). Two recent papers from Santana and colleagues, however, now overthrow this paradigm, by revealing local Ca2+ signals that they identify as arising through L-type Ca2+ channels (LTCCs) operating in functionally ordered clusters (Navedo et al., 2005, 2006).
Navedo et al. (2005) imaged plasma membrane Ca2+ signals in arterial myocytes, after eliminating any intracellular release components by depleting SR stores with thapsigargin. Their results are striking in two regards. First, they observed spontaneous signals that likely arose from Ca2+ influx through constitutive activity of LTCCs at resting, or even hyperpolarized membrane potentials, where these voltage-gated channels might be expected to display a vanishingly low open probability (Fleischmann et al., 1994). Second, in addition to rare, small events that probably correspond to transient openings of single channels (Ca2+ “sparklets;” Wang et al., 2001), they found specific “persistent Ca2+ sparklet” sites (typically just one or a few per cell) at which a much greater Ca2+ influx arose through concerted openings of closely neighboring LTCC. Their paper in the present issue of the Journal of General Physiology (p. 611) now extends these findings by pinning down the major molecular players involved in the generation of persistent sparklets, and begins to elucidate the underlying mechanisms.
Imaging Elementary Ca2+ Signals
The discoveries of puffs, sparks, and now persistent sparklets, owe much to continued developments in Ca2+ imaging technology. Excellent fluorescent indicator dyes, exhibiting fast kinetics and large fluorescence changes on Ca2+-binding have been available for decades (Grynkiewicz et al., 1985), but the imaging of local Ca2+ signals presents particular problems by virtue of their brief durations and tight spatial localization. Optical sectioning techniques are highly advantageous to sharply delineate local fluorescence signals and isolate them from bulk fluorescence in the cell, and studies of intracellular Ca2+ signals have thus made much use of confocal microscopy. Confocal imaging, however, has a relatively poor axial sectioning ability (∼0.8 μm at best) and, being a point-scanning technique, is generally restricted in temporal resolution unless operated in a one-dimensional linescan mode (Pawley, 1995). Although this latter technique was used for the first recording of sparklets from individual LTCC in cardiac myocytes (Wang et al., 2001), it is less suitable for visualizing large areas of the cell membrane, as required to detect sparse events such as the persistent sparklets. Instead, Navedo et al. (2005, 2006) applied the technique of evanescent wave microscopy (also known as total internal fluorescence microscopy [TIRFM]), which is almost ideally suited to imaging plasma membrane Ca2+ signals.
The principle of TIRFM involves the total internal reflection of a light beam incident at a shallow angle on the interface between media with a high refractive index (e.g., a glass coverslip) and a lower refractive index (e.g., an aqueous solution or a cell). Even though the light is totally reflected according to traditional optical theory, a small amount of energy does pass through this interface into the lower refractive index medium in the form of an evanescent wave, which penetrates only a few tens of nanometers from the boundary (Axelrod, 2003). Thus, by letting indicator-loaded cells adhere closely to a coverslip (but not too closely, a thin film of extracellular fluid is required as a source of Ca2+), it is possible to almost perfectly isolate near-membrane Ca2+ fluorescence signals from fluorescence that would otherwise be excited deeper in the cell. Specific advantages of TIRFM are that the optical sectioning is much finer (∼100 nm) than possible with confocal microscopy, and that imaging is done with a camera, potentially allowing very high frame rates. For example, this technique has enabled optical recording of the activity of Ca2+-permeable channels with a fidelity approaching that of patch clamping (Demuro and Parker, 2005). Although TIRF microscopy has long been possible using systems whereby the excitation light is introduced at a shallow angle via a glass prism on which the specimen lies, this has disadvantages that fluorescence must be viewed from above after passing through the specimen, and that the microscope objective lens leaves little space for introduction of microelectrodes. Instead, the recent commercial introduction of a range of specialized TIRFM objectives with very high numerical apertures has made “through-the-lens” TIRFM possible with inverted microscopes, thereby greatly expanding the utility of this technique.
Persistent Sparklet Sites
Navedo et al. (2005) applied TIRF microscopy in conjunction with whole-cell voltage clamp to examine Ca2+ influx across the plasma membrane of rat arterial myocytes. Contrary to their initial expectations, they observed spontaneously active sites of sustained Ca2+ influx in cells clamped at strongly negative potentials, whereas the majority of the plasmalemma was silent or showed only infrequent, small signals. Several findings confirmed that the “persistent sparklet sites” indeed arose through influx of extracellular Ca2+, and not local release from intracellular stores. Among them, experiments were done in the presence of thapsigargin to deplete SR stores; the persistent signals varied in size with the electrochemical gradient for Ca2+ across the plasma membrane (i.e., with extracellular [Ca2+] and membrane potential); and the fluorescence signals were accompanied by, and correlated in magnitude with, inward Ca2+ currents. Moreover, application of dihydropiridine antagonist (nifedipine) and agonist (Bay-K 8644) respectively blocked and potentiated persistent sparklet activity, confirming an extracellular source of Ca2+ and pointing to L-type Ca2+ channels (LTCC) as the likely influx pathway.
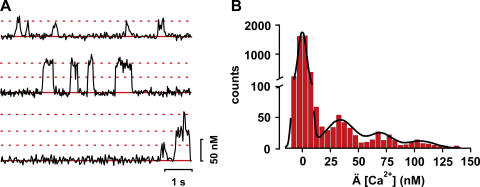
What then constitutes a persistent sparklet site? Is it a single channel with unusually large conductance, or a cluster of channels opening in near unison? Navedo et al. (2005, 2006) make a convincing case for the latter mechanism, by performing a quantal analysis analogous to that classically used to demonstrate the quantal nature of neurotransmitter release (del Castillo and Katz, 1954). Specifically, the distributions of fluorescence amplitudes at persistent sites showed several, clearly separated peaks spaced at integer multiples of a quantal level corresponding to a Ca2+ current of ∼0.5 pA (Fig. 1), consistent with that expected for an LTCC under those experimental conditions. They thus defined a metric for the activity of sparklet sites as nPs, where n is the number of quantal levels and Ps is the probability that a quantal sparklet event is active. On this basis, sparklet sites could be divided into three categories: silent (i.e., regions of the plasmalemma that were silent at rest, but could be provoked into activity by agonists such as Bay-K), low activity (nPs < 0.2), and high activity (nPs > 0.2).
Figure 1.
Quantal distribution of Ca2+ sparklet amplitudes in arterial myocytes. (A) Representative TIRFM Ca2+ fluorescence records from sparklet sites exhibiting one, two, and three quanta events. Dotted lines mark the quantal levels. (B) An all-points histogram generated from 25 representative Ca2+ sparklet traces. The solid curve is a multicomponent Gaussian fit to the data. Note the appearance of peaks at multiples of the quantal level (∼34 nM [Ca2+]). Data are reproduced from Fig. S1 of Navedo et al. (2006).
On a technical note, Navedo et al. (2005, 2006) expressed sparklet amplitudes in terms of free Ca2+ concentration, as calibrated from in vitro measurements of indicator properties and in vivo measurement of maximal fluorescence with saturating [Ca2+]. This provides a measure of average free [Ca2+] throughout the selected imaging region, and is a valid basis for comparison of signals at a given site or between sites. However, it should be noted that cytosolic gradients of free [Ca2+] around an open channel or tight cluster of channels are expected to be extremely steep, falling from tens of μM or more near a channel mouth to tens of nM only a few hundred nanometers away (Shuai and Parker, 2005). Thus, a measure of average [Ca2+] throughout a microdomain provides little or no information regarding the actual concentrations pertaining at different distances from a Ca2+ channel or cluster of channels. Moreover, it will be dependent on the specific parameters of the imaging system, including the point-spread function of the microscope and the dimensions of the selected region of interest.
Nevertheless, the quantal distribution of amplitudes is clear, and argues strongly that persistent sparklets arise because of the concerted activity of several LTCC. The sparklet sites, however, appear not to simply represent physical clusters of LTCC with the remainder of the plasmalemma expressing only sparsely distributed channels, because immunostaining for the CaV1.2 α subunit of LTCC showed a diffuse and strong distribution across the surface membrane. Instead, Navedo et al. (2005) proposed that the persistent sparklet sites are functional units, wherein the gating properties of several adjacent LTCC are modified to increase their opening probability. Given that LTCC activity is promoted by PKC (Fish et al., 1988), they thus examined the effects of the PKC agonist phorbol dibutyrate. In agreement with their hypothesis, PKC activation awoke previously silent sparklet sites, and increased the activity of low nPs sites. Moreover, immunofluorescence staining of PKC was tightly restricted to small clusters, mostly at or close to the plasmalemma. Thus, a spatially confined distribution of PKC may underlie the persistent Ca2+ influx at high activity sparklet sites, and determine the location and number of such sites in the arterial myocyte.
Molecular Machinery of Persistent Sparklets
Navedo et al. (2006) utilized a variety of molecular genetic and pharmacological approaches to further establish this model and pin down the major molecular players. Most directly and elegantly, the authors used a cell culture expression system to recapitulate the general features of Ca2+ sparklets observed in arterial myocytes.
Control (nontransfected) tsA-210 cells showed neither voltage-dependent Ca2+ influx nor sparklets, but these activities were reconstituted after transfection with Cavα1.2 and accessory subunits to express functional LTCC. The quantal unit of Ca2+ elevation during sparklets matched closely to that in the myocytes but, importantly, only low activity sites (nPs ∼0.05) were observed. However, cotransfection to express both Cavα1.2 LTCC and PKCα (an isoform highly expressed in cerebral artery myocytes) resulted in both an overall nPs about threefold greater than in cells expressing LTCC alone, and the appearance of high activity sites with properties (potentiation by Bay-K, dependence upon voltage and extracellular [Ca2+]) mirroring that of sparklets in the myocytes. Cells expressing PKCα alone never showed sparklets. Thus, Cava1.2 and PKCα appear to be the minimal molecular components required for persistent spark activity, and when expressed in a heterologous system, they closely replicated the pharmacology, gating modalities, and amplitudes of sparklets in the arterial myocytes (Navedo et al., 2006).
The authors then went on to identify whether any of the other three PKC isoforms expressed in cerebral artery myocytes also modulate sparklet activity. Both pharmacological and genetic approaches singled out PKCα as being the sole player. Specifically, an inhibitor selective for PKCα and PKCβ completely abolished sparklet activity in the rat myocytes, whereas a PKCβ-specific inhibitor was without effect; and arterial myocytes from a PKCα-knockout mouse were devoid of spontaneous sparklets, although these were plentiful in wild-type mice.
So, if PKCα is the “on” signal that promotes persistent sparklets, what might turn them off? Given earlier findings (Santana et al., 2002) that serine/threonine phosphatases modulate LTCC function, Navedo et al. (2006) examined whether the relative activities of PKCa and opposing protein phosphatases may function as a “rheostat” to regulate the number and activity of persistent sparklet sites. Consistent with this hypothesis, applications of a variety of phosphatase inhibitors activated previously silent sites and increased the activity of low nPs sites, pointing to a constitutive level of inhibitory regulation by phosphatases. Interestingly, though, high nPs sites were unaffected, suggesting that their activity is already maximal under basal conditions. These pharmacological experiments, moreover, singled out both PP2A and PP2B (calcineurin) as the phosphatases responsible.
Conclusions and Questions
Navedo et al. (2006) conclude that PKCα, PP2A, and PP2B together form a molecular signaling module in arterial myocytes that tunes clusters of several LTCC to generate spatially and temporally punctuate Ca2+ influx signals. Their experiments were enabled by the use of TIRF microscopy to resolve single-channel Ca2+ signals across a wide area of the cell membrane, and their results are striking in at least two aspects: the demonstration of appreciable Ca2+ influx through voltage-gated LTCC at resting or even hyperpolarized membrane potentials, and the finding of localized Ca2+ signals that arise through concerted openings of adjacent plasmalemmal channels. In the latter regard, the persistent sparklets may be added to the growing family of “elementary,” localized multichannel Ca2+ signals such as sparks and puffs, of which they are, to date, the only member involving plasmalemmal, as opposed to intracellular, Ca2+-permeable channels.
As with most breakthrough studies, these findings raise many further intriguing questions. What may be the physiological role of persistent sparklet sites, and why are they spatially localized in arterial myocytes? Are they functionally modulated by vasoactive agents that activate PKC? Can analogous events be detected in cardiac and other myocytes? What regulates the assembly and localization of the signaling module, and what is the mechanism that coordinates openings of LTCC during persistent sparklets? Restricting speculation to just the last question, one possibility is that Ca2+ activation of PKC forms a positive feedback mechanism, whereby Ca2+ entering through one channel results in phosphorylation of adjacent LTCC, in turn promoting their opening. However, the apparently simultaneous opening of multiple channels during persistent sparklet activity (e.g., Fig. 1) is difficult to reconcile with the turnover rate of such an enzymatic process, and this mechanism leaves unanswered the issue of how multiple channels can almost simultaneously close. Instead, it may be that the kinase/phosphatase rheostat modulates persistent sparklet activity on a relatively long-term, modal basis, and that a further mechanism must be sought for the rapid synchronization between LTCC during bursts of activity.
Acknowledgments
Work in the author's laboratory is supported by the National Institutes of Health grants GM 48071 and GM65830.
Abbreviations used in this paper: CICR, calcium-induced calcium release; IP3R, inositol 1,4,5-trisphosphate receptor; LTCC, L-type calcium channels; RyR, ryanodine receptor; TIRFM, total internal reflection fluorescence microscopy.
References
- Allbritton, N.L., T. Meyer, and L. Stryer. 1992. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 258:1812–1815. [DOI] [PubMed] [Google Scholar]
- Axelrod, D. 2003. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 361:1–33. [DOI] [PubMed] [Google Scholar]
- Berridge, M.J. 1997. Elementary and global aspects of calcium signaling. J. Physiol. 499:291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell, M.B., H. Cheng, and W.J. Lederer. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744. [DOI] [PubMed] [Google Scholar]
- del Castillo, J., and B. Katz. 1954. Quantal components of the end-plate potential. J. Physiol. 124:560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro, A., and I. Parker. 2005. “Optical patch-clamping”: Single-channel recording by imaging Ca2+ flux through individual muscle acetylcholine receptor channels. J. Gen. Physiol. 126:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish, R.D., G. Sperti, W.S. Colucci, and D.E. Clapham. 1988. Phorbol ester increases the dihydropyridine-sensitive calcium conductance in a vascular smooth muscle cell line. Circ. Res. 62:1049–1054. [DOI] [PubMed] [Google Scholar]
- Fleischmann, B.K., R.K. Murray, and M.I. Kotlikoff. 1994. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc. Natl. Acad. Sci. USA. 91:11914–11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz, G., M. Poenie, and R.Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450. [PubMed] [Google Scholar]
- Hille, B. 2001. Ion Channels of Excitable Membranes. Third edition. Sinauer, Sunderland, MA. 722 pp.
- Jaggar, J.H., G.C. Wellman, T.J. Heppner, V.A. Porter, G.J. Perez, M. Gollasch, T. Kleppisch, M. Rubart, A.S. Stevenson, W.J. Lederer, et al. 1998. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol. Scand. 164:577–587. [DOI] [PubMed] [Google Scholar]
- Neher, E. 1998. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 20:389–399. [DOI] [PubMed] [Google Scholar]
- Marchant, J.S., and I. Parker. 2000. Functional interactions in Ca2+ signaling over different time and distance scales. J. Gen. Physiol. 116:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo, M.F., G.C. Amberg, M. Nieves, J.D. Molkentin, and L.F. Santana. 2006. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J. Gen. Physiol. 127:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo, M.F., G.C. Amberg, V.S. Votaw, and L.F. Santana. 2005. Constitutively active L-type Ca2+ channels. Proc. Natl. Acad. Sci. USA. 102:1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pando, B., S.P. Dawson, D.-O.D. Mak, and J.E. Pearson. 2006. Messages diffuse faster than messengers. Proc. Natl. Acad. Sci. USA. 103:5338–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, I., and Y. Yao. 1991. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc. Biol. Sci. 246:269–274. [DOI] [PubMed] [Google Scholar]
- Pawley, J.B. 1995. Handbook of Biological Confocal Microscopy. Second edition. Plenum Press, New York. 650 pp.
- Santana, L.F., E.G. Chase, V.S. Votaw, M.T. Nelson, and R. Green. 2002. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J. Physiol. 544:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai, J., and I. Parker. 2005. Optical single-channel recording by imaging Ca2+ flux through individual ion channels: theoretical considerations and limits to resolution. Cell Calcium. 37:283–299. [DOI] [PubMed] [Google Scholar]
- Wang, S.Q., L.S. Song, E.G. Lakatta, and H. Cheng. 2001. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 410:592–596. [DOI] [PubMed] [Google Scholar]