Abstract
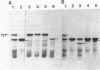
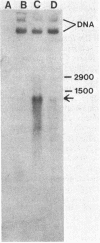
The structural gene region for tetracycline resistance on Tn10 consists of two complementation groups, tetA and tetB (M. S. Curiale and S. B. Levy, J. Bacteriol. 151:209-215, 1982). Using a series of deletion mutants, we have determined that the tetA region is 450 to 600 base pairs long and that the tetB region, which is adjacent to tetA, is 600 to 750 base pairs long. Point mutations in either tetA or tetB affected the amount and size of the inducible inner-membrane Tet protein synthesized in Escherichia coli maxicells. Moreover, deletions in these regions led to the synthesis of an appropriately smaller Tet protein. A single tetracycline-inducible RNA of about 1,200 bases was detected that was homologous with the tetracycline resistance structural gene region. These results indicate that the tetA and tetB complementation regions represent two parts of a single gene encoding two domains of the tetracycline resistance protein Tet.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Mutzel R., Barbé J., Müller W. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J Bacteriol. 1982 May;150(2):633–642. doi: 10.1128/jb.150.2.633-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Shales S. W., Ward J. M., Wallace L. J. Reduced expression of Tn 10-mediated tetracycline resistance in Escherichia coli containing more than one copy of the transposon. J Gen Microbiol. 1981 Sep;126(1):45–54. doi: 10.1099/00221287-126-1-45. [DOI] [PubMed] [Google Scholar]
- Coleman D. C., Chopra I., Shales S. W., Howe T. G., Foster T. J. Analysis of tetracycline resistance encoded by transposon Tn10: deletion mapping of tetracycline-sensitive point mutations and identification of two structural genes. J Bacteriol. 1983 Feb;153(2):921–929. doi: 10.1128/jb.153.2.921-929.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Foster T. J. Analysis of the reduction in expression of tetracycline resistance determined by transposon Tn10 in the multicopy state. Mol Gen Genet. 1981;182(1):171–177. doi: 10.1007/BF00422786. [DOI] [PubMed] [Google Scholar]
- Curiale M. S., Levy S. B. Two complementation groups mediate tetracycline resistance determined by Tn10. J Bacteriol. 1982 Jul;151(1):209–215. doi: 10.1128/jb.151.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biochemical and immunological characterization of an R plasmid-encoded protein with properties resembling those of major cellular outer membrane proteins. J Bacteriol. 1980 Oct;144(1):149–158. doi: 10.1128/jb.144.1.149-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Klock G., Kaffenberger I., Wray L. V., Reznikoff W. S. Purification of the TET repressor and TET operator from the transposon Tn10 and characterization of their interaction. J Biol Chem. 1982 Jun 10;257(11):6605–6613. [PubMed] [Google Scholar]
- Hillen W., Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983 Jan 25;11(2):525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Unger B. Binding of four repressors to double-stranded tet operator region stabilizes it against thermal denaturation. Nature. 1982 Jun 24;297(5868):700–702. doi: 10.1038/297700a0. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Localization of two functions of the phosphoribosyl anthranilate transferase of Escherichia coli to distinct regions of the polypeptide chain. J Bacteriol. 1974 Feb;117(2):502–508. doi: 10.1128/jb.117.2.502-508.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness D. D., Schachman H. K. Genetic characterization of the folding domains of the catalytic chains in aspartate transcarbamoylase. J Biol Chem. 1983 Mar 10;258(5):3266–3279. [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S., Crawford I. P. Complementation in vitro between mutationally altered beta2 subunits of Escherichia coli tryptophan synthetase. J Bacteriol. 1974 May;118(2):551–559. doi: 10.1128/jb.118.2.551-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Plasmid-determined tetracycline resistance involves new transport systems for tetracycline. Nature. 1978 Nov 2;276(5683):90–92. doi: 10.1038/276090a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B. R factor proteins synthesized in Escherichia coli minicells: incorporation studies with different R factors and detection of deoxyribonucleic acid-binding proteins. J Bacteriol. 1974 Dec;120(3):1451–1463. doi: 10.1128/jb.120.3.1451-1463.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Z. Directed mutagenesis method for analysis of mutagen specificity: application to ultraviolet-induced mutagenesis. Proc Natl Acad Sci U S A. 1983 Jan;80(1):237–241. doi: 10.1073/pnas.80.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Marshall B., Tachibana C., Levy S. B. Frequency of tetracycline resistance determinant classes among lactose-fermenting coliforms. Antimicrob Agents Chemother. 1983 Dec;24(6):835–840. doi: 10.1128/aac.24.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Cullinane J. C., Levy S. B. Transport of the lipophilic analog minocycline differs from that of tetracycline in susceptible and resistant Escherichia coli strains. Antimicrob Agents Chemother. 1982 Nov;22(5):791–799. doi: 10.1128/aac.22.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Cullinane J. C., Petrucci R. E., Jr, Levy S. B. Active uptake of tetracycline by membrane vesicles from susceptible Escherichia coli. Antimicrob Agents Chemother. 1981 Sep;20(3):307–313. doi: 10.1128/aac.20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Levy S. B. Two transport systems for tetracycline in sensitive Escherichia coli: critical role for an initial rapid uptake system insensitive to energy inhibitors. Antimicrob Agents Chemother. 1978 Aug;14(2):201–209. doi: 10.1128/aac.14.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Peden K. W. Revised sequence of the tetracycline-resistance gene of pBR322. Gene. 1983 May-Jun;22(2-3):277–280. doi: 10.1016/0378-1119(83)90112-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. H., Rogowsky P., Grinsted J., Altenbuchner J., Schmitt R. The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res. 1983 Sep 10;11(17):6089–6105. doi: 10.1093/nar/11.17.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray L. V., Jr, Jorgensen R. A., Reznikoff W. S. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J Bacteriol. 1981 Aug;147(2):297–304. doi: 10.1128/jb.147.2.297-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Levy S. B. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc Natl Acad Sci U S A. 1976 May;73(5):1509–1512. doi: 10.1073/pnas.73.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabin I., Villarejo M. R. Protein complementation. Annu Rev Biochem. 1975;44:295–313. doi: 10.1146/annurev.bi.44.070175.001455. [DOI] [PubMed] [Google Scholar]