Abstract
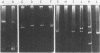
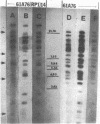
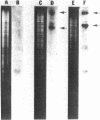
The P group resistance plasmids RP1 and RP4 were introduced into Rhizobium japonicum by polyethylene-glycol-induced transformation of spheroplasts. After cell wall regeneration, transformants were recovered by selecting for plasmid determinants. Plant nodulation, nitrogen fixation, serological, and bacterial genetics studies revealed that the transformants were derived from the parental strains and possessed the introduced plasmid genetic markers. Agarose gel electrophoresis, restriction enzyme analysis, and DNA hybridization studies showed that many of the transformant strains had undergone genome rearrangements. In the RP1 transformants, chromosomal DNA was found to have transposed into a large indigenous plasmid of R. japonicum, producing an even larger plasmid, and the introduced R plasmid DNA was found to be chromosomally integrated rather than replicating autonomously or integrated into the endogenous plasmid. Seemingly, a similar section of chromosomal DNA was involved in all the genomic rearrangements observed in the R. japonicum RP1 and RP4 transformant strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G., Suchanek M. C. Characterization of mutants of Escherichia coli temperature-sensitive for ribonucleic acid regulation: an unusual phenotype associated with a phenylalanyl transfer ribonucleic acid synthetase mutant. J Bacteriol. 1971 Nov;108(2):627–638. doi: 10.1128/jb.108.2.627-638.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisona N. J., Nowak J. A., Nagaishi H., Clark A. J. Transposon-mediated conjugational transmission of nonconjugative plasmids. J Bacteriol. 1980 May;142(2):701–713. doi: 10.1128/jb.142.2.701-713.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Haugland R., Verma D. P. Interspecific plasmid and genomic DNA sequence homologies and localization of nif genes in effective and ineffective strains of Rhizobium japonicum. J Mol Appl Genet. 1981;1(3):205–217. [PubMed] [Google Scholar]
- Holloway B. W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979 Jan;2(1):1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Holsters M., Silva B., Genetello C., Engler G., van Vliet F., de Block M., Villarroel R., van Montagu M., Schell J. Spontaneous formation of cointegrates of the oncogenic Ti-plasmid and the wide-host-range P-plasmid RP4. Plasmid. 1978 Sep;1(4):456–467. doi: 10.1016/0147-619x(78)90004-5. [DOI] [PubMed] [Google Scholar]
- Imsande J., Ralston E. J. Dinitrogen fixation in male-sterile soybeans. Plant Physiol. 1982 Mar;69(3):745–746. doi: 10.1104/pp.69.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L. Acetylene reduction by pure cultures of Rhizobia. J Bacteriol. 1975 Sep;123(3):1265–1268. doi: 10.1128/jb.123.3.1265-1268.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmel'nitskii M. I., Zlotnikov K. M., Baev A. A. Geneticheskii analiz u Rizobium japonicum. Dokl Akad Nauk SSSR. 1981;256(1):191–195. [PubMed] [Google Scholar]
- Kuykendall L. D. Transfer of R factors to and between genetically marked sublines of Rhizobium japonicum. Appl Environ Microbiol. 1979 May;37(5):862–866. doi: 10.1128/aem.37.5.862-866.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Mutant Strains of Rhizobium japonicum with Increased Ability to Fix Nitrogen for Soybean. Science. 1978 Aug 4;201(4354):448–450. doi: 10.1126/science.201.4354.448. [DOI] [PubMed] [Google Scholar]
- Masterson R. V., Russell P. R., Atherly A. G. Nitrogen fixation (nif) genes and large plasmids of Rhizobium japonicum. J Bacteriol. 1982 Nov;152(2):928–931. doi: 10.1128/jb.152.2.928-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Siak J. S., Gray R. H. Characteristics of PRD1, a plasmid-dependent broad host range DNA bacteriophage. J Virol. 1974 Sep;14(3):689–699. doi: 10.1128/jvi.14.3.689-699.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Thomas D. D. Characteristics and purification of PRR1, an RNA phage specific for the broad host range Pseudomonas R1822 drug resistance plasmid. J Virol. 1973 Dec;12(6):1560–1567. doi: 10.1128/jvi.12.6.1560-1567.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Schmidt E. L. Plasmid transfer within and between serologically distinct strains of Rhizobium japonicum, using antibiotic resistance mutants and auxotrophs. J Bacteriol. 1981 Feb;145(2):1025–1030. doi: 10.1128/jb.145.2.1025-1030.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Werner D., Wilcockson J., Zimmermann E. Adsorption and selection of rhizobia with ion-exchange papers. Arch Microbiol. 1975 Sep 30;105(1):27–32. doi: 10.1007/BF00447108. [DOI] [PubMed] [Google Scholar]